Abstract
The potentially hazardous contents of mine tailings can pose a serious threat to the environment. Tailings dispersed around the abandoned Monica mine (Bustarviejo) in the Autonomous Region of Madrid (Central Spain) were studied to determine the concentration of several potential toxic elements and their geochemical impact in the surrounding soils. A total of 17 surface soil samples were collected from both mixed sulfide mine tailings sites and unmined soils, within a radius of 1900 m from the mine entrance. The processing of minerals (basically arsenopyrite, matildite and sphalerite) produced tailings with a pH as low as 2.9. Elements such as As, Cu, Zn, Cd, Pb, W, Ag, Fe were found in very high concentrations, contaminating the soil to varying degrees (these elements were sometimes 10- to 20-times higher in the tailings than in the unmined soils). Given its short distance and accessibility from such a large city as Madrid, it is of undeniable environmental and educational interest. Among other factors, there is a need for improvements to tailings management strategies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mining operations produce waste material after the minerals of interest have been extracted. Such waste materials, known as mine tailings, can often contain high concentrations of potentially toxic material.
As a result, changes in soil morphological, physical and chemical properties can occur, leading to severe soil damage. Moreover, the mining industry produces enormous quantities of fine-rock particles, ranging in size from a grain of sand to a few micrometers (USEPA 1994). Vegetation can also be affected with the destruction of extensive areas.
Different risks have been associated with mine tailings: instability, visual impact, contamination of the water table, acid-mine drainage (AMD) and secondary mineral sedimentation (Grangeia et al. 2011; Bini 2012; He-rong et al. 2015; Mu et al. 2015). Potentially toxic trace elements (PTEs) dumped in high concentrations nearby abandoned mines represent long-term health hazards, as these mine-waste materials percolate into nearby soils (Conde et al. 2008; Martín Peinado et al. 2015; Abreu et al. 2016; Martín-Moreno et al. 2016). They can go on to contaminate land, sub-soil and food crops, causing loss of biodiversity, posing an important risk to human health (Lee et al. 2009; Rodriguez et al. 2009; Fernández-Caliani et al. 2009; Conde et al. 2008), and even causing fatal diseases in humans through crops and water (Lee et al. 2007).
Soils formed on landscapes altered by mining constitute the mine soils and can be classified as either Entisols or Inceptisols (Sencindiver and Ammons 2000; Angel et al. 2008), with few or no natural genetic horizons (Soil Survey Staff 2006). Normally, these very young soils have their origin in fragmented rock mixed with fine earthy material. Their soil profiles are irregular and are mixed or substituted by earthy materials from lower depths.
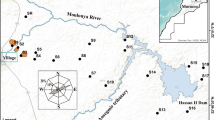
Natural ecological environments around large cities are valued very positively, as they are the nearest natural environments in which to escape from the pressures of urban life. The “Central System” is the main mountain range in the center of the Iberian Peninsula, the southern edge of which is 50 km north of Madrid. The Monica mine (also named the Indiana and the Elvira mine), located at Bustarviejo (in Madrid Province), is in an area of high ecological value, called Cuesta de la Plata (Fig. 1). It was probably the most important metal mining works with the largest reserves of metal in the province of Madrid (Spain) throughout its almost 400 years of intermittent operation (Jordá 2003, 2008; Jiménez et al. 2004). Its principal minerals consist of arsenopyrite and matildite, a sulfide mineral with Bi and Ag, Puche et al. (2000). Its economic exploitation has focused on silver, extracted since the seventeenth century up until the 1890s. Several studies have been undertaken in this area over the last decade, which have been addressed to assess the extent of pollution and particularly arsenic concentrations from the Monica mine (Moreno-Jiménez et al. 2009, 2010). Other studies have reported the presence of As in the uppermost layer of soils nearby mining dumps with concentrations of about 3000 mg kg−1 (Moreno-Jiménez et al. 2009) and almost 1900 μg kg−1 As in vegetation (García-Salgado and Quijano 2014).
Tailings will not always mean contaminated soil, but after mining activity, they could pose a serious environmental threat, because of the presence and concentration of potentially toxic trace elements. Considering that the effects of the Monica mine tailings are still active, it is important to assess the possible levels of contamination of this delicate ecosystem. In fact, field observations would suggest visible indicators of contamination.
The main objective of this study is to assess soil contamination through the concentrations of potentially toxic trace elements that have percolated into the soils from the tailings, as a consequence of small-scale mining activity that has since been abandoned. Secondly, our assessment of soil contamination aims to evaluate soil quality within areas of small-scale mining activity. Our final objective is to evaluate the appropriateness of monitoring as an environmental strategy to contain the problem.
Materials and methods
Study area
The study area, known as Cuesta de la Plata, is located at about 1500 m a.s.l. on the slopes of Cerro de la Braña (Madrid Province, Central System) in the mountain range of the Sierra de Guadarrama in the center of Spain (Fig. 1). The entrance to the mine is at a latitude of N 40°52′2″ and a longitude of W 3°43′50″ (438.45 4524.50). The study area has a subhumid Mediterranean climate with a mean annual air temperature of 12 °C and a mean annual precipitation of 605 mm concentrated between September and April. Deciduous forest is found in the mining area (Quercus pyrenaica), mixed with Pinus sylvestris and Pinus pinaster in the folds of its valley, with shrubby vegetation on the upper slopes and dense hygrophytic vegetation in the streams.
