Abstract
Direct agricultural use of most Australian coal fly ashes is limited by their (1) extreme alkalinity, (2) extreme salinity and (3) trace metal leachability. Therefore, in order to use fly ash as a fertiliser source, the bio-availability of its nutrients needs to be improved by reducing its toxic elements and salinity. Application of fly ash as an amendment to carbonaceous compost has the potential to dilute the toxic effects of fly ash while enhancing the release of more nutrients to the soil through the chemical reaction between compost and fly ash. However, the elevation of pH and salinity levels in compost by the addition of fly ash may reduce the availability of some indigenous micro-organisms in the compost, soil respiration, and nitrogen release and may cause toxic heavy metal intrusion into crops, creating health issues. Therefore, the present study examined the applicability of coal fly ash as an amendment to compost for agricultural use, after neutralization by mineral carbonation, taking into consideration the changes which occur in the geo-chemical properties of fly ash, after the mineral carbonation reactions for CO2 sequestration. The changes in the alkalinity (pH), salinity/electrical conductivity and trace metal concentration of the leachates from column leaching tests were measured and compared before and after the carbonation of three types of Victorian brown coal fly ashes. The results showed a significant drop of pH of the coal fly ash samples from 11–12 to 7.5–8.5 after mineral carbonation, and the salinity reduced from 6–7 to 3.2–3.8 dS/m. Along with the pH reduction, the leachable concentrations of a number of heavy metals, including B, Cd, Cu, Pb, Hg, Zn, As and Se, reduced in the carbonated ash. These favourable changes indicate the enhanced capacity of Victorian brown coal fly ashes as a compost amendment after mineral carbonation reactions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coal fly ash has vast potential as a soil amendment for agriculture, especially due to its physical properties and the presence of macro and micro-nutrients (Pathan 2003). A number of studies have demonstrated the positive effects of coal fly ash for improving soil’s chemical and physical properties, soil nutrient status, plant growth and nutrient uptake (Aitken et al. 1984; Page and Elsiwee 1979). However, the continuous use of fly ash, particularly unweathered and untreated ash, as a soil ameliorant may create problematic situations, depending on the type of soil and the dosage of ash applied (Yunusa et al. 2006). The primary concern is the potential leaching of hazardous metals into the soil from some fly ashes, especially at comparatively high application rates (Palumbo et al. 2005). Fly ash contains some biologically toxic elements in concentrations that greatly exceed their concentrations in soil (Sikka and Kansal 1994; Pandey and Singh 2010). Therefore, the continuous loading of soils with fly ash may lead to a tendency to accumulate these elements in the soil. From the soil, they can be taken up by vegetation or percolated into groundwater sources and cause serious contamination (Sikka and Kansal 1994). Trace elements are persistent global pollutants and accumulation up to toxic levels of these elements is responsible for reductions in crop yields, leading to negative consequences for animal and human health (Sharma and Kalra 2006; Sharma 1989). The toxicity of Boron (B) in particular has become one of the major limiting factors in the agricultural use of coal combustion ash (Pandey and Singh 2010).
Moreover, the addition of fly ash to soil can cause changes in soil alkalinity (expressed as soil pH) and salinity. Soil salinity (total soluble salts) is commonly evaluated using the parameter electrical conductivity (EC). The pH of fly ash can be acidic or alkaline, depending on the type of coal burnt. Accordingly, fly ash is used as a buffering agent to reclaim problem soils with undesirable alkalinity or acidity (Jala and Goyal 2006). However, most coal fly ashes produced worldwide have strong alkalinity in the pH range of 8–12, and therefore their addition as a soil ameliorant to soils can bring about unfavourable changes to soil pH (Pandey and Singh 2010). This may lead to increased bio-availability of some trace metals to levels that are toxic for plants and animals. For example, Riehl et al. (2010) observed an increase in soil pH from 8.1 to 12 in calcaric soils amended with alkaline coal fly ash, which ultimately caused the mortality of soil microfauna and increased mobility of Co, Ni and V. Reduced concentration of water-soluble phosphate nutrients in soil is another result of fly ash addition (Menon et al. 1993). Fly ashes with excessive concentrations of soluble salts (B, Mo, Se, Ca, Mg) may cause salinity problems when applied to soil, and thereby suppress plant growth. Yunusa et al. (2008) observed adverse effects of fly ash application on plant growth at high application rates (<125 Mg/ha), which were attributed to the increased soil salinity. Weathered ash often contains comparatively low concentrations of salts as a result of leaching, which may also limit its nutritional potential due to elemental leaching (Ghodrati et al. 1995). Consequently, salt-tolerant plant species are mostly recommended to grow on soils amended with fly ash in order derive the best agronomic benefits (Yunusa et al. 2006). As the research literature states, the use of coal fly ash in agricultural soil needs to be done paying special attention to the characteristics of the specific fly ash, the properties of the soil and the type of plant (Ukwattage et al. 2013a; Yunusa et al. 2011). Coal combustion fly ash, when applied in conjunction with carbonaceous compost, may act as a favourable amendment to compost as a result of the chemical reactions between the compost and fly ash (Hupenyu et al. 2015). Thermophilic composting is the traditional composting technique during which the high microbial activities enhance soil respiration, creating a greater fertiliser value while killing pathogens. Microbial activities in biological decomposition create organic acids that have the ability to dissolve phosphates containing minerals in fly ash. Therefore, the use of fly ash in composting significantly enhances the phosphates available for plant growth. For example, Menon et al. (1993) observed up to 378 and 348% increments in yields of collard greens and mustard greens, respectively, with 20% of fly ash in organic compost made from grass. According to Basu et al. (2009), fly ash is an ideal amendment for sewage sludge compost, in which lime is normally used to raise the pH, remove pathogens and reduce the heavy metals. Since coal fly ash has significant amounts of CaO, it can be replaced by lime, which reduces the heavy metals through adsorption and precipitation created by its high pH, also considerably reducing the cost. Chou et al. (2005) reported that the addition of fly ash to soil negatively affects plant growth due to the associated high alkalinity and high surface tension. This situation can be overcome by adding around 12.5% of fly ash to a sandy soil and compost mix. This significantly accelerated their tomato plant growth without creating any issue with yield. However, the researchers highlighted that the addition of unnecessarily large quantities of fly ash to compost may negatively affect seed germination. This study clearly showed the necessity to use fly ash appropriately (i.e. in adequate quantity and appropriate medium) when using it in agricultural soils. Likewise, the addition of controlled amounts of fly ash to composts is a very common practice in agriculture to reduce pathogen content and to immobilize heavy metals.
