Abstract
Background, aim, and scope
The mobility of arsenic in soils and its transfer to other environmental components present significant environmental risks. The management of polluted land is determined by the availability, mobility, and transfer of inorganic pollutants to different ecosystem compartments. In this paper, the fate of arsenic at this mining site has been evaluated to determine future management practises to minimise such risk.
Materials and methods
In a field study carried out in the area adjacent to a mining site at Bustarviejo (North Madrid, Spain), samples of soils, plants, and water were collected from areas adjacent to the core of the former mining activity. The following parameters were investigated in soil samples: pH, organic matter, pseudo-total As, P, and Fe, and labile As and P, and a sequential extraction procedure was performed to investigate As speciation in soil. Plant materials were analysed for both As and P. Arsenic concentrations in water samples (surface and soil pore water collected in the field) were also measured. Results are considered in tandem with previous data on metal concentrations in soils and plants from this site.
Results
Despite high As concentrations in soils impacted by former mining activities (spoil accumulation and drainage from spoil heaps resulted in concentrations of up to 3,000 mg kg−1), it was not present in a labile form. Sequential extraction revealed that arsenic was mainly retained by Al- and Fe-(oxihydr)oxides (up to 80%). Therefore, only a small proportion of the total soil pool was potentially available for plant uptake (0.3% and 7% extracted by (NH4)2SO4 and NH4H2PO4, respectively). There was very limited transfer of arsenic from soil to plants, and concentrations of arsenic in shoot tissues were relatively low (<8 μg g−1). There was no evidence of phytotoxic effects in the flora that had colonised this site, indicating that a sustainable ecosystem had been established.
Discussion
High levels of arsenic occur at this site, but arsenic mobility appears to be primarily controlled by the presence of amorphous and crystalline Fe and Al hydrous oxides. Although a low labile As fraction was extracted, concentrations of arsenic in both surface and soil pore water are of concern. The risk of arsenic remobilisation by plant uptake or transfer to the food chain via plant consumption is relatively low in these soils. Large amounts of metals and arsenic remain at the site, and potential risks need to be monitored. Some possible remediation strategies that take into account the presence of both arsenic and heavy metals will be suggested.
Recommendations
Natural attenuation and phytostabilisation processes are taking place in several parts of the study area. These natural processes could be enhanced by application of both compost and a suitable Fe-based amendment. This augmentation of the re-vegetation of the affected area could act to promote both arsenic and metal stabilisation in mine tailings with additional benefits for further vegetation establishment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Background, aim, and scope
Arsenic is a widely distributed trace element in nature as a constituent of some minerals, but the release of arsenic has been promoted by anthropogenic activities, such as mining and carbon combustion (Adriano 2001). Sulphide deposits are the main mineral source of As, in which the element can be present in high concentrations (Milton and Johnson 1999; Smedley and Kinniburgh 2002). These ores have been extensively exploited for some centuries, inducing a wide legacy of arsenic in many soils and mine spoils (Clemente et al. 2006; Strawn et al. 2002; Ongley et al. 2007; Ernst 2005). In Spain, there are now many historic derelict mines (Loredo et al. 1999; Clemente et al. 2003; Conesa et al. 2007; Domínguez et al. 2008; Rufo et al. 2007). At the Mónica mine (NW of Comunidad de Madrid, Spain), silver was extracted since the fifteenth century, but the mine was finally closed in 1980. A geological museum and visitor centre has now been created at the site of the former mine. There are still several areas with abundant pyritic wastes, and the presence and distribution of metals in soils and plants has been recently monitored in this area (Moreno-Jiménez et al. 2009). The arsenic-bearing mineral commonly found there is arsenopyrite (FeAsS), with matildite (AgBiS) appearing as small grains associated with it (Jiménez et al. 2004). There is a need to monitor the presence and behaviour of arsenic because the whole area is now classified as a leisure site inside an environmental reservoir proposed for the ecological network Natura 2000, following the environmental directives of the European Union (92/43/CEE Directive).
The presence of arsenic in soils may have hazardous consequences, but the mechanisms controlling As mobility in soils are not well understood. Arsenic geochemistry is complex, and many variables play a role in determining speciation and, hence, mobility (Fitz and Wenzel 2002; Gulz et al. 2005; Kumpiene et al. 2007; Madejón and Lepp 2007). As a general rule, arsenate is the predominant species present in aerobic soils (Sadiq 1997; Strawn et al. 2002). Arsenate mobility in soils is governed by the presence of iron and manganese hydroxides, organic matter, pH, and phosphate. The weakly retained fractions of As, rather than total soil concentrations, are most useful for current risk assessment procedures, mainly for crops and native flora (Fayiga and Ma 2006; Gulz et al. 2005; Anawar et al. 2008; Vázquez et al. 2008) but also for mammals (Sarkar et al. 2007). The most important factor is the rate of change in the proportion of As that moves from the total soil pool into those fractions that are most accessible by living organisms. Whilst the factors that regulate the rate of this change are poorly understood, such processes can be monitored by the use of sequential extractions (Onken and Adriano 1997; Wenzel et al. 2001; Shiowatana et al. 2001). These are useful tools in long-term management of polluted sites.
Data concerning As uptake by natural vegetation growing on As-polluted soils are not abundant, despite the fact that this can be a good way of monitoring As bioavailability and transfer into the food chain (Milton and Johnson 1999; Madejón et al. 2006). Plant responses to arsenic in the soil should always be investigated for the particular soil–plant system (Kabata-Pendias 2004). Arsenic concentrations in plants are generally found to be low in aboveground tissues (Anawar et al. 2006; Madejón and Lepp 2007; Domínguez et al. 2008), in agreement with a low translocation rate to shoots (Moreno-Jiménez et al. 2008). Many authors have used different calculations to evaluate this transfer from the soil to plant, such as transfer factor (TF) or bioaccumulation factors (BAF; Kloke et al. 1984; Huang et al. 2006). Once inside plant cells, arsenate phytotoxicity has been widely demonstrated, mainly under hydroponic conditions (Hartley-Whitaker et al. 2001; Mascher et al. 2002; Moreno-Jiménez et al. 2008), but field studies on this topic are infrequent. It is particularly well known that phosphate can inhibit arsenate uptake in plants and alleviate its toxicity (Esteban et al. 2003), and from this point of view, phosphate concentration will alter arsenate toxicology in the soil.