The study area includes a smaller subarea of approximately 100 ha of land exposed to erosion. The mining site is conical in shape, due to the waste deposition technology and the topography of the area.
Granite and gneiss dominate the bedrock in the stockwork and brecciation zone. Mine spoils located around the periphery are composed of weathered products of these formations.
Sulfide minerals such as arsenopyrite associated with matildite, chalcopyrite, scorodite, sphalerite, pyrrhotite, galena were identified in the mine and its tailings (Puche et al. 2000; Bouso 2004). Mineralization including As–Fe–Cu–Pb–Ag–Zn and Sn was found in pockets and in small outcropping quartz veins (Jordá et al. 2005; Jordá 2008).
Steep tracks lead up to previously mined areas, where easily extracted soil samples showed A–C and A–R horizons that had formed relatively quickly. When studying the requirements for the formation of these types of horizons, the need to describe Cambic horizons (Bw) should not be ignored. However, in some parts of the area surrounding the mine, all the conditions are present for Bw horizons: thick structure (absence of rock structures found in over half the volume) and higher chroma values, redder hues, and higher clay contents than both the underlying and overlying horizons (Soil Survey Staff 2006; FAO-ISRIC-ISSS 2006).
Therefore, soil in the area around the mine has A–C, A–R, A–C–R and A–Bw–C–R morphologies. The native soil particles in the Bw horizons are sufficiently thick to be classified as Cambic (horizon) Inceptisols (Soil Survey Staff 2006). The floors of the mines are Leptosol or Spolic Tecnosol (FAO-ISRIC-ISSS 2006). The majority of soils in the area are from moderate to very deep Inceptisols and Entisols (Table 1). They form residual soils (due to natural decay) and colluvial soils (with materials drawn down the slope by gravity). Soil formation processes were at the origin of a similar horizon in each native soil, where sequences of A, Bw, and C horizons were found. The granular structures of the horizons were weak or moderate, while the Bw horizons had structures that could be likened to either weak or moderate sub-angular blocks.
Historic mining activities throughout almost four centuries at the Monica mine have determined significant landscape degradation and contamination. The Monica mine wasteland disrupts the aesthetic continuity of the natural landscape in this area of mountain range, degrading soil morphology, drainage, soil structure, etc. Mining operations have left areas of land degraded, due to ecosystem contamination from mine spoils excavated from mines and dumped on the surface in large heaps. The consequence of mining activities on soils are slag heaps, dumps, and alteration of the natural landscape.
In the case of the Monica mine, the dumping sites presented an apparently variable textural and mineral composition. In all cases, the morphological structure of the materials present in the heaps was not proper to the soil conditions in the adjacent natural soil. As a consequence of mine spoil, vegetation cover is now practically absent in the overburden dumps affected by weathering, bringing a wide range of problems, among which the replanting and growth of vegetation.
Soil sampling
A total of 17 soil/sediment samples were collected at different points: tailings, tributary streams at the mine entrance, downstream area and unmined soils. Further soil samples were collected above the mine entrance (Fig. 1). The sampling strategy permitted the study of the concentration gradient and an assessment of the possible mobilization of trace elements. Soil sampling was carried out in May 2014, and at each location, three subsamples were collected at a depth of 0–30 cm. Sample 1 represents the natural soil, situated at higher levels above the location of the mine. Samples 2, 3, 4 and 5 represent the soils/sediments that constitute the waste materials situated around the mouth of the mine. Sample 6 was taken from the bed of the stream where the waters flow that drain the principal dump, whereas samples 10, 11 and 12 were extracted from the bed of the aforementioned stream, located at levels below the mine entrance. Samples 7, 8 and 9 include the accumulation of mining waste from a second dump. Samples 13, 14 and 16 represent the bottom of the geochemical baseline of the basin, and finally samples 15 and 17 represent the environment close to the stream, potentially within reach of the drainage waters. Standard survey procedures were used to classify the pedons (FAO-ISRIC-ISSS 2006).
Laboratory analysis
Following their preparation, the samples underwent air-drying at room temperature (25–30 °C) under laboratory conditions. They were then sieved through a 2-mm screen to separate larger particles, gravel-sized material and other waste and sealed in plastic bags for analysis. Both dry and moist soils were evaluated with the Munsell Soil Color Chart; particle-size distribution was determined using the hydrometer method (Gee and Bauder 1986); a glass electrode in a soil–water ratio of 1:2:5 was used to determine soil pH and electrical conductivity (EC) (Thomas 1996).
Total trace element concentrations of (As, Cu, Pb and Zn) were identified with inductively coupled plasma-optical emission spectrometry (ICP-OES), following soil digestion with a 5:2:3 mixture of HNO3–HClO4–HF (Li et al. 2012). A 50 ml dilution of deionised water and the mineral residues were kept in a volumetric flask and stored in a refrigerator at 4 °C before analysis by Inductively Coupled Plasma–Optical Emission Spectrometry (ICP–OES). Quality control rules required the analysis of at least one in ten random samples. Various reagents verified the accuracy and precision of the analytical data used for trace element determination. Calibration was done with standard PTEs solutions (to establish calibration curves before metal analysis). All chemicals and solutions met analytical standard book-reagent grade. Lastly, quality control was assured in duplicate.
