Abstract
Coal and gas outbursts have occurred in the Zhaozhuang coal mine (a high gas coal mine) since its construction, and these outbursts pose a great threat to lives and property. Therefore, the combination of methane desorption experiments and molecular simulations was adopted to investigate the effects of moisture on the methane desorption characteristics of the Zhaozhuang coal, and the microscopic mechanism was analyzed in this paper. The aim of this work is to provide a method for predicting the effect of moisture on coal seam methane desorption, and quantitatively evaluating the control effect of hydraulic measures on coal and gas outbursts from a molecular perspective. The experimental data (methane desorption amount) were measured under various adsorption equilibrium pressures (0.3 MPa, 0.4 MPa, and 0.5 MPa) and moisture contents (0%, 5%, and 9.8%). Based on the Zhaozhuang coal molecular model, the molecular simulation process was conducted using Materials studio software, which can better match the experimental conditions by setting various pressure parameters (0.3 MPa, 0.4 MPa, and 0.5 MPa) and calculating various numbers of H2O molecules (0, 8, and 16) on the coal molecule. The results show that methane desorption decreases with the increasing moisture content in the experiment. The number of CH4 molecules adsorbed on the coal molecule decreases with the increasing number of H2O molecules in the simulation. This phenomenon can be explained by the competitive adsorption between CH4 and H2O molecule: the interaction between coal molecule and H2O molecules is stronger. The affinity between CH4 molecules and the coal molecule is reduced, and CH4 molecules are less aggregated around the coal molecule because of the presence of H2O molecules.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coal and gas outbursts are dynamic phenomena with violent eruptions of coal and methane from the working coal seam (Liang et al. 2018; An et al. 2018); these outbursts can not only damage seriously facilities and ventilation systems, but can also cause methane explosions and human suffocation due to methane emissions, and such outbursts are among the major serious natural hazards during underground coal mining (Sun et al. 2018). Therefore, during the coal mining process, outbursts cause casualties and economic losses (Wang et al. 2018).
The first reported coal and methane outburst occurred in the Issac Colliery, Loire coal field, France, in 1843, and similar accidents have also occurred in many coal producing countries (Sun et al. 2016). According to the statistics, there have been more than 20,000 coal and methane outburst accidents in China so far, causing thousands of deaths and huge economic losses (Zhai et al. 2016). Taking the Zhaozhuang coal mine (a high methane coal mine) as an example, there have been some coal and gas outburst accidents in the coal mine in recent years, and the most serious accident caused five deaths and the direct economic loss of 3,000,000 RMB. Therefore, researchers have gradually started to study the factors affecting methane adsorption/desorption from the microscopic point of view, such as pore structure (Chen et al. 2018), crystallite structure (Chen et al. 2018), etc. to better understand the nature of coal and gas outbursts and to propose more effective prevention measures.
However, hydraulic measures are now the most widely used in reducing coal and gas outbursts, such as hydraulic flushing (Shen et al. 2018), hydraulic seam cutting (Yang et al. 2016), hydraulic fracturing (Ni et al. 2018), etc. These techniques have been indicated to increase the moisture content of the coal seam. Water molecules can occupy the methane molecule adsorption sites and displace methane molecules in a coal pore, thus reducing the methane content of the coal seam and resulting in reduced methane desorption (Ozdemir and Schroeder 2009; Erdöl et al. 1999; Ding et al. 2014). Therefore, understanding the effects of moisture on methane desorption remains important.
Many researchers have conducted experiments on the effects of moisture on methane desorption characteristics and have arrived at similar conclusions. Nie et al. (2015) researched the amount and rate of methane desorption by designing an experiment under different moisture contents and analysing the mechanism, concluding that moisture plays an inhibitory role in methane desorption. Li et al. (2011) combined simulation testing and theoretical analysis to study the effects of moisture on methane desorption laws in the Xinjing coal mine, concluding that moisture can inhibit methane desorption. Yang (2013) conducted methane desorption experiments to explore the effects of water from the external environment on methane desorption, concluding that methane desorption was reduced due to the presence of moisture. Xiao and Meng (2015), Chen et al. (2013), and Zhao et al. (2011) all designed an experiment involving water injection and observed the corresponding variation of methane desorption, concluding that water injection can inhibit methane desorption. Guo and Su (2010) analysed the mechanism and justification for injecting water to prevent methane outbursts. Ni et al. (2014) researched methane desorption by pulsing water injection, concluding that the methane desorption was reduced by pulsing water injection.
Wang et al. (2017) researched the effect of moisture on methane desorption in hard coals, concluding that water molecules can occupy methane adsorption sites of pore structures with increasing moisture content and weaken methane desorption capacity. Pan et al. (2010) researched the effects of moisture in the coal matrix on methane diffusion and flow; moisture in the coal matrix caused expansion or shrinkage of coal and changed the coal strength, and it was concluded that moisture in the coal matrix plays an inhibition role for methane diffusion and flow. Zeng et al. (2017) conducted limit analysis, approximate analysis, and methane adsorption experiments; experimental data were analysed by regression to obtain the best fitting parameter, and it was concluded that there was competitive adsorption between moisture and methane, and moisture reduced the ability of coal to adsorb methane. These studies show macroscale performance results, but the microscopic mechanism of moisture influence is not reflected. Therefore, this paper focuses on the combination of molecular simulation and experimental desorption.
The most fundamental reason for the change in macroscopic phenomenon is the change in microscopic mechanism of action. Molecular simulation is usually used to illustrate the microscopic mechanism between different molecules.
Liu et al. (2016) used the density functional theory and the grand canonical Monte Carlo method (hereinafter, collectively referred to as ‘the GCMC’) to simulate the adsorption behaviour of CH4, H2O, CO2, and N2 molecules on the surface of coal pores, concluding that the methane adsorption amount decreases with the increasing moisture content at constant temperature and pressure. Sizova et al. (2015) used the GCMC to simulate, respectively, the adsorption behaviour of CH4 and CO2 molecules in wet and dry activated carbon, concluding that the adsorption capacity of CH4 and CO2 molecules is lower in wet activated carbon. Jin et al. (2017) constructed a macromolecular structure model containing 19 Tashan bituminous coal molecules to discuss adsorption isotherms, isosteric heat of adsorption, energy distribution, and probability density distribution under various pressures and temperatures when water molecules were adsorbed on the model; the adsorption mechanism was inferred.