The objectives of the present study were (1) to characterise the fate and dispersal of arsenic in soils around a former mining area, (2) to measure arsenic levels in the wild flora, and (3) to evaluate the transfer and availability of arsenic in the soil–plant system. In summary, we address an initial strategy to manage arsenic risk in this site that also takes into account the presence of significant concentrations of heavy metals.
2 Materials and methods
2.1 Study site and plant and soil collection
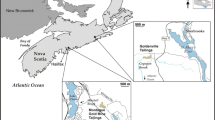
The Mónica mine is within a high valley, wooded at the bottom, and with shrubby vegetation on the upper slopes (Moreno-Jiménez et al. 2009). Soils and plants were sampled in the surrounding of the mine around 200,000 m2 between the following universal transverse Mercator coordinates: 30 T—X = 0438606, Y = 4524302; X = 0437797, Y = 4523518. Sampling was carried out between May and June 2006. Shoots of several plant species were collected, as well as representative soil samples from the soil directly adjacent to the sampled plants (0–30 cm, topsoil layer), obtaining a total of 43 soil samples and 95 shoot samples. Plant species were selected on the basis of their abundance in each vegetation unit of each plant group (ferns, herbaceous plants, shrubs, and trees). Water samples were collected in May from the La Mina and La Barranca streams that flow through the site. One of the water samples was collected upstream the spoil heaps, and the other five were collected downstream the mine. Sampling points were georeferenced by global positioning system, and sampling points for the soils were divided into three groups: (1) soils close to mining dumps (SCMD), (2) soils affected by mine drainage (SAMD), and (3) potentially unaffected soils (UAS). Further details (sampling map and plant species collected) of the site are described by Moreno-Jiménez et al. (2009). Pore water was sampled in spring 2009, using rhizon samplers sited 15 cm below the soil surface, following the methods described by Clemente et al. (2008) in five representatives places: one in a non-polluted soil, two in the soils affected by the mine drainage, and two at the mining spoils.
2.2 Analytical procedures
Bulk soil samples were air-dried for 7 days, sieved to 2 mm, and homogenised. Organic matter was determined by dichromate oxidation, and pH was measured in a soil water suspension 1:2.5 (MAPA (Ministerio de Agricultura, Pesca y Alimentación) 1994). For weakly retained As, 2 g of soils were mixed with 20 ml of (NH4)2SO4 0.1 M, shaken for 4 h, and the resultant suspension filtered (Vázquez et al. 2008). Soils were extracted by HNO3:H2O2 digestion in autoclave prior to determination of pseudo-total element concentrations (Wenzel et al. 2001).
Arsenic fractionation was also studied in four randomised soil samples selected from each group of soils. Five sequential extraction steps were performed with 1 g of soil following Wenzel et al. (2001):
-
1.
Twenty-five millilitre 0.05 M (NH4)2SO4; shaken for 4 h and decanted; F1: non-specifically sorbed
-
2.
Twenty-five millilitre 0.05 M NH4H2PO4; shaken for 16 h and decanted; F2: specifically sorbed
-
3.
Twenty-five millilitre 0.2 M NH4-oxalate buffer, pH = 3.25; shaken for 4 h and decanted; F3: amorphous oxides of Fe and Al
-
4.
Twenty-five millilitre 0.2 M NH4-oxalate buffer + ascorbic acid, pH = 3.25; shaken for 30 min at 96°C and decanted; F4: crystallised oxides of Fe and Al
-
5.
Digestion of soil with HNO3/H2O2 in closed containers under 1,500 Pa and 125°C for 30 min. Mixture was filtered and diluted to 50 mL; F5: residual phase.
All extractions were performed in duplicate.
Arsenic in liquid samples was measured by atomic fluorescence spectroscopy (Millennium Excalibur System, PSAnalytical) using a matrix of HCl 25% (v/v) + ascorbic acid 0.2% (w/v) + KI 2%, and a reductant (NaBH4 0.9% + NaOH 0.4%; Vázquez et al. 2008). The detection limit of the As determination was 0.01 μg l−1. P concentration in liquid extracts was measured spectrophotometrically, and Fe was analysed by atomic absorption spectrometry (MAPA (Ministerio de Agricultura, Pesca y Alimentación) 1994).
Plant samples were carefully washed initially with tap water and subsequently with distilled water, dried at 50°C for 7 days, and ground to 0.5 mm. A known weight of plant material (0.5 g) was digested with 10 mL of mili-Q H2O, 2 mL of H2O2, and 3 mL of HNO3 in an autoclave at 1,500 Pa and 125°C for 30 min. The resultant solution was filtered and made up to constant volume with 25 ml with water. Arsenic and P in plant extracts were measured in the same way as for soil extracts.
Certified reference materials (CTA-VL2, tobacco leaves, 0.97 μg As g−1; CMR048-050, soil, 150 mg kg−1) were also digested and analysed. These were found to contain 0.94 μg As g−1 and 133 mg As g−1, respectively, with a coefficient of variance of <5%.
2.3 Statistical analysis and data processing
The data were analysed using Statistical Package for the Social Sciences 14.0® for Windows. Statistical differences between soil groups were determined using the non-parametric Kruskal–Wallis or Wilcoxon tests. Linear regression, simple, and bivariate correlations for the soil parameters were also performed. Sequential extractions were compared using Tukey’s test. ArcGIS 9.0® was used to geoprocess As concentrations in soils. Principal component analysis (PCA) was performed on soil and plant analysis data, including data on heavy metals from Moreno-Jiménez et al. (2009, with permission). TF and BAF were calculated as follows: \( {\text{TF}} = {\left[ {\text{As}} \right]_{\text{shoot}}}\,/{\left[ {\text{As}} \right]_{\text{pseudo - total}}};\quad {\text{BAF}} = {\left[ {\text{As}} \right]_{\text{shoot}}}/{\left[ {\text{As}} \right]_{{\text{ammonium}}\,{\text{sulphate - extractable}}\,{\text{As}}}} \).