X-ray powder diffraction (XRD) was used to determine the semiquantitative mineralogical composition of the total (≤2 mm) and the fine (≤2 μm) fractions, using both the random powder method for the bulk sample and the oriented slides method. Semiquantitative mineralogical analysis was done by diffraction peak analysis (Kisch 1990).
Data analysis
Trace element pollution levels in the soils/sediments were assayed with the geoaccumulation index (Igeo). The following equation was used for the determination of Igeo (Müller 1979; Boszke et al. 2004):
C n = trace element concentrations measured in the sediment, B n = trace element background value, 1.5 = correction factor for background matrix.
The geoaccumulation index has 7 grades or classes: Igeo ≤ 0 (grade 0), unpolluted; 0 < Igeo ≤ 1 (grade 1), slightly polluted; 1 < Igeo ≤ 2 (grade 2), moderately polluted; 2 < Igeo ≤ 3 (grade 3), moderately severely polluted; 3 < Igeo ≤ 4 (grade 4), severely polluted; 4 < Igeo ≤ 5 (grade 5), severely extremely polluted; Igeo > 5 (grade 6), extremely polluted.
The concentration factor (CF) for each pollutant, defined as:
was calculated by dividing the average concentration of the element in the soil (Ci) by its standardized geochemical classification.
All statistical analyses were performed using SPSS software version 17 for Windows.
Results and discussion
The general soil properties are presented in Table 2. The soils were basically acid with extreme pH values in some case (Table 2). Particle-size classification showed differences in the sand content of the mine spoils and the surrounding unmined soils; though the sand particles tended to decrease, clay and silt particles tended to increase in the mine spoils with respect to the unmined soils. The mine soils that had formed on granite and gneiss bedrock not only had relatively high silt (40%) and clay (35%) contents, but often had high percentages (>50%) of rock fragments (>2 mm). Electrical conductivity of the samples in the study area was under 1 dS m−1 (Table 2), which is quite low overall.
Under these conditions, neither plant nor microbial growth is supported in these spoils, mainly due to low contents of organic matter with unfavorable pH (Table 2), in agreement with Agrawal et al. (1993). Other authors like Pederson et al. (1988) noted that the nutrient status of overburden soil appears to be a major factor limiting plant growth. Physicochemical properties can often inhibit the soil-forming process and plant growth; in environmental terms, mine spoil dumps are very unstable, contaminating the land by increasing heavy-metal soil concentrations that in turn deteriorate soil quality (Maiti 2007).
Mine soils are very young in this area in comparison with natural regional soils, so they show no evidence of soil development. The thicknesses of the A horizons and the solum showed no increases with the age of the mine soil. It has taken hundreds, if not thousands of years for native soils to form, while the mine soils are much younger. As a consequence of topographic conditions, the native soils in the unmined area were gently sloping to very steep terrain. Under these conditions, Entisols and moderately deep Inceptisols (Soil Survey Staff 2006) as well as Leptosol and Cambisol (FAO-ISRIC-ISSS 2006) can form directly on bedrock (granite) or in colluvium (materials transported downslope by gravity) and residuum (formed in place by natural weathering). On the contrary, soils in the mine tailings are classified as Technosols, showing low-chroma redox depletions within the upper 100 cm of the surface, for some time, in normal years. Disturbance has left the soils at the tailings sites with textures that are not very different. Anthropic action includes rock blasting into small fragments, compaction after settlement, and tree planting. Therefore, the mine soils had higher rock-fragment contents compared with the unmined soils; primarily with coarse-textures (loam to coarse sand), with the >2-mm fraction between 20 and 80% (Table 2).
The spoils have suffered weathering and erosion, but they have not achieved equilibrium and homogenization. In any case, rainwater percolates through these tailings dumps causing further soil pollution. Mine dumps/tailings/spoil exposed to the atmosphere usually lead to oxidation, dissolution and precipitation. Sulfide minerals, associated with the ore mine, containing As–Cu–Fe–Zn–Pb–Ag (arsenopyrite, chalcopyrite, etc.), were found in the Monica Mine (Jordá 2008). Natural weathering conditions may cause these exposed mine spoils to break down into small clay-sized particles that are released into the environment (Haigh 1992; Dang et al. 2002). Oxidation of these sulfide minerals results in contamination, especially by the heavy metals contained in sulfide-bearing minerals, (Kim and Jung 2004; Lim et al. 2008), releasing some elements into the surrounding soils/sediments.
The pH of a given mine soil can change rapidly as the rock fragments weather and oxidize. Mine soil pH is somewhat lower than native soil pH, because of the presence of sulfide minerals. In mine soils, pH values are lower than those of unmined soils. The average pH ranges from 2.9 (very strongly acidic) to 5.0 at the tailings sites, while the unmined soil is around 5.2 (acid in nature). This acidic nature is linked to the geology of the rock in the area, but above all to leaching processes in which tailings and impoundments, exposed to atmospheric conditions, are leached by surface runoff and water percolation. Some spoils were therefore extremely acidic.