However, there are many issues associated with the direct use of fly ash in compost (Fang et al. 1997). Composting is a biological process that decomposes micro-organisms and oxidizes organic matter. The high salinity and high pH condition generated by fly ash in compost may cause significant reductions in soil respiration and nitrogen content through ammonia volatilization, which in turn affects micro-organism growth during the composting process (Wong et al. 2009). On the other hand, the large amounts of heavy metals in fly ash may create issues by introducing toxic elements to crops, thereby causing health issues.
When subjected to carbonation, fly ash appears to possess more favourable properties, which help mitigate the adverse health and environmental effects currently associated with its final disposal and re-use. Carbonation of ash is achieved during carbon capture and storage, which is termed mineral sequestration (Huijgen and Comans 2003; Sanna et al. 2014). Coal combustion fly ash contains a variety of thermodynamically unstable oxides, hydroxides and silicates, which can react with CO2 to form carbonates (Sanna et al. 2012). However, the natural ambient reaction takes geological times to complete and is enhanced by accelerated carbonation in gaseous CO2 rich environments and under controlled reaction conditions (Huijgen et al. 2005; Nyambura et al. 2011; Sun et al. 2012; Ukwattage et al. 2013b). As discussed by Pathan (2003), the changes in physico-chemical properties during the carbonation reaction make the end-product of sequestration amendable for beneficial re-uses, such as in construction material and in agriculture. Therefore, mineral sequestration, while achieving carbon sequestration advantages, helps to manage the enormous quantities of solid waste coal fly ash generated each year, through its beneficial re-use as fly ash-based compost. Especially for countries whose agricultural soils need continuous amelioration and liming, such as Australia, fly ash may present an encouraging low-cost substitute to liming agents and costly compost amendments (Yunusa et al. 2011).
Most of the studies to date on the use of fly ash for compost have been primarily focussed on raw or weathered fly ash in combination with other soil ameliorants, in order to overcome the limitations of fly ash in compost. No research work has been reported to date on the carbonation of fly ash as a measure to improve its physico-chemical properties before its use. Hence, the aim of the present study was to investigate the suitability of coal fly ash in applications, including as a compost amendment for agricultural soils, after neutralization by mineral carbonation. The re-use capacity of fly ash was evaluated based on the changes observed in chemical properties (pH and EC) and the trace metal leachability of Victorian brown coal fly ashes. The paper provides a brief description of the properties of Victorian brown coal fly ash used in the present work, the accelerated carbonation and chemical properties testing procedure, the test results and a discussion of the results. Finally, the changes in the chemical properties are discussed in relation to the agricultural utilization of coal fly ash as an amendment to carbonaceous compost.
Materials and experimental methods
This section describes the different fly ashes used in the study, the accelerated carbonation procedure in brief, the testing of alkalinity and salinity and the column leaching test procedure.
Coal fly ashes
Three types of Australian coal fly ash samples (F1, F2, F3) from three power stations located in the Latrobe Valley Victoria were used for the present study. Victorian brown coal is the origin for all three fly ashes (Perera et al. 2011). The ash samples for testing were collected from the waiting ponds at the power plant sites. For the characterization of the fly ash, morphological analysis was carried out using a Nova Nano 450 scanning electron microscope equipped with a field emission gun. According to the images, the Latrobe Valley ash particles are generally irregular and range from sub-angular through to rounded, sometimes spherical. The age and degree of weathering of each ash type were judged using morphological indices such as colour, texture, composition and impurities. The mineralogy of the fly ashes was determined by X-ray diffraction spectroscopy using a Panalytical Empyrean diffractometer coupled with Cu Kα radiation at 45 kV and 40 mA. Chemical analysis was conducted using X-ray fluorescence. Before being used in carbonation, the bulk ash material was sieved with a 1.18 mm sieve to obtain uniform-sized particles. The samples were then oven-dried at 105 °C to a constant weight, to remove all the moisture in the samples.
Accelerated carbonation test procedure
The carbonation of fly ash samples was carried out inside a continuously stirred closed reactor under controlled reaction conditions. Figure 1 shows the front view of the reactor tank after setting up for the test. For each test run, a dried fly ash sample of 300 g mixed with 30% (w/w) deionized water was used. The sample was placed inside the reactor which was charged with gaseous CO2 to an initial pressure of 3 MPa. The inside temperature of 40 °C was maintained throughout the test. Continuous stirring was provided to accelerate the reaction speed. The drop of initial gas pressure was recorded as a measure of the carbonation reactions progression for a period of 48 h.
The reactor set-up used for fly ash carbonation (Ukwattage et al. 2014)
Testing of chemical properties and leachability of fly ash
From each type of fly ash, five replicates of carbonated and non-carbonated ashes were used for alkalinity (pH) and salinity (EC) tests. 1:1 solutions of fly ash to distilled water were used for the tests, according to the ASTM Standard D4972-01. The metal leachability of the fly ashes was measured by analysing the leachates collected from the column leaching tests. The leaching column used in the study is shown in Fig. 2.
The column was 56 mm in diameter and 300 mm in height and made with Perspex material. A 10-mm thick porous disc was fixed at the bottom of the column to hold the sample. The micro-pores of 5 μm diameter in this porous disc facilitated the slow migration of water through the sample. The column was filled with fly ash to a height of 150 mm without applying compaction. A distilled water column of 150 mm was maintained above the fly ash throughout the test. The leachate drops were accumulated into a collection unit. Three replicates of carbonated and non-carbonated for each type of fly ash were used in the column leaching tests. The collected leachate was analysed for metal composition using inductively coupled plasma optical emission spectrometry (ICP-OES) and inductively coupled plasma mass spectrometry (ICP-MS). The trace elements were analysed by ICP-MS owing to its lower detection limit, particularly for heavy metals.
Results and discussion
The pressure drop inside the tank reactor was plotted against time in order to confirm the carbonation of the fly ash samples before using them for chemical testing. A control test with an equivalent weight of water was carried out under the same test conditions and the drop of pressure over time was plotted for comparison. Figure 3 compares a set of tests conducted for F1 fly ash carbonation.