3 Results
3.1 As distribution in soils
Three soil groups were distinguished in the study area: SCMD, soils affected by drainage water from the mine tailings, and UAS. Organic matter, pH, pseudo-total Fe, and pseudo-total and available As and P were measured in samples from each of the three soil groups (Table 1). All soils had an acid pH; no significant differences were observed despite the fact that arsenopyrite oxidation can sharply decrease soil pH (Clemente et al. 2006). No differences were found in organic matter content between soil groups, with values ranging from 0.69% to 13%. Total Fe content was high due to the presence of iron in the geochemical background, but the highest concentrations were present in samples from the mining dumps (P < 0.01), where arsenopyrite minerals are abundant. Total As levels were also very high in the mining dumps (>600 mg kg−1) and in some of the SAMD. Significant differences between soil groups were observed for both total and (NH4)2SO4-extractable As (P < 0.001 for both). Ammonium sulphate extracted between 0.02% and 0.68% of total arsenic from the soil samples. The SAMD showed higher levels of total P (P < 0.05), but no significant differences were observed for (NH4)2SO4-extractable P.
Bivariate correlation analysis between soil variables was used to determine the best correlated variables with As distribution in the soil (Table 2). Total and extractable As were well correlated (P < 0.001), with the highest Pearson’s coefficient (r = 0.840), suggesting that in this particular case, labile As is closely related to the total arsenic concentrations in soils. However, several other variables, such as Fe, total P, and available P were also significantly correlated with (NH4)2SO4-extractable arsenic in these soils.
A linear regression was performed to identify important factors affecting As extractability. Some parameters (pH and organic matter) were removed from the model without significant variations. The resulting equation was \( {\left[ {\text{As}} \right]_{\text{Ext}}} = -0.86 + 0.001 \cdot {\left[ {\text{As}} \right]_{\text{Tot}}} + 4.5 \cdot {10^{ - 5}} \cdot {\left[ {\text{Fe}} \right]_{\text{Tot}}} + 0.024 \cdot {\left[ {\text{P}} \right]_{\text{Ext}}} + 0.001 \cdot {\left[ {\text{P}} \right]_{\text{Tot}}} \); F = 29.4 (P < 0.001), \( R_{adj}^2 = 0.74 \). The significance of the parameters was <0.001 for total As and Fe, <0.005 for available P, and <0.05 for total P.
When arsenic data was included in the PCA carried out by Moreno-Jiménez et al. (2009, with permission) with the soil parameters, As was associated with other pollutants from past mining, such as Cd, Cu, Fe, and Zn, in factor 1 (factor loadings higher than 0.77, data not shown), which is associated with pollution. However, P, Mn, organic matter, and pH had higher loadings in factor 2 (not related to the pollution).
Figure 1 describes the concentration of arsenic in soils using geographic information system tools to clarify the dispersion of arsenic within the valley. The zones with abundant mining spoils showed the highest As levels, appearing as a hot-spot (dark grey polygon). There is also a remarkable pollution plume of arsenic associated with the runoff flow (intermediate greys), but many areas with low levels of arsenic were also present (in lightest grey colour).
Sequential extraction of soil samples was performed to clarify the fractionation of arsenic in these soils (Table 3). The percentage of arsenic extracted by the two first steps (ammonium sulphate and phosphate extractable fractions, respectively) did not show significant differences (3–7%). Steps 3 and 4 (associated to amorphous and crystalline hydrous oxides of Fe and Al) extracted most of the arsenic in the UAS (more than 80%) but also an important portion in polluted soils (41–59%). Finally, the residual step extracted higher significant percentages of arsenic in polluted soils (38–55%).
Arsenic concentrations in pore water were non-detectable (in an unpolluted soil), 0.002, 0.117, 0.062, and 2.901 mg l−1 (just below a spoil heap, close to the main drainage from the heap). Meanwhile, the concentrations in the stream waters were 0.054 (the furthest sampling point downstream the mine), 0.115, 0.147, 0.166, and 0.244 (the nearest) mg As l−1. Upstream the main core of the mine, arsenic concentration in the stream water was 0.028 mg l−1, which could be indicative of the local geochemical background.
3.2 Plants
P and As concentrations in shoots are given in Table 4, as well as TFs and BAF to evaluate arsenic transfer to plants. Means of As content in different plant groups were compared (seedless vascular, herbaceous, and woody plants). Significant differences were observed (P < 0.05), and As and P concentrations in shoots showed similar tends: herbs > ferns > woody plants. Seedless vascular plants tended to accumulate less than 2 mg kg−1 in shoots, despite the three species being sampled from soils with high total As levels; and all species showed a similar behaviour. TFs for the seedless vascular plants were <0.01, meaning that plants are inefficient As accumulators. The BAF ranged between 1 and 2. Herbs showed the highest As concentration in their aboveground tissues (1.44 mg As kg−1). Of all the plant species that were sampled, Glyceria fluitans had the highest As concentration (3 mg As kg−1), followed by Diplotaxis erucoides (2 mg As kg−1). In some species, As was not detected in shoots (i.e., Hypericum perforatum and Daucus carota). The highest BAF was found for Digitalis thapsi and the highest TF for D. erucoides. Woody plants had low As concentrations in shoots. Salix atrocinerea had the highest values (with a mean of 1.93 mg kg−1), while Genista cinerascens and Erica arborea had the lowest (0.20). Depending on the species, shoots were divided into leaves and stems, and significant differences were observed, with As concentration clearly higher in leaves (data not shown). The TF and BAF varied considerably within this group, ranging from 0.0001 to 0.02 and 0.1 to 14, respectively. Betula pendula showed the lowest values of TF and BAF, S. atrocinerea the highest TF, and Cytisus scoparius the highest BAF.