The unmined soils in the area contain large quantities of feldspar in their constituents (Fig. 2). The diffractograms showed that the dominant minerals consisted of phyllosilicates (50–60%), quartz (30–40%) and feldspars (5–10%). The clay minerals were dominated by illite and kaolinite; vermiculite and/or poorly defined mixed-layer phases are also present (Fig. 2).
Total elemental contents
Summary statistics of the total concentrations of all the elements under study in both the mine soils and the unmined soils are reported in Table 3. The total mine-tailing concentrations of elements were up to 20,249 mg kg−1 for As, 347,346 mg kg−1 for Fe, 9075 mg kg−1 for S, 1985 mg kg−1 for Ni, 4391 mg kg−1 for Cu, 23,489 mg kg−1 for Zn, 284 mg kg−1 for Zr, 248 mg kg−1 for Ag, 85 mg kg−1 for Cd, 886 mg kg−1 for Sn, 125 mg kg−1 for W, 4057 mg kg−1 for Pb, 814 mg kg−1 for Bi and 6554 mg kg−1 for Sc. The concentration of some elements (Mo, Pd, Cd, In, etc.) in some samples was under the minimum detection limit. Comparison with background concentrations (De Miguel et al. 2002) revealed that most of the soil samples in the tailings were polluted with As, Cu, Zn, Cd, Ni, Pb and W, among other elements. In addition, large variations in mean, minimum, and maximum values were observed. The concentrations of elements varied widely between the tailings sites, depending on the sampling locations, taken at decreasing distances from the mine spoil sites.
Samples 2, 3, 4 and 8 located in the immediate vicinity of the mine-tailing wastes contained the highest concentrations of some elements; also found in samples 6, 10 and 11 in fluvial sites enriched in organic matter. It is worth highlighting sample 17, which corresponds to mineralization, dragged downstream by water. In some of these soils, total concentrations of As, Cu, Pb, Cd and Zn were between one and two orders of magnitude higher than the regional and the geochemical baseline (De Miguel et al. 2002; BOCM 2007) and levels considered normal in the nearby Community of Castilla La Mancha (Jiménez Ballesta et al. 2010).
High levels of trace element concentration in several samples exceeded the reference value for the Community of Madrid (Table 3; Fig. 3). High concentrations of Fe were detected in soil samples (average 62.14 g kg−1, maximum value 347.35 g kg−1 in mineralization at the bottom of the valley with a minimum value of 20.33 g kg−1).
The following elements are of particular interest: arsenic, copper, lead, chrome, cobalt, nickel, zinc, cadmium, selenium, tin and tungsten. Average concentration values for As were consistently high at 3.15 g kg−1 (Table 3), with the highest value of 20.25 g kg−1 at one tailings dump. This value exceeds the Netherlands soil/sediment intervention guideline value, set at 55 mg kg−1 for remediation; the control site showed 5 mg kg−1 or less, and the reference value was 10 mg kg−1 (De Miguel et al. 2002). Moreover, the geochemical evaluations showed that As contamination was very high and that it therefore posed a threat to environmental quality. There were significant differences between the As levels around the active site and mean As concentration in the control soils. Tailing dams certainly introduced considerable amounts of As into the surrounding soils well over background As levels.
Most of the samples had a concentration of Sb moderately above the reference value; only in a peaty soil (M11) and a tailing sample (M4), it was intensely accumulated at 15 and at 11 mg kg−1, respectively. The standard deviation for Bi was high (225.55 mg kg−1), indicating its variability; accumulations were found in slag heaps, particularly in sample 8 (814 mg kg−1).
Copper is both a significant and a key microelement in plant nutrient supply. The average Cu concentration ranged from 4 to 4391 mg kg−1 (Fig. 3). Although in some soil samples it was hardly detected, in most samples it was doubled, tripled or even multiplied by more than 200, especially in dumps and peat soils. The highest value was 4391 mg kg−1 in soil sample 8 (Table 2); the lowest value was found in the control soil (samples 13 and 14 with values of 4 and 5 mg kg−1, respectively). The Cu concentration of the control soils fell within the recommended limit of 36 mg kg−1 for ordinary uncontaminated soil. On-site contamination remained under the Cu contaminated-site intervention limit of 190 mg kg−1 (VROM 2000). The mean Cu concentrations in soils from the active tailings dams were over the recommended limit of 36 mg kg−1 for standard uncontaminated soil, but some values were over the Cu intervention limit of 190 mg kg−1 at contaminated sites (VROM 2000).
Pb is known to be one of the principal contaminants in the environment (Adriano 2001). Practically, all samples exceeded the reference value for Pb (the mean is 569.94 mg kg−1). In some samples, the reference value was multiplied by more than 100 and in another by more than 30. The maximum value was 4057 mg kg−1, and the minimum 14 mg kg−1 (Fig. 3).
Most samples contained less than 32 mg kg−1 of Cr, which is the benchmark for the Region of Madrid. Its moderate accumulation in sample 11 (peat stream soil), and especially its intense accumulation in sample 12 (also peaty), denotes mobility and refocuses the effect of organic matter, while sample 17, corresponding to an accumulation point, showed mineralization. In the case of W, its highly variable concentrations that accumulated in tailing dumps ranged between 8 and 125 mg kg−1 (Fig. 3). Traces of molybdenum were insignificant.