Xiang et al. (2014) used the Yanzhou coal molecular model to simulate the adsorption behaviour of CH4, H2O, and CO2 molecules, concluding that the adsorption is highest for H2O molecules, which are adsorbed preferentially; and the adsorption law is the opposite for CH4 molecules. Zhou et al. (2016) and Yu et al. (2017) used the density functional theory and the GCMC to research the adsorption behaviour of CH4 and H2O molecules on coal molecule, and it was concluded that the adsorption capacity of H2O molecules is stronger than that of CH4 molecules. At present, these studies are mainly in the theoretical simulation stage, so it is necessary to conduct further research in combination with experiments.
The objective of this paper is to combine methane desorption experiments and molecular simulations to probe the effects of moisture on methane desorption, and analyse the mechanism. The simulation results can provide a method for predicting the effects of moisture on coal seam methane desorption and quantitatively evaluating the control effect of hydraulic measures on coal and methane outbursts from a molecular perspective.
Experiment
Sample preparation
Sample basic information
Experimental coal samples were collected from the No. 3 coal seam of the Zhaozhuang coal mine of the Jinmei Coal Group in Shanxi Province, China. The coal-bearing strata of the coal mine is the Late Paleozoic Carboniferous-Permian, mostly covered by the quaternary system. The coal mine is controlled by the neocathaysian structural system, and its structural trend is arranged in xi-type. On the basis of gentle strata and small dip angle, the strata are also accompanied by wide and gentle folds, small-scale faults, and a collapse column. The exposed faults are all normal faults, and the dip angle of the strata near these faults is more than 15°.
The average thickness is 4.68 m, and the average buried depth is 667 m for the coal seam. The main component of coal seam roof includes sandy mudstone, siltstone, and dark grey mudstone, and the coal seam floor consists of black mudstone, sandy mudstone, and dark grey siltstone.
The ground stress of the coal seam ranges from 1.40 to 6.5 MPa, and the ground stress of coal body at the sampling site is approximately 3.012 MPa. The No. 3 coal seam of the Zhaozhuang coal mine has large thickness, good stability, and excellent coal quality, and it is the primary mineable coal seam in the Zhaozhuang coal mine. For the coal body, the overall structure is relatively broken, mechanical strength is low, the appearance is brighter, and the fracture is jagged and stepped.
According to the file entitled “Measurement report on the basic parameters of gas in No. 3 coal seam of the Zhaozhuang coal mine of the Jinmei Coal Group”, the initial gas pressure in the coal seam ranges from 0.31 to 0.46 MPa, the initial gas content ranges from 3.55 to 18.16 m3/t, and the average initial gas content is 9.13 m3/t.
The sample was collected according to Chinese standard GB/T 482-2008 and placed in a sampling bag, and then, the bag was tightened and vacuum-sealed to prevent contamination and oxidation of the sample. After collection, the sample was immediately delivered to the laboratory for sample preparation.
The sample was pulverized and screened to through pass 60–80 mesh sieves, and it was dried continuously for 12 h at 80 °C. The proximate analysis of samples was performed following the international standard ISO 11,722: 2013 and ISO 1171: 2010. The ultimate analysis of samples was conducted in the light of international standard ISO 17,247: 2013 and ISO 19,579: 2006. The vitrinite reflectance (Ro) and maceral composition of the coal were determined in accordance with international standard ISO 74,045: 2009 and Chinese standard GB/T 8899–1998, respectively. The density was measured using a 3H-2000 TD1 automatic true density analyser. The pore structure characteristics were measured using an ASAP-2020 automatic surface area and pore diameter distribution analyser. Basic information on the coal sample is shown in Table 1.
Different moisture content samples
Many researchers have already described the preparation methods for coal samples with different water content. Zhao et al. (2018) used a drying method, and dry and wet cylindrical coal samples were weighed to calculate the moisture content. Zhang and Ma (2008) put steam into coal until it was saturated, and coal samples were vacuumed successively to obtain different moisture content coal samples. Gao et al. (2018) put three coal samples into water, and they were moistened until they reached saturation, and then, two of these coal samples were dried with different times to obtain the desired coal samples. Nie et al. (2016) adsorbed steam in coal to obtain different moisture content coal samples, with less error. Wang et al. (2014) prepared different moisture content coal samples by setting the value of relative humidity for the incubator.
Due to the low mechanical strength of the Zhaozhuang coal sample, it is difficult to obtain raw coal by drilling. Therefore, cylindrical coal samples made of pulverized coal were used to study the methane desorption characteristics of the sample in this paper. Zhang et al. (2014), Cai et al. (2016), and Zhai (2018) have shown that it is feasible to study the universal law of methane adsorption–desorption with cylindrical coal samples instead of raw coal. Considering the actual condition of coal samples required in the experiment, the drying method was adopted to prepare different moisture content coal samples.
Dry pulverized coal with a mass of 260 g was weighed, and then, a small amount of coal tar was added and mixed fully. Next, the pulverized coal was put into the standard abrasive tool with a container size of 50 mm × 100 mm for pressurization to obtain the cylindrical coal sample. During drying the prepared cylindrical coal sample, the coal sample was weighed every hour until the two adjacent weight changes did not exceed 0.1%. At this time, the coal sample with 0% moisture content was successfully prepared, and the weight was \(M_{\text{coal}}\). Several groups of dry pulverized coal with mass of 260 g were weighed and added coal tar according to the above process, and then, different volumes of water were added to each group of pulverized coal using a measuring cylinder, and were mixed fully with pulverized coal. The mixture was put in the standard abrasive tool for pressurization until no moisture was extruded, and the cylindrical coal samples with varying moisture content were prepared successfully. Finally, these coal samples were removed and placed in a constant temperature (25 °C) curing box, and weighed every hour until the weight changes did not exceed 0.1%; their weight is \(M_{\text{coal/moisture}}\). All prepared coal samples were placed in a container that does not absorb moisture and is sealed. The moisture content of the coal sample was calculated according to formula (1); two groups of coal samples with 5% and 9.8% moisture content were selected. Figure 1 presents these coal samples.
where \(W\%\) is the moisture content of the coal sample, \(M_{{{\text{coal}}/{\text{moisture}}}}\) is the mass of the wet coal sample (g), and \(M_{{{\text{coal}}}}\) is the mass of the dry coal sample (g).