Total and (NH4)2SO4-extractable As concentrations were correlated with arsenic concentrations in the shoots of the dominant plant species (Table 5). This relationship was separately evaluated for each plant species. High and significant positive correlations were generally obtained for plant arsenic concentrations and their (NH4)2SO4-extractable fraction in soils, while this correlation was frequently non-significant when the total As concentration was the other variable.
4 Discussion
4.1 Arsenic in soils
Table 1 shows that about 30% of the soils contained levels of arsenic 30–125 times higher than the limit of 24 mg As kg−1 for soils. This is the standard threshold in the Community of Madrid for human health protection that requires a toxicological assessment (BOCM 2007). Whilst arsenic exceeded the threshold value to larger extent (up to 125 times), values for Cd, Cu, and Zn were only around ten, eight, and two times higher, respectively (Moreno-Jiménez et al. 2009). Within the site boundaries, an area of more than 20,000 m2 had soil As concentrations that exceeded 1,000 mg kg−1 (see Fig. 1). However, total element concentration is not a good indicator of As mobility, availability and, subsequently, risk (Sheppard 1992; Adriano 2001). Concentrations determined by chemical analysis using soft extractant agents and sequential extractions are more suitable to evaluate As transfer from soils to other ecosystem compartments (such as plants, animals, or water) than total As (Madejón et al. 2006; Menzies et al. 2007; Clemente et al. 2008; Vázquez et al. 2008). Thus, levels of easily extractable As in soil and As in plants (see Table 3) are actually very low in comparison with total amounts in the soils. Weak neutral salt solutions seem to be adequate to assess the impact of trace elements on plants and soil biological activity (Kabata-Pendias 2004), and (NH4)2SO4-extractable As has been successfully used as an indicator of As availability (Vázquez et al. 2008). This method was also successfully used with metals in field experiments (Moreno-Jiménez et al. 2009) and with multi-polluted soils in controlled experiments (Vázquez et al. 2008). In the present study, there were no significant correlations between organic matter or pH with either total or extractable As concentrations in soil. The bivariate analysis and the linear regression model showed the influence of total As on extractable arsenic in this particular case (with the highest significance in bivariate correlation and in the regression linear model), but other parameters were also shown to influence the model (P and Fe). In the same soils, total metal concentrations were also the main factor controlling the extractable metal fraction (Moreno-Jiménez et al. 2009). The influence of available P was particularly interesting but, despite its significance, it was not as important as total As or Fe. There was a negative correlation between (NH4)2SO4-extractable P and extractable As, but the corresponding parameter is positive in the linear regression model. This means that P could mobilise As from soils but, in this case, soils with a high extractable As content also had low concentrations of labile P. The effect of phosphate on arsenate mobility in soils is well known, as they compete to be absorbed in the same soil constituents (Peryea and Kammereck 1997; Cao et al. 2003). The proportion of the total soil As pool extracted with (NH4)2SO4 was always <0.7%. This is in agreement with other studies on As-polluted soils (Anawar et al. 2008; Vázquez et al. 2008; King et al. 2008), but contrasts with some experiments carried out with As-spiked soils where NaNO3- or (NH4)2SO4-extractable As were around 1–3% and 8–20%, respectively (Gulz et al. 2005; Carpena et al. 2008), knowing that NaNO3 extracts As with a 50% less efficiency than (NH4)2SO4 (Vázquez et al. 2008). Although many studies have used As-spiked soils (Fitz and Wenzel 2002), artificially spiked soils are unrepresentative of As geochemistry in mine tailings (Ko et al. 2008), as arsenic extractability was excessive after the spiking. Plants growing on spiked soils usually show unrealistic As uptake. The low mobility of As in the studied soils is particularly evident if we compare arsenic data with other metals in this site (Moreno-Jiménez et al. 2009). The best correlation using (NH4)2SO4-extractable fraction than total As in soils was found with As concentrations in shoots (see Table 5), indicating that phytoavailability is better predicted by the ammonium sulphate-extractable fraction of As than by the total concentrations. When taken together with previous findings both under controlled and under field conditions (Vázquez et al. 2008; Moreno-Jiménez et al. 2009), these data support the use of (NH4)2SO4 extraction as a robust indicator for the phytoavailable fraction of trace elements present in multi-polluted soils. Percentages of (NH4)2SO4-extractable Cd are at least 20 times higher than As ones. Moreover, when we extracted with phosphate (see F2 in Table 3), the values were always lower than 7% of the total arsenic. The extraction of Cd using (NH4)2SO4 is much more efficient than for As in these soils. In addition, ammonium phosphate extraction removes <7% of the total soil As pool. This fraction has also been used as an indicator of phytoavailable As (Huang et al. 2006). Arsenic mobility in soils is known to be dependent on absorption by iron, aluminium, and manganese hydroxides, clay minerals, and mineral oxyanions such as phosphates, carbonates, and sulphates (Aguilar et al. 2007; Gulz et al. 2005). The sequential extraction of these soils indicated a principal role of Fe- and Al-(oxyhydr)oxides in As geochemistry in soils (see Table 3). Arsenic in fractions 3 and 4 is mainly associated with amorphous and crystalline hydrous oxides of iron and aluminium (Wenzel et al. 2001), and up to 60–80% of As was retained in these fractions. This is in clear agreement with previous findings for different soils (Wenzel et al. 2001; Fitz and Wenzel 2002; Kumpiene et al. 2007; Clemente et al. 2008), and arsenate absorption onto Fe oxides is a frequent phenomenon after mineral oxidation in pyritic soils (Strawn et al. 2002). The residual step extracted higher significant percentages of arsenic in polluted soils (in SCMD and SAMD samples), probably due to the presence of As minerals such as arsenopyrite and matildite (Jiménez et al. 2004). Finally, arsenic in pyritic soils was associated with Fe both in pyrite minerals and in (oxyhydr)oxides, resulting in a high correlation between total Fe and As. Arsenic retained in these fractions is not readily available or mobile, although it could be released if environmental conditions change. Moreover, in highly polluted soils, As is also a constituent of the least soluble fraction, i.e., as a constituent of minerals (arsenopyrite). Thus, more than 70–90% of arsenic in soils could be in the most inert fractions in mining-affected soils (Conesa et al. 2008). According to Rodríguez et al. (2003), who demonstrated that the arsenic fraction bound to Fe and Mn oxides can be dissolved in the stomach, there are arsenic risks associated with soil ingestion at this site. Soil ingestion is a major pathway of metals into livestock grazing in polluted lands (Thornton and Abrahams 1983). Cattle and horses are frequently feeding in the studied zone, and they are directly exposed to vegetation and soil particles ingestion. Regarding to humans, soil particles can be also voluntarily or involuntarily ingested, but there are also other possible pathways involving the risk assessment of trace elements in these soils such as soil particles inhalation or dermal absorption (Abrahams 2002). There are still many mine spoils uncovered by vegetation in the studied site and, subsequently, the dump particles can be easily disturbed by wind and transported to the atmosphere, enhancing the risk of mining spoils.