There was a similar trend for Co and Ni. The Ni concentration was high in samples 12, corresponding to peat (1985 mg kg−1) and 17 corresponding to mineralization. Its average value was 214.12 mg kg−1 (Table 3). For Co, the highest amount was for sample 8 (tailings dump). It has been previously reported that, at a pH of below 5, the toxic-metal bioavailability in the form of iron, nickel, lead and cadmium also increased (Maiti 2003).
Practically, all samples (average 1.94 g kg−1) exceeded the Zn reference value for the Madrid Community of 80 mg kg−1 (De Miguel et al. 2002) and in some cases exceeded the reference value by a factor of 300 (maximum value 23.49 g kg−1); it hardly appears in some dumps due to washing and is therefore a highly polluting element. However, significant differences were found in Zn concentrations between soil samples from around the tailings dam and from the control site.
Hardly detected in some samples, Cd levels were abundant in one dump (85 mg kg−1) and levels in many others were above the reference value, 0.27 mg kg−1 (De Miguel et al. 2002). Selenium (Se) tends to be found in the tailings further away; in several samples, it was above the reference value for Castilla La Mancha (near Madrid), Jiménez Ballesta et al. (2010), and similar to other soils in the Madrid region, (Moreno et al. 2005). The average was 3.24 mg kg−1. A dispersion of Ti values was observed: some above 2000 mg kg−1 and others below 300 mg kg−1. The average was 1122.41 mg kg−1. Manganese is of low toxicity and of considerable biological significance (Hasan et al. 2013). There was only marginal Mn contamination from the active tailings dams, as their Mn levels remained within the soil protection guidelines at values of 320 mg kg−1.
Accumulation of Ag, the primary product of the mine, was expected in the tailings dumps. Its concentrations were high in some samples such as M8 and low in soil outside the mine (M10 and 11). Hg was not detected in several samples, including waste dumps; there are two points, one in dump M8, with a value of 364 mg kg−1, and another in M11 (peaty soil), with a lower concentration.
Vanadium, niobium, barium and tantalus are insignificant elements (indicating large-scale regional patterns), and most of the values were around or under the reference value; with only one sample nearly double this value for V. In the case of Nb, the values were under 17 mg kg−1, although with some variations, while Ta was under 4 mg kg−1. High concentrations of Ba showed the reference value doubling in natural soil samples and in some tailings. In contrast, the presence of Hg was significant (some values exist of up to 364 mg kg−1) with an average of 22.84 mg kg−1 and with a very high standard deviation of 85.32.
Other elements such as sulfur are found in widely varying amounts, reaching values of up to 2.00–3.00 g kg−1, and in one sample (17), a value of 9.08 g kg−1; in other cases, it was hardly over 200 mg kg−1. In the case of one heap, very high levels of Ti and Sn were detected, while it hardly appeared in others; its levels in the most natural soils were low.
In the B, Al, Ga, In and Tl groups, the concentration of B was quite variable (standard deviation 2.70 g kg−1), reaching a very high value in one sample (11.66 g kg−1) and a minimum value of 7 mg kg−1 in sample 7. Ga and Tl showed negligible concentrations with little variability, like Al, although this element in sample 8 showed a high content. Finally, rare earth elements (REE) were concentrated in the natural soil (samples 13 and 14).
Mining represents a significant source of potentially toxic trace element contamination in adjacent environmental soils and sediments (Wray 1998; Aucamp and van Schalkwyk 2003; Bruce et al. 2003; Liu et al. 2005; Acosta et al. 2011; Bini 2012; Li et al. 2014; Smart et al. 2015; Soulard et al. 2016). Hu et al. (1992), expressed the opinion that soil with a higher stone content than 50% should be rated as poor quality. Physicochemical properties that are adverse (such as texture) will tend to inhibit soil formation processes and plant growth (Koushik et al. 2012). In this regard, issues such as tailing dam instabilities, the volumes of tailings that they might be retaining at any one time, and the programs and at times nonexistent response to tailing dam management were all considered contributory factors in contamination by some elements around the dams. It may be understood from the study that, once water filters through the dumps, the highest PTEs contamination will be found in the soils located in the area around the riverbed.
There are ever so many significant differences in trace element concentrations in the Monica mine tailings and in unmined soils. Trace element concentration data clearly demonstrate the accumulation of some trace elements in the tailings and main stream sediments compared with the unmined soil sites. Therefore, all pollutant elements from the mine were not exclusively, but principally transported through the main stream. The high concentration of sulfides and metal (loid)s in the tailings gave rise to spots with very acidic and highly polluted soils devoid of vegetation, as corroborated by Martín Peinado et al. (2015).
The assessment of soil enrichment with elements can be done in many ways. The most widely used is the index of geoaccumulation and enrichment. The concentration factor (CF), defined in terms of the ratio between each trace element from the soil sample and its background value, was particularly high for some elements, indicating soil pollution (Table 3). For example, see the case of As (up to 104), Cd (up 70), Cu (up to 35), Zn (up 25), Ag (up 500), Sc (up 16), Pb (up to 11) and Ni (up 10). The ratio was under 1 for many elements. The geoaccumulation index (Igeo) for some elements was arrived at using Muller’s (1979) expression and regional data (De Miguel et al. 2002; BOCM 2007; Jiménez Ballesta et al. 2010). The geoaccumulation index (Table 3) indicated the presence of As, Ag and Cd in high quantities in an extremely polluted area; extremely severe pollution levels of Cu and Zn; Mo and Sc, with moderately severe levels of Ni, W and Pb.