Experimental equipment and procedure
Methane desorption equipment under triaxial stress was used to complete the experiment, as it can provide stable pressure and temperature. The equipment was composed of a mechanical press, triaxial holding device, confining pressure loading device, gas cylinder, temperature controller, mass flowmeter, pressure gauge, and water-ring vacuum pump. The coal sample was placed in the cavity of triaxial holding device. Methane can enter the cavity from the air inlet, pass through the coal sample, and outflow from the air outlet. The interfaces on both sides of the cavity were used to connect the temperature controller and confining pressure loading device, so the temperature and confining pressure required in the experiment can be controlled. The way of the experimental system connection and a picture of the triaxial holding device are shown in Figs. 2 and 3, respectively.
Before the experiment started, the temperature controller was set to 25 °C and maintained until stable. Next, the experimental system was vacuumed by a vacuum pump; then, the coal sample was put into the triaxial holding device, and confining pressure and axial pressure were adjusted continuously until reaching 3 MPa. After the above steps were complete, the methane cylinder outlet pressure was set to 0.3 MPa, and methane was used to fill the triaxial holding device via the methane cylinder. The air inlet was closed, and the air outlet was opened when the pressure in the triaxial holding device no longer changed. The methane desorption was measured using a mass flowmeter. When methane desorption did not change substantially with time, the desorption process was considered basically completed. The above steps were repeated changing the methane cylinder outlet pressure (0.4 and 0.5 MPa) and coal sample.
Results and analysis
The variations of methane desorption with time under different moisture content conditions are shown in Fig. 4. It can be seen that the methane desorption curves all show a monotone increasing trend; the growth rate of methane desorption amount is faster in the first 20 min, and then, it slows down and gradually becomes gentler after 20 min. The methane desorption velocity and methane desorption quantity of dry coal samples is much larger than that of wet coal samples in the early stage of methane desorption, and the difference gradually decreases with the methane desorption. It indicates that moisture has a significant inhibitory effect on the methane desorption in coal. There is difference in methane desorption velocity and methane desorption quantity for both coal samples with 5% and 9.8% moisture contents, but the difference is smaller than that between dry coal samples and 5% moisture content coal samples. It indicates that the methane desorption process is still inhibited with the increase of moisture content in wet coal samples, but the inhibition is weaker than that of moisture on dry coal samples.
After the methane desorption process is completed, the accumulated desorption of methane measured under different moisture contents and adsorption equilibrium pressures are shown in Table 2. As seen from the experimental data, the accumulated desorption amount of methane in the saturated moisture content coal sample decreases by 60.2% compared to dry coal samples at 0.3 MPa, and decreases by 56.6% and 37.1% at 0.4 and 0.5 MPa, respectively. These decreases indicate that the adsorption equilibrium pressure can reduce the inhibition of moisture on methane desorption.
Molecular simulation
Model construction
In this section, the Zhaozhuang coal molecular model (C183H130O20N2) constructed by Meng et al. (2018) was used to simulate adsorption behaviours of methane in coal and analyse adsorption mechanism under various moisture contents (0%, 5% and 9.8%). The molecular model is in good agreement with the results of proximate analysis, elemental analysis, 13C nuclear magnetic resonance (13C-NMR), and X-ray photoelectron spectroscopy (XPS). Additionally, the size of the aromatic core in the coal sample and the existing form of elements (C, H, O, and N) on the coal sample surface were considered when the Zhaozhuang coal molecular model was constructed to allow the model to comprehensively reflect the macromolecular structural characteristics of the Zhaozhuang coal.
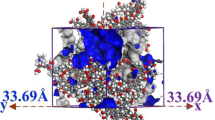
The planar graph of the Zhaozhuang coal molecule is shown in Fig. 5. It was imported into the Materials Studio 8.0 software (hereinafter, collectively referred to as ‘the MS’) to obtain the three-dimensional space graph, and geometrically optimized and annealed to obtain a new three-dimensional space graph with the lowest energy. The optimum density of the coal molecule (1.15 g/cm3) was obtained by analysing the relationship between energy and density. Then, the coal molecule was enclosed in the periodic boundary condition, as shown in Fig. 6.
CH4 and H2O molecules are described using a united-atom model. They can be constructed in the MS and optimized for geometric structures in the Forcite module. The three-dimensional structure graph is shown in Fig. 7.
Simulation details
According to previous research, the GCMC is an effective tool to analyse the adsorption characteristics and adsorption mechanisms of porous materials (Liu and Wilcox 2012; Mosher et al. 2013; Brochard et al. 2012). Therefore, we used the sorption module in the MS to simulate the adsorption behaviour of CH4 molecule under various pressures and moisture content combining the GCMC.
Experimental pressure (0.3 MPa, 0.4 MPa, and 0.5 MPa) was converted to fugacity according to the Peng–Robinson (PR) formula (Li et al. 2017), and fugacity was used as an input condition in the simulation by the GCMC (You et al. 2016). Experimental moisture contents (0%, 5%, and 9.8%) were converted to the number of H2O molecules (0, 8, and 16) required for the simulation by calculation, so the moisture content of coal molecule in the simulation is consistent with that of the experiment. The simulation temperature was set to 298 K, and force field was set to COMPASS; Yang et al. (2000) and Hu et al. (2010) had proved that the COMPASS force field can be used to describe the interaction between gas molecules and coal molecules. Ewald and Group were used to calculate the electrostatic force (Xiang et al. 2014); the Ewald accuracy was set to 4.18 × 10–3 kJ/mol, and the atom-based method was used to describe the van der Waals force (Karasawa and Goddard 1992), and the cut-off distance was set to 12.5 Å. Furthermore, to achieve a true balance of the system, 2 × 107 configurations were generated in each the GCMC simulation; the former 107 configurations were discarded to guarantee equilibration, and the latter 107 configurations were used to calculate the ensemble averages.