4.2 Transference of arsenic to plants and waters
The risk assessment was completed with data concerning plants and waters as receptors of soil pollution. All the data in our study supported the low mobility of arsenic in acidic soils. The majority of plant species preferentially store As in roots, as opposed to shoots (Moreno-Jiménez et al. 2008), with the exception of As hyperaccumulators (Zhang et al. 2002). Despite the high concentrations in soil, arsenic in plants is usually below the toxicity threshold for aboveground tissues of 3–10 mg kg−1 dry weight (Chaney 1989). Arsenic concentrations in shoots of many plants exceeded the value of 1–1.7 mg kg−1 quoted for shoots of plant growing in unpolluted soils (Kabata-Pendias and Pendias 1992), but this could also be attributed to incomplete removal of surface-borne As-containing particulates by the washing procedure. Because of the very elevated concentrations of As found in some of the mine spoils at this site, a small volume of unremoved particulates could have a significant influence on shoot As concentrations. No phytotoxic symptoms were observed in plants growing at the site even in G. fluitans and S. atrocinerea, which had shoot concentrations of 7 and 3 mg kg−1, respectively. The low TFs calculated from the data suggest insignificant movement of As to plant shoots in this ecosystem. The TF values fluctuated between 0 and 0.037, all within the range of 0.0001–0.1 reported as normal (Kloke et al. 1984; Warren et al. 2003). Previous studies have commonly reported low transfer of arsenic from soil to plant shoots (Bunzl et al. 2001; Jung et al. 2002; Anawar et al. 2006; Domínguez et al. 2008), which progressively decreased when total soil As concentrations increased (Huang et al. 2006). Warren et al. (2003) found out differences between this TF depending on the source of As in soils. In the current study, we were studying a mining site with elevated As concentrations in soils and a significant proportion of the total soil As pool in non-labile fractions and, in consequence, a low TF was expected. If we compare this with other trace elements at the site, the TF for As is between 1 and 3 orders of magnitude lower than those calculated for Cd, Cu, or Zn (Moreno-Jiménez et al. 2009), following the same trend as the percentage of (NH4)2SO4-extractable As and metals in these soils. Not only the low mobility of As in soils is determining a low transfer rate to aboveground tissues, but some physiological factors could also be affecting the low concentrations of arsenic in shoots, such as limited trans-membrane mobility of arsenate in comparison to other metals such as Hg or Cd (Esteban et al. 2003, 2008; Esteban et al. unpublished data), arsenate-phosphate interactions (Esteban et al. 2003), and low As translocation from roots (Moreno-Jiménez et al. 2008). The BAF, based on (NH4)2SO4-extractable As, was also calculated. This coefficient reduces the contribution of soil-related differences in mobility, emphasising differences in the ability of plant species to concentrate As into target organs (Huang et al. 2006). Pteridium aquilinum, D. thapsi, and C. scoparius were the species with the highest BAF found in ferns, herbs, and woody plants, respectively. The main aspects of the risk to the environment through arsenic uptake by plants are (1) introduction into the food chain, (2) loss of vegetation cover induced through phytotoxicity, and (3) cycling of metals to surface soil horizons by tolerant plants to induce toxic effects on flora and fauna (Kabata-Pendias 2004). As a result, arsenic and metals at a site must be monitored over the long-term in order to monitor changes in environmental risk. Milton and Johnson (1999) found that low levels of arsenic in plants were associated with low transfer to the food chain. There is also clear evidence for sustainable As-phytostabilisation in some natural habitats (Madejón and Lepp 2007). Annual plant shoots (i.e., G. fluitans and D. erucoides) and leaves of trees showed higher As concentrations than other plants or aboveground tissues, respectively. Both are principal components of litter fall, which could play a role in remobilisation of trace elements in soils (Mertens et al. 2004). Nevertheless, its influence on arsenic biogeochemistry in soils seemed to be low in this particular case due to the low As concentrations in plant shoots in comparison with total As in soil. In agreement with previous reports (French et al. 2006; Madejón and Lepp 2007), woody plants had the lowest concentrations in shoots, supporting them as good candidates for As-phytostabilisation.
Stream waters contained elevated concentrations of As, exceeding legal limits for surface and potable waters (BOE 2000; Steinmaus et al. 2006). There is abundant literature showing high levels of As in waters impacted by sites with As geological backgrounds (Smedley and Kinniburgh 2002), arsenate being the predominant species (Williams 2001). There is a need for additional hydrogeological studies taking into account sources and discharges of surface and ground waters in this area before a suitable risk assessment can be formulated. Pore water provides a realistic picture of the mobility of trace elements in the soil (Clemente et al. 2008). Up to 3 ppm (40 μM) of As was detected in pore water from one sample site, but at other sites, concentrations of <1.6 μM confirmed the low mobility of As despite the high total concentrations present. This agrees with Fitz and Wenzel (2002), who estimated <2.3 μM arsenate in soil solution, and with Clemente et al. (2008), who found levels of up to 1.6 μM As in pore water under field conditions.