Conclusions
Several conclusions may be drawn from this study: trace element contaminants released from the Monica mine have affected the neighboring area, in the course of mining activity and after its closure. Considerable amounts of some PTEs, particularly (As, Cu, Zn, Cd, Pb, W, Ag, Fe), were associated with mine tailings dams. Some of their concentrations were extremely high, such as arsenic (As), selenium (Se) and cadmium (Cd) in addition to high concentrations of chromium (Cr), and moderate amounts of lead (Pb). We assume that factors such as the stability of the tailings dams, the amount of waste that they may at any given time contain, and climatic and topographic conditions in the area have all contributed to the extension of total pollution and the dispersion of chemical elements around the spoils.
This potential impact was greater near the tailings; levels of contamination have been observed to decrease farther downstream at some distance from the source of contamination: the tailings dams. The water that passes over and percolates the dumps will incorporate some PTEs and subsequently run down the river bed and contaminate the soils in the river basin.
The evaluation of total chemical element concentrations in the Monica mine spoil and surrounding soils provides baseline data for future research. The study not only yields information to compare the asymmetries of mine-dump and native soils, but it also helps to understand future perspectives for the regional environment. So, if recovery is the process by which highly degraded lands are returned to productivity in the natural environment, land reclamation around the abandoned Monica mine is a much-needed process. In doing so, the soil is the basis for this process, because of its composition and density that directly affect the future stability of plants. The restoration of vegetation cover in landfills can meet the objectives of stabilization, monitoring pollution, visual improvements to the landscape and the removal of hazardous threats to humans.
This situation makes it imperative to establish an environmental monitoring scheme that will check the concentration levels of some elements found in and around the Monica mine area. So, regular monitoring (i.e., every 2 or 3 years) of the spoil/soil conditions is therefore recommended, in order to assess and control certain elements such as Cu, Zn, Pb and Cd; and to take measures to counter soil acidification. Future research would do well to focus on the balance of some elements in the surrounding forest-soil-ecosystem, to gain a more precise diagnosis of soil contamination. It might also categorize the quantification of elements transported from mine soils to the environment.
References
Abreu MM, Matias MJ, Magalhães MC, Basto MJ (2016) Impacts on water, soil and plants from the abandoned Miguel vacas copper mine, Portugal. J Geochem Explor 96(2–3):161–170
Acosta JA, Faz A, Martinez-Martinez S, Zornoza R, Carmona DM, Kabas S (2011) Multivariate statistical and GIS-based approach to evaluate heavy metals behavior in mine sites for future reclamation. J Geochem Explor 109:8–17
Adriano DC (2001) Trace elements in terrestrial environments. Biogeochemistry, bioavailability and risk of metals, 2nd edn. Springer, New York
Agrawal M, Singh J, Jha AK, Singh JS (1993) Coal-based environmental problems in a low rainfall tropical region. In: Keefer RF, Sajwan KS (eds) Trace elements in coal combustion residues. Lewis Publishers, Boca Raton, pp 27–57
Angel P, Barton C, Warner R, Agouridis C, Taylor T, Hall S (2008) Tree growth, natural regeneration, and hydrologic characteristics of three loose-graded surface mine spoil types in Kentucky. In: Barnhisel RI (ed) Proceedings, 25th conference of the American Society of mining and reclamation. Lexington, pp 28–65
Aucamp P, van Schalkwyk A (2003) Trace element-pollution of soils by abandoned gold-mine tailings near Potchefstroom. S Afr Bull Eng Geol Environ 62:123–134
Bini C (2012) Environmental impact of abandoned mine waste: a review. Nova, Hauppauge
BOCM (2007) Orden 2720/2006, de 11 de agosto, por la que se establecen niveles genéricos de referencia de metales pesados y otros elementos de traza de suelos contaminados de la Comunidad de Madrid, vol 204. Comunidad de Madrid, Madrid, pp 29–30
Boszke L, Sobczynski T, Kowalski A (2004) Distribution of mercury and other heavy metals in bottom sediments of the Middle Odra River (Germany/Poland). Polish J Environ Stud 13:495–502
Bouso JL (2004) La antigua mina de plata de Bustarviejo. Rocas y minerales: Técnicas y procesos de minas y canteras, 391:24–29. ISSN:0378-3316
Bruce SL, Noller BN, Grigg AH, Mullen BF, Mulligan DR, Ritchie PJ, Currey N, Ng JC (2003) A field study conducted at Kidston Gold Mine, to evaluate the impact of arsenic and zinc from mine tailings to grazing cattle. Toxicol Lett 137:23–34