Simulation results
The adsorption behaviour of CH4 molecule on the coal molecule was simulated under different fugacity (matching experimental pressure), but the premise was that the coal molecules had adsorbed different numbers of H2O molecules (0, 8, and 16) to match experimental moisture content (0%, 5%, and 9.8%). From Fig. 8, the maximum number of CH4 molecules adsorbed on the coal molecule decreased as the number of water molecule increased when the simulation pressure was the same.
At 0.3 MPa, after 8 H2O molecules were adsorbed on the coal molecule, the adsorption sites of the CH4 molecules numbered 1–0.3 and 2–0.3 were occupied by the H2O molecules numbered 1–0.3* and 2–0.3*, respectively, and the adsorption distance of the CH4 molecule numbered 3–0.3 became larger. After 16 H2O molecules were adsorbed on the coal molecule, the adsorption site of the CH4 molecule numbered 4–0.3 was occupied by the H2O molecule numbered 4–0.3*, and the adsorption distance of the CH4 molecule numbered 3–0.3 was further increased.
At 0.4 MPa, after 8 H2O molecules were adsorbed on the coal molecule, and the adsorption distances of the CH4 molecules numbered 1–0.4 and 3–0.4 all increased; furthermore, the adsorption site of the CH4 molecule numbered 2–0.4 was occupied by the H2O molecule numbered 2–0.4*, and the CH4 molecule numbered 5–0.4 was no longer adsorbed by the coal molecule. After 16 H2O molecules were adsorbed on the coal molecule, the adsorption distance of the CH4 molecule numbered 3–0.4 was further increased, the adsorption site of the CH4 molecule numbered 4–0.4 was occupied by the H2O molecule numbered 4–0.4*, and the CH4 molecule numbered 1–0.4 was no longer adsorbed by the coal molecule.
At 0.5 MPa, after 8 H2O molecules were adsorbed on the coal molecule, the adsorption distances of the CH4 molecules numbered 1–0.5 and 3–0.5 were all increased, the CH4 molecule numbered 5–0.5 was no longer adsorbed, and the adsorption sites of the CH4 molecules numbered 2–0.5 and 6–0.5 were occupied by the H2O molecules numbered 2–0.5* and 6–0.5*, respectively. After 16 H2O molecules were adsorbed on the coal molecule, the CH4 molecule numbered 1–0.5 was no longer adsorbed on the coal molecule, the adsorption distance of the CH4 molecule numbered 3–0.5 was further increased, and the adsorption site of the CH4 molecule numbered 4–0.5 was occupied by the H2O molecule numbered 4–0.5*.
It can be seen from the above results that H2O molecules can occupy effective adsorption sites in coal molecule and weaken the adsorption of CH4 molecules on the coal molecule. Therefore, the more adsorption sites occupied by H2O molecules, the fewer adsorption sites occupied by CH4 molecules. The conclusion is consistent with the findings of previous studies (Li et al. 2018; Zhao et al. 2017; Lin et al. 2016).
Since a single coal molecule is used in this paper, the maximum adsorption number of CH4 molecules hardly changes as a part of the simulation environment. In view of this, we introduced the average number of CH4 molecules adsorbed on the coal molecule, and it is shown in Fig. 9. From Fig. 9, there are differences in the average number of CH4 molecules adsorbed on the coal molecule at different simulation pressures.
Comparison between experimental and simulation results
The adsorption process in the simulation was conducted under an ideal state, and non-adsorbed components in the coal sample were not considered; therefore, the adsorption amount in the simulation is equivalent to the desorption amount (in simulation, desorption amount is expressed by adsorption amount). The number of CH4 molecules absorbed on a single coal molecule was obtained by simulation, and the desorption amount of methane adsorbed on a 260 g coal sample was obtained by experiment. Therefore, we need to convert the results of simulation and experiment to be able to compare and analyse them, as shown in Table 3 and Fig. 10. From Table 3 and Fig. 10, the adsorption of CH4 molecules in the simulation was higher than the methane desorption in the experiment, and the difference decreased as moisture content increased.
There is a certain error between the experiment and simulation results, but the two curves have the same trend. Research has shown that fixed carbon in coal plays a controlling role in the adsorption capacity of coal, but the adsorption capacity of coal will be reduced due to the existence of moisture and ash (Zhao et al. 2010); thus, the methane desorption amount in the experiment is lower. Apart from this, the error is also caused by a variety of reasons: the pore structure of coal may be destroyed in the process of making cylindrical coal samples, the overestimation of the simulations may result from general assumptions used in the input of the MS, the immobility of the coal model may cause difficulties, bias sampling for the Monte Carlo iterations may be done incorrectly, etc. Based on the above analysis, it is rational to study the methane desorption behaviour with a single coal molecule through simulation in this paper, and the method can be used for follow-up research.
Mechanism analysis
Competitive adsorption
The average number of CH4 and H2O molecules under a pressure of 0–1.5 MPa were calculated by MS software simulation and fitted by Langmuir model in Fig. 11. It can be seen that the two curves can meet well the Langmuir model (the values of R2 are 0.97 and 0.93, respectively), and the average number of CH4 and H2O molecules adsorbed on the coal molecule increase continuously as pressure increases, which indicates that pressure can promote the adsorption of CH4 and H2O molecules, but the promotion is gradually weakened as the pressure increases. According to the Langmuir model, the average number order of molecules adsorbed on the coal molecule is H2O > CH4, and the increasing average number of H2O molecules adsorbed on the coal molecule with the increase of pressure is higher than that of CH4 molecule; thus, the adsorption capacity order is H2O > CH4. Xiang et al. (2014) also studied the competitive adsorption for CH4 and H2O molecule, and used it to characterize the adsorption capacity of CH4 and H2O molecules; the conclusions are consistent with the above.