4.3 Proposals for soil management in this site
At this site, arsenic mainly affected water quality, while Cd and Zn were more labile in soils and showed significant soil-plant transfers (Moreno-Jiménez et al. 2009). The dispersal of mine wastes is the main source of arsenic and metals to soils. Subsequently, plants and surface waters are also affected. Techniques commonly used in soil remediation such as sanitary disposal and on-site or ex situ soil leaching disturb soil functions and ecological equilibrium. Here, where maintaining ecological equilibria is a priority objective, environmentally friendly alternatives should be adopted to conserve the ecosystem during any reclamation procedure. Mine wastes are difficult to treat with phytotechnologies: their properties do not allow plant establishment, and toxic elements are also leached to deeper layers (Ernst 2005). Treating multi-polluted soils with soil amendments is complex (Kumpiene et al. 2008), and many edaphic and ecological factors must be evaluated. Mine wastes should be amended to improve soil fertility and stability but also to immobilise toxic elements. One type of amendment that could fulfil these criteria is composted materials (Mench et al. 2003; Gadepalle et al. 2007; Tandy et al. 2009). The amendment would reduce metal dispersion (Gadepalle et al. 2007) that has taken place in the studied soils as well (Moreno-Jiménez et al. 2009). However, some controversial results have been reported after compost supply in As-polluted soils: some authors reported As immobilisation (Gadepalle et al. 2007), but some other authors reported an increase of the labile As fraction due to compost supply (Mench et al. 2003; Kumpiene et al. 2008; Hartley et al. 2009). Thus, compost might be carefully used in As-polluted soils because there are scientific evidences of mobilisation of arsenic from soil after compost supply, and monitoring the soil could support the safety successful of the amendment. On the other hand, compost amendment can improve the fertility on multi-polluted soils and can facilitate the establishment of a healthy vegetation cover (Bernal et al. 2006). In order to reduce As availability, there are some available alternatives such as iron amendments (Warren et al. 2003; Hartley et al. 2004), but they can release metals from soils. A combination of both compost (5%) and iron grit (1%) was successfully used as a compromise option to treat spoils from an abandoned gold mine (Mench et al. 2003) in order to assure the low mobility of As. Re-vegetation is a good and cheap solution to use in derelict areas (French et al. 2006; Robinson et al. 2006) and has been successfully used in polluted Mediterranean areas (Domínguez et al. 2008). Plant establishment in gold mine waste was also improved after compost plus iron grit supply (Mench et al. 2003). Finally, the vegetation cover would prevent soil ingestion and re-entrainment (Vamerali et al. 2009) and metalloid mobility and leaching in soils (Robinson et al. 2006). Nowadays, many authors doubt the current feasibility of phytoextraction because many questions are still open, but trace element phytostabilisation (natural or anthropogenic) could be a competitive technology (Ernst 2005; Robinson et al. 2006; Madejón and Lepp 2007). In places with low vegetation cover, S. atrocinerea or C. scoparius could be suitable candidates for reforesting, depending on the humidity of the soils. The presence of perennial plants will definitively play an important landscape and ecological role (Vamerali et al. 2009).
This site presents three potential hazards (large amounts of trace elements in mine spoils, arsenic in waters, and cadmium in plants) that need to be remediated by soil amendments and site phytostabilisation. In this context, the area should be carefully monitored in order to detect changes in the long-term risk due to the presence of elevated concentrations of trace elements in soils. Toxicological tests and risk assessment will also be carried out with highly polluted soils from this site to evaluate the actual environmental risk of trace elements and their transfer to the food chain.
References
Abrahams PW (2002) Soils: their implications to human health. Sci Total Environ 291:1–32
Adriano DC (2001) Trace elements in terrestrial environments: biogeochemistry, bioavailability, and risks of metals. Springer, New York, pp 219–262
Aguilar J, Dorronsoro C, Fernández E, Fernández J, García I, Martín F (2007) Arsenic contamination in soils affected by a pyrite-mine spill (Aznalcóllar, SW Spain). Water Air Soil Pollut 180:271–281
Anawar HM, Garcia-Sanchez A, Murciego A, Buyolo T (2006) Exposure and bioavailability of arsenic in contaminated soils from the La Parrilla mine, Spain. Environ Geol 50:170–179
Anawar HM, Garcia-Sanchez A, Regina IS (2008) Evaluation of various chemical extraction methods to estimate plant-available arsenic in mine soils. Chemosphere 70:1459–1467
Bernal MP, Clemente R, Walker DJ (2006) The role of organic amendments in the bioremediation of heavy metal-polluted soils. In: Gore RW (ed) Environmental research at the leading edge. Nova Science Pub, New York, pp 1–58
BOCM (2007) Orden 2720/2006, de 11 de agosto, por la que se establecen niveles genéricos de referencia de metales pesados y otros elementos de traza de suelos contaminados de la Comunidad de Madrid, vol 204. Comunidad de Madrid, Spain, pp 29–30
BOE (2000) Real Decreto 995/2000. Núm 147, pp 21558–21562
Bunzl K, Trautmannsheimer M, Schramel P, Reifenhäuser W (2001) Availability of arsenic, copper, lead, thallium, and zinc to various vegetables grown in slag-contaminated soils. J Environ Qual 30:934–939
Cao X, Ma LQ, Shiralipour A (2003) Effects of compost and phosphate amendments on arsenic mobility in soils and arsenic uptake by the hyperaccumulator Pteris vittata L. Environ Pollut 126:157–167