According to the results, in both tests, the initial pressure inside the reactor reduced gradually over time and came to a constant. In the control test, the pressure drop was caused by the dissolution of CO2 in water. When the water becomes saturated, further dissolution reduces and equilibrium is achieved. Therefore, the pressure drop is stabilized. In contrast, in the carbonation test, the pressure drop was caused by (1) dissolution of CO2 in water and (2) participation of CO2 in the carbonation reaction. Therefore, the difference between the two curves simply represents the drop of CO2 pressure caused by the carbonation of the fly ash sample. This method of carbonation estimation has been reported in a number of other research studies (Nyambura et al. 2011; Montes-Hernandez et al. 2009; Ukwattage et al. 2013b).
Effect of carbonation on pH of fly ash
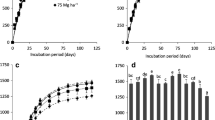
The results of the pH tests before and after carbonation are shown in Fig. 4.
According to the results, the Latrobe Valley fly ashes as received are extremely alkaline, with initial pH values of 11.3–11.95. This can be explained in relation to the chemical composition of the three ash samples. Table 1 shows the chemical composition of the three Latrobe Valley fly ashes used in the study.
The pH of an ash-water system is mainly controlled by the ratio of Ca and S concentrations in the fly ash (Querol et al. 2002) and other minor alkalis or alkaline earth cations such as Mg (Ward et al. 2009). Accordingly, the existence of significant levels of alkaline Ca/Mg oxides makes Latrobe Valley fly ashes extremely alkaline, which is detrimental for their re-use (Montes-Hernandez et al. 2009). The differences in the initial pH of the three ash types can be discussed in relation to their degree of weathering and the available alkaline oxide content. By the time of collection, the F1 fly ash sample had already been significantly weathered in the waiting ponds and hence it showed the lowest pH at the time of collection (Ukwattage et al. 2013b). Both F2 and F3 ashes were comparatively fresh and showed higher initial alkalinity than F1. After carbonation, the fly ash pH dropped significantly to average values of around 8.15, 8.1 and 7.85, representing percentage pH drops of 28.5, 32.3 and 33.8%, respectively for F1, F2 and F3 fly ashes. After carbonation, the drop of pH is mainly attributed to the conversion of free lime into stable carbonates (Pérez-López et al. 2008). The chemical and mineralogical changes in the aqueous fly ash CO2 system throughout the carbonation process can be evaluated to better understand the pH and EC changes. For this purpose, some researchers have analysed samples drawn at regular time intervals during the progression of carbonation (Druckenmiller and Maroto-Valer 2005; Back 2012).
As the research literature suggests, the pH of the reaction between CO2 and fly ashes in aqueous solutions is mainly driven by the rates of alkalinity-providing reactions and the rates of alkalinity-consuming reactions. For example, the dissolution of lime and periclase (MgO) is an alkalinity-providing step, whereas the precipitation of calcite is an alkalinity-consumption step. According to Back et al. (2008), three reaction phases can be identified within the entire carbonation process, with respect to two pH inflection points. The first phase (phase I) is characterized by high pH values accompanied by a strong increase of EC. These high pH values reflect the caustic alkalinity generated by the dissolution of lime, and the relevant reaction is shown in Eq. 1.
Because of the above lime dissolution reaction, a high Ca concentration occurs soon after the start of the test. In addition, the dissolution of gaseous CO2 into the aqueous phase to form HCO3 − and CO3 2− also occurs through the reaction path described in Eq. 2. The dissolution of CO2 in water is dependent on temperature, pressure, and brine salinity, and it reduces the pH of the system (Druckenmiller and Maroto-Valer 2005).
The pH determines which steps dominate in the above reaction sequence and accordingly the proportions of the carbonic species (Bond et al. 2002). At low pH (~4), the production of H2CO3 dominates, at mid pH (~6) HCO3 − production dominates, and at high pH (~9) CO3 2− dominates (Soong et al. 2002). Therefore, at a basic pH, the precipitation of carbonate minerals is favoured because of the availability of carbonate ions. Conversely, the dissolution of carbonates may increase as the solution becomes increasingly more acidic. After the formation of carbonate ions, they react with the metals to form carbonate minerals (Eq. 3). According to Stumm and Morgan (1996), in the initial phase, the carbonation reaction appeared to proceed quickly relative to the transfer of CO2 and therefore no build-up of total dissolved inorganic carbon (HCO3 − and CO3 2−) could be observed. With the onset of the carbonation reaction, a decrease of Ca in solution accompanied by a drop of EC occurred.
With the rapid calcite precipitation, a continuous drop in pH occurs, which is related to the direct transfer of Ca from the lime pool into calcite. While the reaction progresses, the depletion of the lime pool decreases the further dissolution rate of lime to values significantly lower than the carbonation rate. This leads to a significant shift of the pH regime into the carbonate buffer system. Phase II is dominated by the carbonation reaction, which consumes alkalinity and drives the pH towards lower values. However, at the end of phase II, the onset of HCO3 − formation indicates a deceleration of the carbonation reaction, which is due to exhaustion of the lime pool. The pH of the solution is now buffered by the equilibrium between dissolved CO3 2− and HCO3 −, so that the pH rapidly decreases to values below the equivalence point of the buffering system. CO2 uptake in phase III is to a significant extent controlled by the formation of dissolved inorganic carbon. Periclase starts to dissolve only at the beginning of phase III (since the dissolution rate of MgO is low at high pH of phase I and phase II) which helps to neutralize CO2 mainly as dissolved Mg bicarbonate. Therefore, the pH remains nearly unchanged during this phase. Moreover, Santos et al. (2013) state that the formation of a carbonate layer blocks the access of inner un-reacted alkaline minerals to the solution, which may also contribute to a gradual pH reduction as a function of carbonation progression.
As an overall effect, a pH drop can be observed in alkaline materials after carbonation. Previous researchers have reported significant reductions in alkalinity in materials subjected to carbonation. According to Hjelmar and van der Sloot (2011), carbonation typically reduces the pH of municipal solid waste incineration (MSWI) bottom ash leachate from 11 to 12 to a level of 7 to 8. Similarly, Li et al. (2007) and Fernandez Bertos et al. (2004) observed the lowering of the pH of fresh MSWI fly ash from 12–12.5 to 7–10 and from 12 to below 8 (nearly neutral), respectively. In the present study, the ash was collected from waiting ponds at power plant sites. Hence, the initial pH values were slightly lower than the pH of fresh Latrobe Valley fly ashes, which was in the range of 11.5–12, as observed by Mudd (2000). This is a result of natural ageing or weathering over time. According to Li et al. (2007), fresh fly ash showed a greater drop of pH with its higher reactivity compared to the slight drop observed in aged ash. Therefore, to a large extent, the pH drop of ash is related to the reactivity of carbonation and the amount of CO2 sequestrated.