Theoretically, the adsorption potential well of H2O molecules is higher than that of CH4 molecules; H2O is more easily adsorbed on coal (Song et al. 2017; Jiang et al. 2007), and this view also explains the reason for the phenomena in this paper. Therefore, the interaction between coal and H2O is stronger than that of CH4, the coal molecule adsorbing H2O molecules can form a more stable structure, and the structure is not easily changed when CH4 molecules are adsorbed in the structure again. From a macroperspective, there is a competitive adsorption relationship between moisture and methane, and the existence of moisture can reduce the adsorption of methane.
Isosteric heat of adsorption and adsorption energy
Isosteric heat of adsorption is an important parameter to describe the affinity between gas molecules and a framework material; furthermore, it is also a crucial parameter to characterize the energetics of such systems (Li et al. 2018; Liu et al. 2017), which can be calculated by the following equation:
where \(R\) is perfect gas constant (m3 Pa mol−1 K−1), \(P\) is pressure (Pa), \(N\) is the number of adsorbate (loading), \(T\) is temperature (K), \(Q_{{{\text{st}}}}\) is the isosteric heat of adsorption (KJ/mol), and C is constant (Cimino et al. 2017).
Figure 12 depicts the isosteric heat of adsorption of CH4 molecules under various moisture contents and various pressures. The isosteric heat of adsorption of CH4 molecules fluctuates within a small range with the increasing pressure, ranging from 21.77 to 23.12 kJ/mol, 18.06 to 19.1 kJ/mol, and 17.15 to 17.99 kJ/mol when the moisture content is 0%, 5%, and 9.8%, respectively. Xiang et al.’s research showed that the isosteric heat of adsorption is less than 42 kJ/mol for physical adsorption (Xiang et al. 2014), so the adsorption process of the CH4 molecule is typically physical adsorption. At the same pressure, it is obvious that the isosteric heat of adsorption of CH4 molecule on the dry coal sample is much larger than that of CH4 molecule on the wet coal sample; the isosteric heat of adsorption of CH4 molecule on the coal sample with 5% moisture content is larger than that of CH4 molecule on the coal sample with 9.8% moisture content, indicating that the lower moisture content, the stronger affinity between CH4 and coal molecule. This stronger affinity leads to the larger CH4 molecule adsorption (Dong et al. 2019). Apart from this, the isosteric heat of adsorption of CH4 molecules exhibits a more significant decrease when moisture content changes from 0 to 5% than those of CH4 molecules when moisture content changes from 5 to 9.8%, which suggests that moisture has a more obvious influence on the affinity between CH4 molecules and dry coal sample. This explains the reason for the decrease in the number of CH4 molecules adsorbed on the coal molecule from another angle.
In addition, adsorption energy can also be used to characterize the force between the CH4 molecule and coal molecule, and it is calculated according to:
where \({E}_{\text{ads}}\) is the adsorption energy of CH4 molecule (KJ/mol), \({E}_{\text{coal}}/{\text{CH}}_{4} \) is the total energy of system (KJ/mol), \({E}_{\text{coal}} \) is the energy of coal molecule (KJ/mol), and \({E}_{{\text{CH}}_{4}} \) is the energy of CH4 molecule (KJ/mol).
The value is generally negative, the smaller value, the stronger adsorption capacity (Huang et al. 2007). First, geometric structure of the Zhaozhuang coal and CH4 molecules were optimized to make them the most stable, and the energy was calculated by MS software, namely \({E}_{\text{coal}} \) and \({E}_{{\text{CH}}_{4}} \), respectively. Different amounts of H2O molecules (0/8/16) were adsorbed on coal molecules, and then, the overall geometry optimization was carried out. At this point, coal molecular models with different moisture content were constructed. Finally, CH4 molecules were adsorbed on coal molecules with different moisture content under different pressure, and then, geometric structure of models were optimized to obtain the most stable state. The energy of these models was calculated by MS software to obtain \({E}_{\text{coal}}/{\text{CH}}_{4} \).
Figure 13 shows that the adsorption energy of CH4 molecule decreases with increasing pressure under different moisture contents, and it can be concluded that the adsorption capacity increases with the increase of pressure. At the same pressure, the lower the moisture content of the coal sample is, the smaller the adsorption energy and the stronger adsorption capacity of CH4 molecule. This conclusion is consistent with the findings of the isosteric heat of adsorption.
Radial distribution function (RDF)
To further research the effect of moisture in coal on the desorption behaviour of methane, the radial distribution function (RDF) between CH4 molecules and oxygen-containing functional groups (carboxyl, carbonyl, and ether bonds) were simulated under different moisture contents by the MS. The RDF is a physical quantity characterizing the microscopic structure of the particle and can reflect the orderly nature of particle aggregation; this value also explains that the ratio of the local density and the average density (An et al. 2014), and the equation is as follows:
where \(r\) is the distance from the oxygen atom on the oxygen-containing functional group (Å), \({\text{d}}N\) is the number of particle that is distributed in the range from \(r\) to \({\text{r } + \text{ }}d{\text{r}}\) around oxygen atoms on the oxygen-containing functional groups (loading), \(\rho\) is the number density of particle, and \({\text{g}}_{ab} ({\text{r}})\) is the degree of aggregation of particle.
Figure 14 shows the radial distribution function \(g(r)\) between CH4 molecules and oxygen-containing functional groups (carboxyl, carbonyl, and ether bonds) at 0%, 5%, and 9.8% moisture content, respectively. According to Fig. 14, there are many adsorption peaks; the distinct peaks are generated by stronger interaction between oxygen atoms on oxygen-containing functional groups and CH4 molecules; other peaks may be attributed to the interaction between remaining atoms on oxygen-containing functional groups and CH4 molecules.
For CH4 molecules and carboxyl groups (Fig. 14a–c), the most distinct adsorption peaks are approximately observed at \(r\) = 3 Å, \(r\) = 2.6 Å, and \(r\) = 4.8 Å under different moisture contents (0%, 5%, and 9.8%), and the \(g(r)\) values are 4.36, 2.92, and 2.18, respectively, which suggests that CH4 molecules are aggregated in large amounts around carboxyl groups at 0% moisture content. Thus, CH4 molecules have a stronger interaction with carboxyl groups at 0% moisture content and a weaker interaction with carboxyl at 9.8% moisture content.