Carpena RO, Pérez Sanz A, Vázquez S, Moreno E, Peñalosa J, Hernández LE, Alarcón R, Garcia P, Lobo MC (2008) Respuesta de Silene vulgaris en suelos contaminados con arsénico y metales pesados. Capacidad fitorrecuperadora. In: Actas XII Simposio Ibérico sobre Nutrición Mineral de las Plantas. Granada
Chaney RL (1989) Toxic element accumulation in soils and crops: protecting soil fertility and agricultural food chains. Inorganic contaminants in the vadose zone. Springer-Verlag, Berlin, pp 140–158
Clemente R, Walker DJ, Roig A (2003) Heavy metal bioavailability in a soil affected by mineral sulphides contamination following the mine spillage at Aznalcollar (Spain). Biodegradation 14:199–205
Clemente R, Almela C, Bernal MP (2006) A remediation strategy based on active phytoremediation followed by natural attenuation in a soil contaminated by pyrite waste. Environ Pollut 143:397–406
Clemente R, Dickinson NM, Lepp NW (2008) Mobility of metals and metalloids in a multi-element contaminated soil 20 years after cessation of the pollution source activity. Environ Pollut 155:254–261
Conesa HM, García G, Faz A, Arnaldos R (2007) Dynamics of metal tolerant plant communities’ development in mine tailings from the Cartagena-La Unión Mining District (SE Spain) and their interest for further revegetation purposes. Chemosphere 68:1180–1185
Conesa HM, Robinson BH, Schulin B, Nowack B (2008) Metal extractability in acidic and neutral mine tailings from the Cartagena-La Unión Mining District (SE Spain). Appl Geochem 23:1232–1240
Domínguez MT, Marañón T, Murillo JM, Schulin R, Robinson BH (2008) Trace element accumulation in woody plants of the Guadiamar Valley, SW Spain: a large-scale phytomanagement case study. Environ Pollut 152:50–59
Ernst WHO (2005) Phytoextraction of mine wastes—options and impossibilities. Chem Erde 65:29–42
Esteban E, Carpena RO, Meharg AA (2003) High-affinity phosphate/arsenate transport in white lupin (Lupinus albus) is relatively insensitive to phosphate status. New Phytol 158:165–173
Esteban E, Moreno E, Peñalosa J, Cabrero JI, Millán R, Zornoza P (2008) Short and long-term uptake of Hg in white lupin plants: kinetics and stress indicators. Environ Exp Bot 62:316–322
Fayiga AO, Ma LQ (2006) Using phosphate rock to immobilize metals in soil and increase arsenic uptake by hyperaccumulator Pteris vittata. Sci Total Environ 359:17–25
Fitz WJ, Wenzel WW (2002) Arsenic transformations in the soil-rhizophere-plant system: fundamentals and potential application to phytoremediation. J Biotechnol 99:259–278
French CJ, Dickinson NM, Putwain PD (2006) Woody biomass phytoremediation of contaminated brownfield land. Environ Pollut 141:387–395
Gadepalle VP, Ouki SK, Van Herwijnen R, Hutchings T (2007) Immobilization of heavy metals in soil using natural and waste materials for vegetation establishment on contaminated sites. Soil Sediment Contam 16:233–251
Gulz PA, Gupta SK, Schulin R (2005) Arsenic accumulation of common plants from contaminated soils. Plant Soil 272:337–347
Hartley W, Edwards R, Lepp NW (2004) Arsenic and heavy metal mobility in iron oxide-amended contaminated soils as evaluated by short- and long-term leaching tests. Environ Pollut 131:495–504
Hartley W, Dickinson NM, Clemente R, French C, Piearce TG, Sparke S, Lepp NW (2009) Arsenic stability and mobilization in soil at an amenity grassland overlying chemical waste (St. Helens, UK). Environ Pollut 157:847–856
Hartley-Whitaker J, Ainsworth G, Meharg AA (2001) Copper- and arsenate-induced oxidative stress in Holcus lanatus L. clones with differential sensitivity. Plant Cell Environ 24:713–722
Huang R, Gao S, Wang W, Staunton S, Wang G (2006) Soil arsenic availability and the transfer of soil arsenic to crops in suburban areas in Fujian Province, southeast China. Sci Total Environ 368:531–541
Jiménez R, Jordá L, Jordá R, Prado P (2004) La minería metálica en Madrid. Bocamica 14:50–89
Jung MC, Thornton I, Chon HT (2002) Arsenic, Sb and Bi contamination of soils, plants, waters and sediments in the vicinity of the Dalsung Cu–W mine in Korea. Sci Total Environ 295:81–89
Kabata-Pendias A (2004) Soil-plant transfer of trace elements-an environmental issue. Geoderma 122:143–149
Kabata-Pendias A, Pendias H (1992) Trace elements in soils and plants. CRC Press, Boca Raton, Florida, p 365
King DJ, Doronila AI, Feenstra C, Baker AJM, Woodrow IE (2008) Phytostabilisation of arsenical gold mine tailings using four Eucalyptus species: growth, arsenic uptake and availability after five years. Sci Total Environ 406:35–42
Kloke A, Sauerbeck DR, Vetter H (1984) The contamination of plants and soils with heavy metals and the transport of metals in terrestrial food chains. In: Nriagu J (ed) Changing metal cycles and human health. Springer, Berlin, pp 113–141
Ko BG, Anderson CWN, Bolan NS, Huh K-Y, Vogleler I (2008) Potential for the phytoremediation of arsenic-contaminated mine tailings in Fiji. Aust J Soil Res 46:493–501
Kumpiene J, Montesinos IC, Lagerkvist A, Maurice C (2007) Evaluation of the critical factors controlling stability of chromium, copper, arsenic and zinc in iron-treated soil. Chemosphere 67:410–417
Kumpiene J, Lagerkvist A, Maurice C (2008) Stabilization of As, Cr, Cu, Pb and Zn in soil using amendments—a review. Waste Manage 28:215–225
Loredo J, Ordonez A, Gallego JR (1999) Geochemical characterisation of mercury mining spoil heaps in the area of Mieres (Asturias, northern Spain). J Geochem Explor 67:377–390
Madejón P, Lepp NW (2007) Arsenic in soils and plants of woodland regenerated on an arsenic-contaminated substrate: a sustainable natural remediation? Sci Total Environ 379:256–262
Madejón P, Murillo JM, Marañón T, Espinas JL, Cabrera F (2006) Accumulation of As, Cd and selected trace elements in tubers of Scirpus maritimus L. from Doñana marshes (South Spain). Chemosphere 64:742–748