Natural weathering can also bring about similar pH changes to the accelerated carbonation reactions of solid waste materials over time. For instance, Elseewi et al. (1980) observed an initial increase of Ca content and pH of agricultural soil with the addition of fresh fly ash, which was attributed to the dissolution of Ca2+ and OH− ions from the fly ash. However, over seasons, the enrichment of these ions in the acid soil eventually led to CaCO3 precipitation and the resultant pH was similar to that of a calcite system. According to Hjelmar and van der Sloot (2011), after dumping into landfills, fly ash can be subjected to mineral carbonation over time through contact with rainwater and atmospheric CO2. Due to preferential flow, the carbonation-associated pH lowering may be surprisingly rapid. This may negatively affect the leaching of some contaminants (Pb and Zn) which are soluble and hence released at high pH values. However, even after several years, if the landfill is disturbed and the particles are crushed, the alkalinity can emerge again since the ash particles may still have a strong alkaline core which may be exposed. According to above results, the use of fly ash after the carbonation reactions in compost amendment should be a better method of using it for crop cultivation, since extreme pH may harm the micro-organisms in soil.
Effect of carbonation on soluble salt concentration of fly ash
The changes in the total soluble salts of fly ash solution before and after carbonation were evaluated using EC measurements, and the results are shown in Fig. 5.
Before carbonation, the soluble salt content of the fly ashes was in the range of 6–7 dS/m, which represents the moderately saline class, based on soil salinity classifications (Richards 1954). According to mineralogical analysis, this high EC in leachate can be directly attributed to the presence of a large proportion of minerals in soluble form (e.g. SO4 2−, Cl−, CaO, MgO) in the ash (Back et al. 2008; Rai and Ainsworth 1987). Previous researchers have observed even higher salinity of 18–19 dS/m for Victorian brown coal fly ashes (Yunusa et al. 2011). However, weathering and leaching over time in ash ponds has made present fly ashes less saline than fresh precipitator ash. After the carbonation reaction, the soluble salt content reduced on average by 39, 46 and 47% for F1, F2 and F3 fly ashes, respectively. Accordingly, the final ECs of the carbonated fly ashes were between 3.2 and 3.8 dS/m. The reduction of the soluble salt content of fly ashes after carbonation mainly occurs due to the conversion of oxides and/or hydroxides into carbonates. This conversion changes most of the soluble forms of metals into insoluble or less soluble forms, which can result in reduced levels of salinity in fly ash solutions. Rai and Ainsworth (1987), on the basis of thermodynamic evaluations, suggested that in raw fly ash samples, the dissolved Ca concentrations are controlled by CaO and/or Ca(OH)2, whereas in CO2 reacted samples the concentration would be controlled by calcite (CaCO3). Moreover, most of the other metals, such as Ba, Cd and Pb, are probably present in the form of oxides and/or hydroxides [e.g. Ba(OH)2, CdO, Pb(OH)2] in raw fly ash soon after the combustion process. During carbonation, these oxides and/or hydroxides are converted to carbonates (e.g. BaCO3, CdCO3, PbCO3) which are more insoluble than oxides and/or hydroxides (Fernandez Bertos et al. 2004; Rai and Ainsworth 1987). In addition, the concentrations of the key trace elements B, Mo and Se are generally tied to the total amounts of soluble salts and hence to the salinity of fresh fly ash (Yunusa et al. 2011). The physical retention of these metals following carbonation also leads to the reduced salinity of carbonated fly ash, which is discussed in detail in relation to metal leachability in the following section.
Effect of carbonation on heavy metal leachability of fly ash
The leachability of trace metals was analysed in carbonated and non-carbonated fly ashes using the column leaching tests and the results of the leachate analysis are shown in Fig. 6.
The Latrobe Valley fly ashes contain significant quantities of B, Cu, Zn and Se as trace metals in the ash matrices. Taking into consideration the availability of trace metals (especially B), their extreme alkalinity and high level of salinity, Victorian brown coal ashes are the least suitable Australian fly ashes for soil application (Yunusa et al. 2011). However, as the results of the leachate analysis stand, the concentration of trace elements in the leachate was reduced after carbonation in all three types of brown coal ashes. In particular, the reductions in leachable B in the carbonated ash samples are remarkable. In F1, F2 and F3 ashes, respectively, 33, 36 and 48% of B removal was observed after carbonation. In fresh fly, ash B may occur in several modes which directly influence its mobility. Nevertheless, earlier studies have shown that a large fraction of B in fly ash is in soluble forms that can cause toxicity problems in soils and plants when used in agriculture (Cox et al. 1978; Manoharan et al. 2010). The significant reduction of B removal from the leachate can be explained by the pH changes following carbonation. The pH of the fresh fly ash as received was in the range of 11–12, which is extremely alkaline. After carbonation, the ash pH dropped to around 7.5–8.5, within which the solubility of B has been found to be the lowest. For example, Tabelin et al. (2014) observed the minimum leachability of B around the circumneutral pH range due to its strong adsorption onto minerals like Al-/Fe-oxyhydroxides and clays. Under acidic pH, the dissolution of mineral phases, which incorporated and/or adsorbed the element, creates increased B release into the solution, while under strongly alkaline conditions the enhanced desorption causes increased leachability (Tabelin et al. 2014). In addition, the results showed that CO2 treatment was effective in significantly reducing the concentrations of contaminants such as Zn, Pb, As, Cu and Se in the leachate. As mentioned above, the effect of pH change following carbonation appeared to be the dominant mechanism for a number of heavy metals when considering the possible leachability reduction mechanisms (Wang et al. 2010). At the alkaline pH of fresh fly ash, the leaching concentration of heavy metals, especially Pb, Zn and Cu, is very high. The final ash pH achieved after carbonation coincides with minimum solubility pH of a number of heavy metals. For instance, Cu, Pb and Zn show their minimum solubilities at a pH range of 7–9 in their V-shaped leaching versus pH curves. Accordingly, the metals are soluble at low and high pH values and are relatively insoluble at neutral to slightly alkaline pH values. The leachability of Ni is also known to reduce after carbonation due to pH reduction (Santos et al. 2013; Wang et al. 2010). However, in the present study, Ni leaching in both fresh and carbonated ash was minute, making it difficult to use the varying pH results to confirm the mechanism. Hg leaching reduced due to the basicity reduction after carbonation. According to Zhang et al. (2008), Hg is relatively stable under weak acidic conditions (pH up to 6) and starts releasing when the acidity increases. In addition, the decrease of pH may cause the co-precipitation of certain metals as carbonates, which can lead to the reduced leachability of those elements (Wang et al. 2010). Metals such as Pb, Cd, Cu and Zn have a strong affinity with calcite and they are reduced by sorption onto CaCO3, leading to co-precipitation (Freyssinet et al. 2002).