For CH4 molecules and carbonyl groups (Fig. 14d–f), the most distinct peaks are about found at \(r\) = 4.2 Å, \(r\) = 2.8 Å, and \(r\) = 4.5 Å under different moisture contents (0%, 5%, and 9.8%), and the \(g(r)\) values are approximately 4.8, 3.9, and 2.7, respectively. CH4 molecules are aggregated in large amounts around carbonyl groups at 0% moisture content. Therefore, there is a stronger interaction between CH4 molecules and carbonyl groups at 0% moisture content and a weaker interaction at 9.8% moisture content.
In terms of CH4 molecule and ether bonds (Fig. 14g–i), the \(g(r)\) values of the most significant peaks are approximately 3.25, 2.4, and 2.25, respectively, under different moisture contents (0%, 5%, and 9.8%), which indicates that CH4 molecules are aggregated in large amounts around ether bonds at 0% moisture content, so CH4 molecules have a stronger interaction with ether bonds at 0% moisture content and a weaker interaction at 9.8% moisture content. In addition, we can also observe that the \(g(r)\) value is the largest for CH4 molecules and carbonyl groups compared to other two oxygen-containing functional groups, so CH4 molecules are more aggregated around carbonyl groups, and there is a stronger interaction between CH4 molecules and carbonyl groups.
Based on the above discussions, there is a stronger interaction between CH4 molecules and the coal molecule at 0% moisture content. As the moisture content increases, the adsorption capacity of CH4 molecules at the adsorption site of oxygen-containing functional groups is weakened, so the number of CH4 molecules adsorbed on the coal molecule is decreased, resulting in a decrease in the desorption amount of methane.
Engineering significance
Through the comparison of experiment and simulation results, the feasibility of using the coal molecular model to study the effects of moisture on methane desorption has been verified, which can provide a method for predicting the influence of moisture on coal seam methane desorption and quantitatively evaluating the control effect of hydraulic measures on coal and methane outbursts in engineering practice from a molecular perspective. Specific steps are as follows:
- 1.
Collect and process coal sample.
- 2.
Conduct experiment, including elemental analysis, 13C nuclear magnetic resonance (13C-NMR), X-ray photoelectron spectroscopy (XPS), etc.
- 3.
Obtain the atomic ratio by elemental analysis. Different aromatic structures of the coal sample are assigned by analysing the results of 13C-NMR, and the distribution of heteroatoms (O, N, etc.) is determined by analysing the results of XPS. Then, the coal molecule model is constructed, and the structure is adjusted constantly to match the experimental results of 13C-NMR.
- 4.
Calculate the moisture content of coal molecule according to Eq. (5).
$$ W_{{\text{m}}} \% = \left[ {\left( {\frac{{N_{{{\text{H}}_{{2}} {\text{O}}}} }}{{N_{{\text{A}}} }} \times M_{{{\text{H}}_{{2}} {\text{O}}}} } \right)/\left( {\frac{{N_{{{\text{coal}}}} }}{{N_{{\text{A}}} }} \times M_{{{\text{coal}}}} } \right)} \right] \times 100\% , $$(5)where \(W_{\text{m}} {\text{\% }}\) is the moisture content of coal molecule, \(N_{\text{A}}\) is the Avogadro constant (6.02 × 1023 mol−1), \(N_{{{\text{H}}_{2} {\text{O}}}}\) is the number of H2O molecule adsorbed by coal molecule (loading), \({{N}}_{\text{coal}}\) is the number of coal molecule (loading), \(M_{{{\text{H}}_{{2}} {\text{O}}}}\) is the molar mass of H2O molecule (g/mol), and \(M_{\text{coal}}\) is the molar mass of coal molecule (g/mol).
- 5.
The adsorption process of the H2O molecule is conducted at fixed loading using the sorption module of the MS, which can better control the number of H2O molecules adsorbed by the coal molecule. After the adsorption process is completed, the geometric structure of the entire system is optimized to obtain the most stable configuration.
- 6.
Adjust the parameters of fixed loading and repeat the step (5); the coal molecular models with different moisture content are constructed.
- 7.
The CH4 molecule adsorption process is conducted on the coal molecular models with different moisture content.
Taking the No. 3 coal seam of the Zhaozhuang coal mine as an example, the coal molecular models with 0%, 2.6%, 5%, 7.5%, and 9.8% moisture content were constructed, and the CH4 molecule adsorption process was conducted at 0.3, 0.4, and 0.5 MPa. The results are shown in Fig. 15. It can be seen that the methane adsorption amount decreased by 75.17%, 61.83%, and 48.21% at 0.3, 0.4, and 0.5 MPa, respectively, when the moisture content increased from 0% to 9.8%. This conclusion is consistent with “Results and analysis”.
Conclusion
-
1.
Methane desorption curves of the Zhaozhuang coal all show monotone increasing trends, and the growth rate of methane desorption is faster in the first 20 min. At the same pressure, methane desorption decreases with increasing moisture content, and moisture has an inhibitory effect on the desorption process of methane. When moisture content increased from 0 to 9.8%, the degree in reduction in methane desorption is the largest at 0.3 MPa and the smallest at 0.5 MPa. Pressure can reduce the inhibition of moisture on methane desorption.
-
2.
The maximum and average number of CH4 molecules adsorbed on the coal molecule all decreased as the number of water molecule increased when the simulation pressure was the same. H2O molecules can occupy effective adsorption sites in the coal molecule and weaken the adsorption of CH4 molecules on the coal molecule. The same law is observed in both the experimental results and molecular simulation, and the error is acceptable; thus, they can be mutually verified, so the coal molecule can be used in the next step of research.
-
3.
Through analysis of the mechanism, there is competitive adsorption between CH4 and H2O molecules, and the interaction between the coal molecule and H2O molecules is stronger. Therefore, the affinity and interaction between CH4 and coal molecules is reduced because of the presence of H2O molecules, and the adsorption capacity of CH4 molecule is decreased. Various analysis methods verify each other, which ensure the accuracy of the microscopic mechanism.