MAPA (Ministerio de Agricultura, Pesca y Alimentación) (1994) Métodos oficiales de análisis: Tomo III. Secretaría General Técnica, Madrid, Spain
Mascher R, Lippmann B, Holzinger S, Bergman H (2002) Arsenate toxicity: effects on oxidative stress response molecules and enzymes in red clover plants. Plant Sci 163:961–969
Mench M, Bussiere S, Vangronsveld J, Manceau A (2003) Progress in remediation and revegetation of the barren Jales gold mine spoil after in-situ treatments. Plant Soil 249:187–202
Menzies NW, Donn MJ, Kopittke PM (2007) Evaluation of extractants for estimation of the phytoavailable trace metals in soils. Environ Pollut 145:121–130
Mertens J, Vervaeke P, De Schrijver A, Luyssaert S (2004) Metal uptake by young trees from dredged brackish sediment: limitations and possibilities for phytoextraction and phytostabilisation. Sci Total Environ 326:209–215
Milton A, Johnson M (1999) Arsenic in the food chains of a revegetated metalliferous mine tailings pond. Chemosphere 39:765–779
Moreno-Jiménez E, Peñalosa JM, Carpena-Ruiz RO, Esteban E (2008) Comparison of arsenic resistance in Mediterranean woody shrubs used in restoration activities. Chemosphere 71:466–473
Moreno-Jiménez E, Peñalosa JM, Manzano R, Carpena-Ruiz RO, Gamarra R, Esteban E (2009) Heavy metals distribution in soils surrounding an abandoned mine in NW Madrid (Spain) and their transference to wild flora. J Hazard Mater 162:854–859
Ongley LK, Sherman L, Armienta A, Concilio A, Salinas CF (2007) Arsenic in the soils of Zimapan, Mexico. Environ Pollut 145:793–799
Onken BM, Adriano DC (1997) Arsenic availability in soil with time under saturated and subsaturated conditions. Soil Sci Soc Am J 61:746–752
Peryea EJ, Kammereck R (1997) Phosphate-enhanced movement of arsenic out of lead arsenate-contaminated topsoil and through uncontaminated subsoil. Water Air Soil Pollut 93:243–254
Robinson B, Schulin R, Nowack B, Roulier S, Menon M, Clothier B, Green S, Mills T (2006) Phytoremediation for the management of metal flux in contaminated sites. Forest Snow Landsc Res 80:221–234
Rodríguez RR, Basta NT, Casteel SW, Armstrong FP, Ward DC (2003) Chemical extraction methods to assess bioavailable arsenic in soil and solid media. J Environ Qual 32:876–884
Rufo L, Rodríguez N, Amils R, de la Fuente V, Jiménez-Ballesta R (2007) Surface geochemistry of soils associated to the Tinto River (Huelva, Spain). Sci Total Environ 378:223–227
Sadiq M (1997) Arsenic chemistry in soils: an overview of thermodynamic predictions and field observations. Water Air Soil Pollut 93:117–136
Sarkar D, Makris KC, Parra-Noonan MT, Datta R (2007) Effect of soil properties on arsenic fractionation and bioaccessibility in cattle and sheep dipping vat sites. Environ Int 33:164–169
Sheppard SC (1992) Summary of phytotoxic levels of soil arsenic. Water Air Soil Pollut 64:539–550
Shiowatana J, McLaren RG, Chanmekha N, Samphao A (2001) Fractionation of arsenic in soil by a continuous-flow sequential extraction method. J Environ Qual 30:1940–1949
Smedley PL, Kinniburgh DG (2002) A review of the source, behaviour and distribution of arsenic in natural waters. Appl Geochem 17:517–568
Steinmaus CM, George CM, Kalman DA, Smith AH (2006) Evaluation of two new arsenic field test kits capable of detecting arsenic water concentrations close to 10 µg/L. Environ Sci Technol 40:3362–3366
Strawn D, Doner H, Zavarin M, McHugo S (2002) Microscale investigation into the geochemistry of arsenic, selenium, and iron in soil developed in pyritic shale materials. Geoderma 108:237–257
Tandy S, Healey JR, Nason MA, Williamson JC, Jones DL (2009) Remediation of metal polluted mine soil with compost: co-composting versus incorporation. Environ Pollut 157:690–697
Thornton I, Abrahams P (1983) Soil ingestion—a major pathway of heavy metals into livestock grazing contaminated land. Sci Total Environ 28:287–294
Vamerali T, Bandiera M, Coletto L, Zanetti F, Dickinson NM, Mosca G (2009) Phytoremediation trials on metal- and arsenic-contaminated pyrite wastes (Torviscosa, Italy). Environ Pollut 157:887–894
Vázquez S, Moreno E, Carpena RO (2008) Bioavailability of metals and As from acidified multicontaminated soils: use of white lupin to validate several extraction methods. Environ Geochem Health 30:193–198
Warren GP, Alloway BJ, Lepp NW, Singh B, Bochereau FJM, Penny C (2003) Field trials to assess the uptake of arsenic by vegetables from contaminated soils and soil remediation with iron oxides. Sci Total Environ 311:19–33
Wenzel WW, Kirchbaumer N, Prohaska T, Stingeder G, Lombi E, Adriano DC (2001) Arsenic fractionation in soils using an improved sequential extraction procedure. Anal Chim Acta 436:309–323
Williams M (2001) Arsenic in mine waters: an international study. Environ Geol 40:267–278
Zhang WH, Cai Y, Tu C, Ma LQ (2002) Arsenic speciation and distribution in an arsenic hyperaccumulating plant. Sci Total Environ 300:167–177
Acknowledgements
This study was supported by the Spanish Ministry of Education and Science, project CTM 2007-66401-CO2/TECNO, and by Comunidad de Madrid, project S-0505/AMB/0296. The authors are grateful to the Excmo. Ayuntamiento de Bustarviejo, for admittance to the mine zone and the facilities offered and to Dr. R. Gamarra for his assistance with plant identification. Authors are grateful to L. Beesley for his comments and language corrections.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Jaco Vangronsveld
Rights and permissions
About this article
Cite this article
Moreno-Jiménez, E., Manzano, R., Esteban, E. et al. The fate of arsenic in soils adjacent to an old mine site (Bustarviejo, Spain): mobility and transfer to native flora. J Soils Sediments 10, 301–312 (2010). https://doi.org/10.1007/s11368-009-0099-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-009-0099-4