Conde P, Bellido E, Martín Rubí JA, Jiménez Ballesta R (2008) Concentration and spatial variability of mercury and other heavy metals in surface soil samples of periurban waste mine tailing along a transect in the Almadén mining district (Spain). Env Geol 56(5):815–824
Dang Z, Liu C, Haigh MJ (2002) Mobility of heavy metals associated with the natural weathering of coal mine soils. Environ Pollut 118:419–426
De Miguel E, Callaba A, Arranz JC, Cala V, Chacón E, Gallego E, Alberuche E. Alonso C, Fernández-Canteli P, Iribarren I, Palacios H (2002) Determinación de niveles de fondo y niveles de referencia de metales pesados y otros elementos traza en suelos de la Comunidad de Madrid. Serie Medio Ambiente, Terrenos contaminados, no 2, Instituto Geológico y Minero de España, Madrid, p 167
Duffus JH (2002) Heavy metals—a meaningless term? Pure Appl Chem 74(5):793–807
FAO-ISRIC-ISSS (2006) World reference base for soil resources. A framework for international classification, correlation and communication. World soil resources reports 103, p 132
Fernández-Caliani JC, Barba-Brioso C, González I, Galán E (2009) Heavy metal pollution in soils around the abandoned mine sites of the Iberian pyrite belt (South-west Spain). Water Air Soil Pollut 200:211–226
García-Salgado S, Quijano MA (2014) Levels of toxic arsenic species in native terrestrial plants from soils polluted by former mining activities. Environ Sci Process Impacts 16:604–612
Gee GW, Bauder JW (1986) Particle-size analysis. In: Klute A (ed) Methods of soil analysis. Part 1. Physical and mineralogical methods, 2nd edn. Madison, ASA-SSSA, pp 383–411 (Agronomy Monograph No. 9)
Grangeia C, Avila P, Matias M, Ferreira da Silva E (2011) Mine tailings integrated investigations: the case of Rio tailings (Panasqueira Mine, Central Portugal). Eng Geol 123(4):359–372
Haigh M (1992) Problems in the reclamation of coal-mine disturbed lands in Wales. Int J Surf Min Reclam 6:31–37
Hasan AB, Sohail Kabir AHM, Reza S, Zaman MN, Ahsan A, Rashid M (2013) Enrichment factor and geo-accumulation index of trace metals in sediments of the ship breaking area of Sitakund Upazilla (Bhatiary–Kumira), Chittagong, Bangladesh. J Geochem Explor 125:130–137
He-rong G, Man-li L, Wei-hua P (2015) Heavy metals in coal mine groundwater responding to mining activity: concentration, temporal variation and speciation. Water Pract Technol 10(2):390–401
Hooda PS (ed) (2010) Trace elements in soils. Wiley, Chichester. http://ec.europa.eu/environment/integration/research/newsalert/pdf/230na6.pdf
Hu Z, Caudle RD, Chong SK (1992) Evaluation of firm land reclamation effectiveness based on reclaimed mine properties. Int J Surf Min Reclam Environ 6:129–135
Jiménez Ballesta R, Conde Bueno P, Martín Rubí JÁ, García Giménez R (2010) Pedo-geochemical baseline content levels and soil quality reference values of trace elements in soils from the Mediterranean (Castilla la Mancha, Spain). Cent Eur J Geosci 2(4):441–454
Jiménez R, Jordá L, Jordá R, Prado P (2004) Madrid: la minería metálica desde 1417 hasta nuestros días. Bocamina 14:89–91
Jordá L (2003) Breve historia de la minería de los metales en la Comunidad de Madrid. Tierra y Tecnología 25:63–68 (in Spanish)
Jordá L (2008) La Minería de los metales en la provincia de Madrid: Patrimonio Minero y puesta en valor del espacio subterráneo. Doctoral thesis. Universidad Politécnica de Madrid, p 780 (in Spanish)
Jordá L, Puche O, Mazadiego LF (2005) La minería de los metales y la metalurgia en Madrid (1417–1983). Ed Instituto Geológico y Minero de España. Serie Recursos Minerales n. 7, p 192 (in Spanish)
Kim M, Jung Y (2004) Vertical distribution and mobility of arsenic and heavy metals in and around mine tailings of an abandoned mine. J Environ Sci Health Part A 39:203–222
Kisch H (1990) Recommendations on illite crystallinity. IGCP Project 294, VIGM, pp 1–9
Koushik S, Kalyan A, Anuruddha G (2012) Effect of mine spoil on native soil of Lower Gondwana coal fields: Raniganj coal mines areas, India. Int J Environ Sci 2(3):1675–1687
Lee M, Paik I, Kim I, Kang H, Lee S (2007) Remediation of heavy metal contaminated groundwater originated from abandoned mine using lime and calcium carbonate. J Hazard Mater 144:208–214
Lee H, Kabir MI, Kwon PS, Kim JM, Kim JG, Hyun SH, Rim YT, Bae MS, Ryu ER, Jung MS (2009) Contamination assessment of abandoned mines by integrated pollution index in the Han River watershed. Open Environ Pollut Toxicol J 1:27–33
Li XY, Liu LJ, Wang YG, Luo GP, Chen X, Yang XL, Gao B, He XY (2012) Integrated assessment of heavy metal contamination in sediments from a coastal industrial basin, NE China. PLoS One 7(6):e39690. doi:10.1371/journal.pone.0039690