Another mechanism is the absorption of trace metals into other forms of metal compounds which causes significant immobilization of those elements following carbonation. For example, the reduction in leachable As and Se is attributable to increased sorption by iron oxides at reduced pH (Theis and Wirth 1977). Moreover, a geochemical study suggested that carbonation can play a role in the solubility control of As and Se through co-precipitation into pure calcite (Román-Ross et al. 2006). Change in speciation and formation of calcium arsenate complexes in the aqueous phase was observed by Yokoyama et al. (2012). Physical retention following carbonation is another reason for the reduction of heavy metal leaching (Wang et al. 2010). The carbonation reaction starts at the surface of the granular mineral waste particles and slowly moves inwards (Hjelmar and van der Sloot 2011). With time, the formation of an encapsulating carbonate layer, although detrimental to the progression of the carbonation reaction, may play an important role in limiting heavy metal leaching by reducing exposure of the unreacted or unreactive phases in the particle core to the aqueous medium (Santos et al. 2013). In addition, it is well established that by decreasing the soluble concentration of a contaminant, the mobility of that contaminant can also be reduced (Reddy et al. 1986; Schramke 1992).
As discussed thus far, the carbonation reaction exerts numerous favourable effects on the reduction of the leachability of a range of trace metals. Nevertheless, as the results of the study stand, the release of V and Sb from coal fly ash is enhanced after carbonation. This is caused by the reduction of basicity after carbonation, since V is known to be more prone to solubilization with the lowering of pH (Reddy et al. 1986; van Zomeren et al. 2011). Similarly, the leaching of the oxyanion Cr also shows a slight increase after carbonation. According to Rai and Ainsworth (1987), both Cr and V leaching is proportional to the basicity reduction in the ash-water system. Leaching of Cr decreases with carbonate formation, whereas the decrease in pH increases leaching more substantially (Cornelis et al. 2008; van Gerven et al. 2005). Fernandez Bertos et al. (2004) found that there is the possibility of Cr associating with calcium silicate hydrate (CSH) as a silicon substitute at higher pH values, and this mineral phase can undergo solubilization at lower pH, re-releasing Cr. However, as observed by Santos et al. (2013), Cr leaching only becomes significant below pH 5 and therefore does not cause significant problems under the ultimate ash pH achieved in the present study. At the same time, Van Gerven et al. (2005) showed that Cr-containing untreated bottom ashes brought to the same lower pH as carbonated ashes by acidification show much higher Cr leaching, meaning that Cr speciation in carbonated samples at least offers a buffer that hinders drastic solubilization. Molybdenum leaching remained constant or slightly increased after carbonation due to pH reduction. Nonetheless, all the increased elemental concentration of the leachate after carbonation still appeared to remain below regulatory defined limits for the safe disposal of coal fly ash.
In addition to the above analytical results, scanning electron microscopic (SEM) imaging and EDX analysis were conducted for the fly ash samples before and after carbonation and after leaching, to determine the morphological changes which occurred in the ash. Figure 7 shows the resultant SEM images.
In the SEM images, the non-carbonated ash sample shows the spherical shape of the ash particles with some irregularities. In the carbonated fly ash sample, the formation of calcite crystals is visible as hexagonal rod-shaped objects. It is evident that there has been a transformation of the dense particles of raw fly ash into particles that are more porous, after leaching.
Effect of carbonation on final disposal and reuse of fly ash
As discussed in the above sections, accelerated carbonation is shown to have a significant potential to improve the chemical stability and the leaching behaviour of potentially hazardous solid waste residues. The reactions involving Ca and CO2 are expected to mainly control the pH, solubility and mobility of inorganic contaminants in fly ash (Schramke 1992). In terms of the final disposal of fly ash, the changes brought about through carbonation are of great importance. When considering the use of fly ash as an amendment for composting, the reduction of the fly ash pH to values corresponding to the minimum solubility ranges of a number of heavy metals helps to reduce potential soil and crop contamination following application. When considering the land-filling of fly ash, the lowering of the fly ash pH to values corresponding to the minimum solubility of many heavy metals helps to reduce potential soil and groundwater contamination following disposal. Moreover, at pH < 9.5, the reduced leachability of trace elements can be sometimes expected up to within regulatory defined limits. (Wong et al. 2009). In addition, the decrease in free oxides and hydroxides and the formation of calcites during accelerated carbonation can improve the acid neutralization capacity (ANC) or the pH buffering capacity of the ash, which enhances its capacity for use in reclaiming problem soils (Chimenos et al. 2000). Fresh fly ash is characterized by poor pH buffering capacity and therefore when applied to soil the resulting pH sometimes becomes closer to the pH of the soil rather than the pH of the fly ash (Yunusa et al. 2011). However, after carbonation, the ANC of fly ash appeared to be improved. For instance, under acidic conditions, the ANC of bottom ash increased from 0.46–0.48 to 0.88 meq/g (at pH 5) after 48 h of accelerated carbonation (Sharma and Kalra 2006). Similarly, according to Cappai et al. (2011), MSWI fly ash showed a significant buffering capacity between neutral and alkaline values at pH around 7–8 after carbonation treatment.