-
4.
This research can provide a method for predicting the influence of moisture on coal seam methane desorption and quantitatively evaluating the control effect of hydraulic measures on coal and methane outburst in engineering practices from a molecular perspective.
References
An D, Guo X, Zheng C (2014) Structure and adsorption properties of microporous amorphous carbon by molecular dynamics simulation. J Eng Thermophys 35(3):614–617
An F, Yuan Y, Chen X, Li Z, Li L (2018) Expansion energy of coal gas for the initiation of coal and gas outbursts. Fuel 235:551–557
Brochard L, Vandamme M, Pellenq R, Fenchong T (2012) Adsorption-induced deformation of microporous materials: coal swelling induced by CO2–CH4 competitive adsorption. Langmuir 28(5):2659–2670
Cai Y, Yang X, Cai B, Li Q (2016) Experimental study on gas desorption laws of loading coal sample. Saf Coal Mine 47(10):5–8
Chen R, Qin Y, Zhang P, Wang Y (2017) Changes in pore structure of coal caused by CS2 treatment and its methane adsorption response. Geofluids. https://doi.org/10.1155/2018/7578967
Chen R, Wang Y, Wang Z (2018) Variations in adsorption of two step solvent extracted vitrain and their coal crystallites response. Teh Vjesn 25(3):663–669
Chen X, Cheng Y, Wang L (2013) Experimental study on the inhibition of injection water to the gas desorption of coal. J Min Saf Eng 30(2):296–301
Cimino RT, Kowalczyk P, Ravikovitch PI, Neimark AV (2017) Determination of isosteric heat of adsorption by quenched solid density functional theory. Langmuir 33(8):1769–1779
Ding H, Miao X, Ju F, Wang X, Wang Q (2014) Strata behavior investigation for high-intensity mining in the water-rich coal seam. Int J Min Sci Technol 24(3):299–304
Dong K, Zeng F, Jia J, Chen C, Gong Z (2019) Molecular simulation of the preferential adsorption of CH4 and CO2 in middle-rank coal. Mol Simulat 45(1):15–25
Erdöl N, Çalli L, Okutan H, Arisoy A, Ekinci E (1999) Effect of coal moisture on particulate emission in a fixed bed combustion appliance. Fuel Process Technol 58(2):109–117
Gao J, Li S, Yang M, Liu Y (2018) Experimental study of the impact of the moisture content on the coal gas adsorption through the low-field NMR relaxation method. J Safety Environ 18(1):151–155
Guo H, Su X (2010) Research on the mechanism of gas emission inhibition in water-flooding coal seam. J China Coal Soc 35(6):928–931
Huang X, Zhang X, Ji Q, Yang X (2007) Molecular mechanics (MM) study on the surface adsorption energy. Comput Appl Chem 24(3):375–379
Hu H, Li X, Fang Z, Wei N, Li Q (2010) Small-molecule gas sorption and diffusion in coal: Molecular simulation. Energy 35(7):2939–2944
Jiang W, Cui Y, Zhang Q, Zhong L, Li Y (2007) The quantum chemical study on different rank coals surface interacting with methane. J China Coal Soc 32(3):292–295
Jin Z, Wu S, Deng C, Dai F (2017) H2O adsorption mechanism in coal basing on Monte Carlo method. J China Coal Soc 42(11):2968–2974
Karasawa N, Goddard W (1992) Force fields, structures, and properties of poly (vinylidene fluoride) crystals. Macromolecules 25(26):7268–7281
Li Q, Ruan M, Zheng Y, Mei X, Lin B (2017) Investigation on the selective adsorption and separation properties of coal mine methane in ZIF-68 by molecular simulations. Adsorption 23:163–174
Li S, Li Y, Lu X (2018) The influence of moisture content on methane adsorption in lignite: a molecular simulation study. Sci China Phys Mech 48(6):91–97
Li X, Wang Z, Li Q, Qi X (2011) Moisture affected to gas desorption law of No 3 seam in Xinjing mine. Coal Sci Technol 39(5):47–50
Liang Y, Wang F, Luo Y, Hu Q (2018) Desorption characterization of methane and carbon dioxide in coal and its influence on outburst prediction. Adsorpt Sci Technol 36(7–8):1484–1495
Lin K, Yuan Q, Zhao Y, Chen C (2016) Which is the most efficient candidate for the recovery of confined methane: water, carbon dioxide or nitrogen? Extreme Mech Lett 9(1):127–138
Liu L, Zhang H, Do DD, Nicholson D, Liu JJ (2017) On the microscopic origin of the temperature evolution of isosteric heat for methane adsorption on graphite. Phys Chem Chem Phys 19(39):27105–27115
Liu X, He X, Qiu N (2016) Molecular simulation of CH4, CO2, H2O and N2 molecules adsorption on heterogeneous surface models of coal. Appl Surf Sci 389(23):894–905
Liu Y, Wilcox J (2012) Effects of surface heterogeneity on the adsorption of CO2 in microporous carbons. Environ Sci Technol 46(3):1940–1947
Meng J, Zhong R, Li S, Yin F, Nie B (2018) Molecular model construction and study of gas adsorption of Zhaozhuang coal. Energy Fuels 32(9):9727–9737
Mosher K, He J, Liu Y, Rupp E, Wilcox J (2013) Molecular simulation of methane adsorption in micro-and mesoporous carbons with applications to coal and gas shale systems. Int J Coal Geol 109:36–44
Ni G, Dong K, Li S, Sun Q (2018) Gas desorption characteristics effected by the pulsating hydraulic fracturing in coal. Fuel 236:190–200
Ni G, Lin B, Zhai C, Li Q, Peng S, Li X (2014) Kinetic characteristics of coal gas desorption based on the pulsating injection. Int J Min Sci Technol 24(5):631–636
Nie B, Liu X, Guo J, Zhu F (2015) Effect of moisture on gas desorption and diffusion in coa mass. J China U Min Technol 44(5):781–787
Nie B, Liu X, Yuan S, Ge B, Jia W, Wang C, Chen X (2016) Sorption charateristics of methane among various rank coals: impact of moisture. Adsorption 22(3):315–325