Li Z, Ma Z, Jan T, van der Kuijp Z, Yuan LH (2014) A review of soil heavy metal pollution from mines in China: pollution and health risk assessment. Sci Total Environ 468–469:843–853
Lim HS, Lee JS, Chon HT, Sager M (2008) Heavy metal contamination and health risk assessment in the vicinity of the abandoned Songcheon Au–Ag mine in Korea. J Geochem Explor 96:223–230
Liu HY, Probst A, Liao BH (2005) Metal contamination of soils and crops affected by the Chenzhou lead/zinc mine spill (Hunan, China). Sci Total Environ 339:153–166
Maiti SK (2003) Moef report, an assessment of overburden dump rehabilitation technologies adopted in CCL, NCL, MCL, and SECL mines (Grant No. J-15012/38/98-IA IIM)
Maiti SK (2007) Bioreclamation of coalmine overburden dumps—with special emphasis on micronutrients and heavy metals accumulation in tree species. Environ Monit Assess 125:111–122
Martín Peinado FJ, Romero-Freire A, García Fernández I, Sierra Aragón M, Ortiz-Bernad I, Simón Torres M (2015) Long-term contamination in a recovered area affected by a mining spill. Sci Total Environ 514:219–223
Martín-Moreno C, Martín Duque JF, Nicolau Ibarra JM, Hernando Rodríguez N, Sanz Santos MA, Sánchez Castillo L (2016) Effects of topography and surface soil cover on erosion for mining reclamation: the experimental spoil heap at El Machorro Mine (Central Spain). Land Degrad Dev 27(2):145–159
Moreno MR, Cala Rivero V, Jiménez Ballesta R (2005) Selenium distribution in topsoils and plants of a semi-arid Mediterranean environment. Environ Geochem Health 27:513–519
Moreno-Jiménez E, Peñalosa JM, Manzano R, Carpena-Ruiz RO, Gamarra R, Esteban E (2009) Heavy metals distribution in soils surrounding an abandoned mine in NW Madrid (Spain) and their transference to wild flora. J Hazard Mater 162:854–859
Moreno-Jiménez E, Manzano R, Esteban E, Peñalosa J (2010) The fate of arsenic in soils adjacent to an old mine site (Bustarviejo, Spain): mobility and transfer to native flora. J Soils Sediments 10:301–312
Mu Y, Yuee H, Jun L, Chaopin L (2015) Characterization of heavy metals in soil near coal mines and a power plant in Huainan, China. Anal Lett 48(4):726–737
Müller G (1979) Index of geoaccumulation in sediments of the Rhine River. Geojournal 2:108–118
Pederson TA, Rogowski AS, Pennock R (1988) Physical characteristics of some mine spoils. Soil Sci Soc Am J 144:131–140
Puche O, García I, Mazadiego LF (2000) El Patrimonio Minero de la mina del Cerro de la Plata de Bustarviejo (Madrid). III Reunión Científica de la SEDPGYM. Patrimonio Minero- Metalúrgico, 13–15 Noviembre 1998. La Rábida (Huelva), 15 pp, in: Actuaciones sobre Patrimonio Minero- Metalúrgico. Servicio de Publicaciones de la Universidad de Huelva (in Spanish)
Rodríguez L, Ruiz E, Aloso-Azcárate J, Rincón J (2009) Heavy metal distribution and chemical speciation in tailing and soils around a Pb–Zn mine in Spain. J Environ Manag 90:1106–1116
Sencindiver JC, Ammons JT (2000) Minesoil genesis and classification. In: Barnhisel RI, Darmody RG, Daniels WL (eds) Reclamation of drastically disturbed lands. Agronomy Monograph No. 41, ASA, CSSA, SSSA, Madison, pp 595–613
Smart D, Callery S, Courtney R (2015) The Potential for waste-derived materials to form soil covers for the restoration of Mine Tailings in Ireland. Land Degrad Dev 27:542–549. doi:10.1002/ldr.2465
Soil Survey Staff (2006) Keys to soil taxonomy, 10th edn. U.S Department of Agriculture, Natural Resources Conservation Service, Washington, DC
Soulard CE, Acevedo W, Stehman SV, Parker OP (2016) Mapping extent and change in surface mines within the United States for 2001 to 2006. Land Degrad Dev 2(2):248–257. doi:10.1002/ldr.2412
Thomas GW (1996) Soil pH and soil acidity. In: Sparks DL (ed) Methods of soil analysis: chemical methods. Part 3. Soil Sci Soc Am, Madison
USEPA (1994) Design and evaluation of tailings dams. Technical report—EPA530-R-94-038. USEPA, Office of Solid Waste, Special Waste Branch, Washington
VROM (2000) Circular on target values and intervention values for soil remediation Netherlands Ministry of Housing. Spatial Planning and Environment, The Hague
Wray DS (1998) The impact of unconfined mine tailings and anthropogenic pollution on a semi-arid environment—an initial study of the Rodalquilar mining district, South East Spain. Environ Geochem Health 20:29–38
Acknowledgements
The authors thank the reviewers of this paper for providing constructive comments, which have contributed to the improvement of the present version.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
García-Giménez, R., Jiménez-Ballesta, R. Mine tailings influencing soil contamination by potentially toxic elements. Environ Earth Sci 76, 51 (2017). https://doi.org/10.1007/s12665-016-6376-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-016-6376-9