In relation to the agricultural utilization of coal fly ash, Yunusa et al. (2009) identified extreme alkalinity, salinity and elemental toxicity as the most important environmental considerations. In Australia, almost half of the 100 million ha of agricultural soils are acidic with a pH below 5.5 and as much as 11 million ha of land is identified as extremely acidic (pH < 4.5; Yunusa et al. 2006). Therefore, gypsum is routinely applied as a liming agent to correct the problematic pH of the soil. Furthermore, fly ash is a perfect amendment for sewage sludge compost, in which lime is normally used to raise the pH, remove pathogens and reduce the heavy metals. Coal fly ash has significant amounts of CaO and therefore it can be replaced by lime, which reduces the heavy metals through adsorption and precipitation through its high pH, also considerably reducing the cost (Basu et al. 2009). As shown by Ukwattage et al. (2013a), Australian coal fly ashes, especially alkaline coal ashes, have the potential to substitute this costly soil and compost amendment when used at appropriate application rates. Depending on the soil salinity level and the fly ash EC, it is advisable that the EC of the fly ash not exceed 1.5 dS/m, and the salt concentration should be <960 mg/L (Yunusa et al. 2011). However, according to the results of the current study, even after carbonation, the salinity of Victorian brown coal fly ash did not achieve this low level, which is desirable for application to most crops. Nevertheless, the EC of carbonated ash ranged between 3.2 and 3.8 dS/m, which is below the prohibited level of fly ash salinity of 4 dS/m for agricultural application, as regulated by the Protection of the Environmental Operations Act 2005 prepared by the Environment Protection Authority and the Department of Environment and Climate Change, New South Wales, Australia (PEOR 2005). Hence, carbonated fly ash can be applied to crop lands of moderately salt-tolerant crops such as oats, sorghum, wheat, canola, safflower, soybean, and sunflower, which can be grown in 4 dS/m salinity (Richards 1954). In this regard, the use of this carbonated fly ash as an amendment to carbonaceous compost would be a better option, as it will assist in diluting the toxic effects of fly ash, further reducing the salinity level through the chemical reactions initiated between the compost and the fly ash (Menon et al. 1993).
Based on the trace metal concentrations in Australian fly ashes, elemental toxicity does not cause any serious issues, except for the concentration of B. Rather, Victorian brown coal fly ash is a good source of a range of essential nutrients for soil fertility and plant growth, including Ca, Mg, S, K and N. Other elements of major concern (such as As, Cd, Hg, Mo, Pb and Se) and of moderate concern (such as Cr, Cu, Ni, V, Zn and F), in terms of environmental risk are present in comparatively low concentrations in Australian fly ashes (Yunusa et al. 2006). However, it should be noted that there is only a small difference between beneficial and toxic levels of trace elements. Therefore, full chemical characterization of the ash should be repeated every three years if the fly ash is used for agricultural applications, even as compost amendment (PEOR 2005). In addition, special care should be taken with the B concentration of fly ash, since it has been reported to be phytotoxic to a number of crops planted under fly ash amendment. For instance, Manoharan et al. (2010) observed B phytotoxicity in canola plants grown at fly ash dosages above 39 Mg/ha. However, certain soils, such as those with high clay content, can absorb most of the fly ash-derived B, making it less available to plants (Goldberg 1997). Moreover, according to the guidelines of the Protection of the Environmental Operations Act 2005, almost all Australian fly ashes readily pass the 60 mg/kg of B concentration limit for land application. According to Yunusa et al. (2011), especially when used at prudent rates not more than 10 Mg/ha, Australian fly ashes do not have any negative impacts on the environment. Nevertheless, after carbonation, the significant reduction observed in B concentration of fly ash is highly important in terms of the establishment of acceptable soil application rates for Victorian brown coal fly ashes. As an overall effect, accelerated carbonation helps to fully or partly overcome the three major environmental concerns regarding fly ash application as a compost amendment.
However, it should be noted that soil characteristics vary greatly. Therefore, fly ash should only be used under specific conditions. In particular, the pH and salinity of the fly ash should be counter-checked with the above characteristics before its application to soil. For example, in Australia, almost half of the 100 million ha of agricultural soils are acidic with a pH below 5.5 (Yunusa et al. 2006). Therefore, fly ash with higher pH values may be suitable for such soils, but not suitable for other soils with relatively higher pH. Furthermore, soils with high clay content can absorb most of the fly ash-derived B, making it less available to plants (Goldberg 1997). Therefore, fly ash with high B concentration can be safely applied to such soils, which is certainly not the case for other soils, such as sandy soils. The interaction of fly ash with soil causes properties such as pH, salinity and heavy metal concentrations to vary over time, and it is therefore necessary to frequently check soil conditions upon the application of fly ash, even as a compost amendment.
Conclusion
The present study examined the effect of carbonation on the alkalinity, salinity and trace metal leachability of Victorian brown coal combustion fly ashes collected from the waiting ponds of three power plants in the Latrobe Valley, Australia. According to the results, the alkalinity of all three types of fly ashes significantly reduced from extremely alkaline pH values of 11–12 to slightly alkaline or neutral pH values of 7.5–8.5. This reduction of pH is solely attributable to the conversion of alkaline metal oxides (Ca/Mg) to metal carbonates during the carbonation reaction. Of the three tested fly ashes, the lowest percentage pH drop of 28% was recorded for the F1 fly ash, which had been initially weathered to a significant degree in the collection pond. With regard to the salinity (EC) of the fly ashes, all three types of ashes fall into the classification category of moderately saline, with the initial EC in the range of 6–7 dS/m. This dropped to values below 4 dS/m, which is the threshold EC for land application of Australian coal fly ash. However, even with the EC range achieved after carbonation (3.2–3.8 dS/m), the brown coal fly ashes can only be recommended for application to crops which can tolerate moderate to slight salinity. The leachability of a number of trace metals, including B, Cd, Cu, Pb, Hg, Zn, As and Se, reduced after carbonation, mainly due to the change of pH and mineral speciation during carbonation. The leachable concentration of Cr, Sb and V appeared to increase with the drop of alkalinity in fly ash after carbonation, but with slight to moderate increments which do not exceed the safe disposal limits. Considering these improvements, carbonated fly ash provides a better option than fresh fly ash as an amendment for compost. However, more research is needed on crop responses to carbonated fly ash to better understand its value as a compost amendment. As an overall conclusion, the mineral carbonation of coal fly ash not only serves to capture CO2, but is also beneficial in improving the geo-chemical properties of the material, thus helping to meet safe disposal or re-utilization requirements.