Ozdemir E, Schroeder K (2009) Effect of moisture on adsorption isotherms and adsorption capacities of CO2 on coals. Energy Fuels 23(5):2821–2831
Pan Z, Connell LD, Camilleri M, Connelly L (2010) Effects of matrix moisture on gas diffusion and flow in coal. Fuel 89(11):3207–3217
Shen R, Qiu L, Lv G, Wang E, Li H, Han X, Zhang X, Hou Z (2018) An effect evaluation method of coal seam hydraulic flushing by EMR. J Nat Gas Sci Eng 54:154–162
Sizova AA, Sizov VV, Brodskaya EN (2015) Computer simulation of CO2/CH4 mixture adsorption in wet microporous carbons. Colloid J 77(1):82–90
Song Y, Jiang B, Li W (2017) Molecular simulation of CH4/CO2/H2O competitive adsorption on low rank coal vitrinite. Phys Chem Phys Chem 19(27):17773–17788
Sun H, Cao J, Li M, Zhao X, Dai L, Sun D, Wang B, Zhai B (2018) Experimental research on the impactive dynamic effect of gas-pulverized coal of coal and gas outburst. Energies 11(4):797
Sun Q, Zhang J, Zhang Q, Yin W, Germain D (2016) A protective seam with nearly whole rock mining technology for controlling coal and gas outburst hazards: a case study. Nat Hazards 84(3):1793–1806
Wang C, Yang S, Li X, Yang D, Jiang C (2018) The correlation between dynamic phenomena of boreholes for outburst prediction and outburst risks during coal roadways driving. Fuel 231:307–316
Wang K, Zang J, Feng Y, Wu Y (2014) Effects of moisture on diffusion kinetics in Chinese coals during methane desorption. J Nat Gas Sci Eng 21(2):1005–1014
Wang L, Chen E, Liu S, Cheng Y, Cheng L, Chen M, Guo H (2017) Experimental study on the effect of inherent moisture on hard coal adsorption–desorption characteristics. Adsorption 23(5):723–742
Xiang J, Zeng F, Liang H, Li B, Song X (2014) Molecular simulation of the CH4/CO2/H2O adsorption onto the molecular structure of coal. Sci China Earth Sci 57(8):1749–1759
Xiao Z, Meng L (2015) Experimental study and mechanism analysis of impact of pressure water on coal seam gas desorption. China Saf Sci J 25(1):122–127
Yang J, Ren Y, Tian A, Sun H (2000) COMPASS force field for 14 inorganic molecules, He, Ne, Ar, Kr, Xe, H2, O2, N2, NO, CO, CO2, NO2, CS2, and SO2, in liquid phases. J Phys Chem B 104(20):4951–4957
Yang L (2013) Experimental research on influence of free water from external environment on rule of coal sample’s methane desorption. Coal Min Technol 18(6):10–11
Yang W, Lin B, Gao Y, Lv Y, Wang Y, Mao X, Wang N, Wang D, Wang Y (2016) Optimal coal discharge of hydraulic cutting inside coal seams for stimulating gas production: a case study in Pingmei coalfield. J Nat Gas Sci Eng 28:379–388
You J, Tian L, Zhang C, Yao H, Dou W, Fan B, Hu S (2016) Adsorption behavior of carbon dioxide and methane in bituminous coal: a molecular simulation study. Chinese J Chem Eng 24(9):1275–1282
Yu S, Bo J, Wu L (2017) Molecular simulation of CH4/CO2/H2O competitive adsorption on low rank coal vitrinite. Phys Chem Chem Phys 19(27):17773–17788
Zeng Q, Wang Z, Mcpherson B, MclennaN J (2017) Modeling competitive adsorption between methane and water on coals. Energy Fuels 31(10):10775–10786
Zhai C, Xiang X, Xu J, Wu S (2016) The characteristics and main influencing factors affecting coal and gas outbursts in Chinese Pingdingshan mining region. Nat Hazards 82(1):507–530
Zhai S (2018) Experimental study on gas adsorption-desorption deformation characteristics of briquette coal samples with different granularity. J Saf Sci Technol 14(6):84–89
Zhang Z, Ma P (2008) Experimental study on effects of moisture on the gas adsorption speciality of different kinds of coal. J China Coal Soc 33(2):144–147
Zhang Z, Cao S, Guo P, Liu Y (2014) Comparison of the deformation characteristics of coal in gas adsorption-desorption process for raw and briquette coals. J China U Min Technol 43(3):388–394
Zhao D, Zhao Y, Feng Z (2011) Laboratory Experiment on coalbed-methane desorption influenced by water injection and temperature. J Can Petrol Technol 50(7–8):24–33
Zhao T, Li X, Zhao H, Li M (2017) Molecular simulation of adsorption and thermodynamic properties on type II kerogen: Influence of maturity and moisture content. Fuel 190:198–207
Zhao X, Tang D, Xu H (2010) Control mechanism of coal metamorphism on coal adsorption capacity. In: Proceedings of Chinese CBM Symposium in 2010; 2010 Sep 01; Suzhou, China. Petroleum Industry Press, pp 59–65
Zhao Y, Gao S, Li Y, Yang H, Gao P, Liu G, Pan P (2018) Experimental and numerical investigation on the effect of moisture on coal permeability. Nat Hazards 90(3):1201–1221
Zhou Y, Sun W, Chu W, Liu X, Jing F, Xue Y (2016) Theoretical insight into the enhanced CH4 desorption via H2O adsorption on different rank coal surfaces. J Energy Chem 25(4):677–682
Acknowledgements
This work was financially supported by the Fundamental Research Funds for State Key Research Development Program of China (no. 2016YFC0600708) and Central Universities (no. 2011QZ02); the authors are grateful for their support. We sincerely thank the editor and anonymous reviewers’ who improved this paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Meng, J., Li, S., Niu, J. et al. Effects of moisture on methane desorption characteristics of the Zhaozhuang coal: experiment and molecular simulation. Environ Earth Sci 79, 44 (2020). https://doi.org/10.1007/s12665-019-8788-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-019-8788-9