References
Aitken RL, Campbell DJ, Bell LC (1984) Properties of Australian fly ashes relevant to their agronomic utilization. Aust J Soil Res 22:443–453
Back M (2012) Mineral sequestration of CO2 by reaction with alkaline residues. Doctoral Dissertation, University of Bayreuth, Bayreuth
Back M, Kuehn M, Stanjek H, Peiffer S (2008) Reactivity of alkaline lignite fly ashes towards CO2 in water. Environ Sci Technol 42:4520–4526
Basu M, Pande M, Bhadoria PBS, Mahapatra SC (2009) Potential fly-ash utilization in agriculture: a global review. Prog Nat Sci 19(10):1173–1186
Bond GM, McPherson B, Stringer J, Wellman T, Abel A, Medina M (2002) Preprints of papers-American chemical society. Div Fuel Chem 47(1):39
Cappai G, Cara S, Muntoni A, Piredda M (2011) Application of accelerated carbonation on MSW combustion APC residues for metal immobilization and CO2 sequestration. J Hazard Mater 207–208:159–164
Chimenos JM, Fernández AI, Miralles L, Segarra M, Espiell F (2000) Short-term natural weathering of MSWI bottom ash. J Hazard Mater 79(3):287–299
Chou SFJ, Chou MIM, Stucki JW, Warnock D, Chemler JA, Pepple M (2005) Plant growth in sandy soil/compose mixture and commercial peat moss both amended with Illinois coal fly ash. World of Coal Ash (WOCA), Kentucky USA. http://www.flyash.info/2005/206cho.pdf
Cornelis G, Johnson CA, Gerven TV, Vandecasteele C (2008) Leaching mechanisms of oxyanionic metalloid and metal species in alkaline solid wastes: a review. Appl Geochem 23(5):955–976
Cox JA, Lundquist GL, Przyjazny A, Schmulbach CD (1978) Leaching of boron from coal ash. Environ Sci Technol 12(6):722–723
Druckenmiller ML, Maroto-Valer MM (2005) Carbon sequestration using brine of adjusted pH to form mineral carbonates. Fuel Process Technol 86:1599–1614
Elseewi AA, Straughan IR, Page AL (1980) Sequential cropping of fly ash-amended soils: effects on soil chemical properties and yield and elemental composition of plants. Sci Total Environ 15(3):247–259
Fang M, Wong JWC, Li GX, Wong MH (1997) Effect of coal ash residues on the microbiology of sewage sludge composting. In: Wise DL (ed) Global environmental biotechnology, proceedings of the third international symposium on the international society for environmental biotechnology, vol 66. Elsevier Publications, Boston, pp 511–523
Fernandez Bertos M, Li X, Simons JR, Hills CD, Carey PJ (2004) Investigation of accelerated carbonation for the stabilisation of MSW incinerator ashes and the sequestration of CO2. Green Chem 6(8):428–436
Freyssinet P, Piantone P, Azaroual M, Itard Y, Clozel-Leloup B, Guyonnet D, Baubron JC (2002) Chemical changes and leachate mass balance of municipal solid waste bottom ash submitted to weathering. Waste Manag 22(2):159–172
Ghodrati M, Sims JT, Vasilas BL, Hendricks SE (1995) Enhancing the benefits of fly ash as a soil amendment by pre-leaching. Soil Sci 159(4):244–252
Goldberg S (1997) Reactions of boron with soils. Plant Soil 193:35–48
Hjelmar O, van der Sloot HH (2011) Landfilling: mineral waste landfills. In: Christensen T (ed) Solid waste technology and management. Wiley, Chichester. doi:10.1002/9780470666883.48
Huijgen WJJ, Comans RNJ (2003) Carbon dioxide sequestration by mineral carbonation: literature review. Energy Research Centre of the Netherlands 31-60. http://www.ecn.nl/docs/library/report/2005/c05022.pdf
Huijgen WJJ, Witkamp GJ, Comans RNJ (2005) Mineral CO2 sequestration by steel slag carbonation. Environ Sci Technol 39(24):9676–9682
Hupenyu AM, Ernest D, Pearson NSM (2015) Fly ash composting to improve fertiliser value: a review. S Afr J Sci. doi:10.17159/sajs.2015/20140103
Jala S, Goyal D (2006) Fly ash as a soil ameliorant for improving crop production: a review. Bioresour Technol 97(9):1136–1147
Li X, Fernandez Bertos M, Hills CD, Carey PJ, Simon S (2007) Accelerated carbonation of municipal solid waste incineration fly ashes. Waste Manag 27(9):1200–1206
Manoharan V, Yunusa IAM, Loganathan P, Lawrie R, Murray BR, Skilbeck CG, Eamus D (2010) Boron content and solubility in Australian fly ashes and its uptake by canola (Brassica napus L.) from the ash amended soils. Aust J Soil Res 48:480–487
Menon MP, Sajwan KS, Ghuman GS, James J, Chandra K (1993) Fly ash-amended compost as a manure for agricultural crops. J Environ Sci Health A 28(9):2167–2182
Montes-Hernandez G, Pérez-López R, Renard F, Nieto JM, Charlet L (2009) Mineral sequestration of CO2 by aqueous carbonation of coal combustion fly-ash. J Hazard Mater 161(2–3):1347–1354
Mudd G (2000) Solute transport modelling of Latrobe Valley ash disposal sites. PhD Thesis, Faculty of Engineering and Science, Victoria University, Melbourne, p 417
Nyambura MG, Mugera WG, Felicia PL, Gathura NP (2011) Carbonation of brine impacted fractionated coal fly ash: implications for CO2 sequestration. J Environ Manag 92:655–664
Page AL, Elsiwee AA (1979) Physical and chemical properties of fly ash from coal fired power plants with reference to environmental impacts. Residue Rev 71:83–120
Palumbo AV, Tarver JR, Fagan LA, McNeilly MS, Ruther R, Amonette JE (2005) Potential for metal leaching and toxicity from fly ash applied for increasing carbon sequestration in soil. World of Coal Fly Ash, Lexington. http://www.worldofcoalash.org/2005/ashpdf/185pal.pdf. Retrieved on 2 Feb 2014
Pandey VC, Singh N (2010) Impact of fly ash incorporation in soil systems. Agric Ecosyst Environ 136(1–2):16–27
Pathan S (2003) Fly ash amendment of sandy soils to improve water and nutrient use in Turf. Thesis in Agriculture and Plant Sciences, University of Western Australia
PEOR Protection of the Environment Operations (Waste) Regulations (2005) General excemption under Part 6. http://www.environment.nsw.gov.au/resources/waste/residue/flyashnov2006.pdf. Retrived on 10th Nov 2013
Perera M, Ranjith PG, Choi SK, Bouazza A, Kodikara J, Airey D (2011) A review of coal properties pertinent to carbon dioxide sequestration in coal seams: with special reference to Victorian brown coals. Environ Earth Sci 64(1):223–235
Pérez-López R, Montes-Hernandez G, Nieto JM, Renard F, Charlet L (2008) Carbonation of alkaline paper mill waste to reduce CO2 greenhouse gas emissions into the atmosphere. Appl Geochem 23(8):2292–2300
Querol X, Moreno N, Umaña JC, Juan R, Hernández S, Fernandez-Pereira C, Ayora C, Janssen M, García-Martínez J, Linares-Solano A, Cazorla-Amoros D (2002) Application of zeolitic material synthesised from fly ash to the decontamination of waste water and flue gas. J Chem Technol Biotechnol 77(3):292–298
Rai D, Ainsworth CC (1987) Inorganic and organic constituents in fossil fuel combustion residues. Interim report, Electric Power Research Institute, Pacific Northwest Laboratory
Reddy KJ, Lindsay WL, Boyle FW, Redente EF (1986) Solubility relationship and mineral transformations associated with recarbonation of retorted oil shales. J Environ Qual 15:129–133
Richards LA (1954) Diagnosis and improvement of saline and alkali soils. USDS Agricultural Handbook, Department of Agriculture
Riehl A, Elsass F, Duplay J, Huber F, Trautmann M (2010) Changes in soil properties in a fluvisol (calcaric) amended with coal fly ash. Geoderma 155(1–2):67–74
Román-Ross G, Cuello GJ, Turrillas X, Fernández-Martínez A, Charlet L (2006) Arsenite sorption and co-precipitation with calcite. Chem Geol 233(3–4):328–336
Sanna A, Dri M, Hall MR, Maroto-Valer MM (2012) Waste materials for carbon capture and storage by mineralisation (CCSM): a UK perspective. Appl Energy 99:545–554
Sanna A, Uibu M, Caramanna G, Kuusik R, Maroto-Valerac MM (2014) A review of mineral carbonation technologies to sequester CO2. Chem Soc Rev 43:8049–8080
Santos RM, Van Bouwel J, Vandevelde E, Mertens G, Elsen J, Van Gerven T (2013) Accelerated mineral carbonation of stainless steel slags for CO2 storage and waste valorization: effect of process parameters on geochemical properties. Int J Greenh Gas Control 17:32–45
Schramke JA (1992) Neutralization of alkaline coal fly ash leachates by CO2. Appl Geochem 7(5):481–492
Sharma S (1989) Fly ash dynamics in soil water systems. Crit Rev Environ Control 19(3):251–275
Sharma SK, Kalra N (2006) Effect of fly ash incorporation on soil properties and productivity of crops: a review. J Sci Ind Res 65:383–390
Sikka R, Kansal BD (1994) Characterization of thermal power-plant fly ash for agronomic purposes and identify pollution hazards. Bioresour Technol 50:269–273
Soong Y, Jones R, Hedges SW, Harrison DK, Knoer JP, Baltrus JP, Thompson RL (2002) Preprints of papers-American chemical society. Div Fuel Chem 47(1):43
Stumm W, Morgan JJ (1996) Aquatic chemistry: chemical equilibria and rates in natural waters, 3rd edn. Wiley, New York
Sun Y, Parikh V, Zhang L (2012) Sequestration of carbon dioxide by indirect mineralization using Victorian brown coal fly ash. J Hazard Mater 209–210:458–466
Tabelin CB, Hashimoto A, Igarashi T, Yoneda T (2014) Leaching of boron, arsenic and selenium from sedimentary rocks: II. pH dependence, speciation and mechanisms of release. Sci Total Environ 473–474:244–253
Theis TL, Wirth JL (1977) Sorptive behavior of trace metals on fly ash in aqueous systems. Environ Sci Technol 11(12):1096–1100
Ukwattage NL, Ranjith PG, Bouazza M (2013a) The use of coal combustion fly ash as a soil amendment in agricultural lands (with comments on its potential to improve food security and sequester carbon). Fuel 109:400–408
Ukwattage NL, Ranjith PG, Wang SH (2013b) Investigation of the potential of coal combustion fly ash for mineral sequestration of CO2 by accelerated carbonation. Energy 52:230–236
Ukwattage NL, Ranjith PG, Yellishetty M, Bui HH, Xu T (2014) A laboratory-scale study of the aqueous mineral carbonation of coal fly ash for CO2 sequestration. J Clean Prod 103:665–674
van Gerven T, Van Keer E, Arickx S, Jaspers M, Wauters G, Vandecasteele C (2005) Carbonation of MSWI bottom ash to decrease heavy metal leaching, in view of recycling. Waste Manag 25:291–300
van Zomeren A, van der Laan SR, Kobesen HB, Huijgen WJ, Comans RN (2011) Changes in mineralogical and leaching properties of converter steel slag resulting from accelerated carbonation at low CO2 pressure. Waste Manag 31(11):2236–2244
Wang L, Jin Y, Nie Y (2010) Investigation of accelerated and natural carbonation of MSWI fly ash with a high content of Ca. J Hazard Mater 174(1–3):334–343
Ward CR, French D, Jankowski J, Dubikova M, Li Z, Riley KW (2009) Element mobility from fresh and long-stored acidic fly ashes associated with an Australian power station. Int J Coal Geol 80(3–4):224–236
Wong JWC, Fung SO, Selvam A (2009) Coal fly ash and lime addition enhances the rate and efficiency of decomposition of food waste during composting. Bioresour Technol 100(13):3324–3331
Yokoyama Y, Tanaka K, Takahashi Y (2012) Differences in the immobilization of arsenite and arsenate by calcite. Geochim Cosmochim Acta 91:202–219
Yunusa IAM, Eamus D, De Silva DL, Murray BR, Burchett MD, Skilbeck GC, Heidrich C (2006) Fly-ash: an exploitable resource for management of Australian agricultural soils. Fuel 85(16):2337–2344
Yunusa IAM, Manoharan V, De Silva DL, Eamus D, Murray BR (2008) Growth and elemental accumulation by canola on soil amended with coal fly ash. J Environ Qual 37(3):1263–1270
Yunusa IA, Burchett MD, Manoharan V, De Silva DL, Eamus D, Skillbeck CG (2009) Photosynthetic pigment concentrations, gas exchange and vegetative growth for selected monocots and dicots treated with two contrasting coal fly ashes. J Environ Qual 38(4):1466–1472
Yunusa IAM, Loganathan P, Nissanka SP, Manoharan V, Burchett MD, Skilbeck CG, Eamus D (2011) Application of coal fly ash in agriculture: a strategic perspective. Crit Rev Environ Sci Technol 42(6):559–600
Zhang H, He PJ, Shao LM, Lee DJ (2008) Temporary stabilization of air pollution control residues using carbonation. Waste Manag 28(3):509–517
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ukwattage, N.L., Ranjith, P.G. & Perera, M.S.A. Effect of accelerated carbonation on the chemical properties and leaching behaviour of Australian coal fly ash, to improve its use as a compost amendment. Environ Earth Sci 75, 1398 (2016). https://doi.org/10.1007/s12665-016-6188-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-016-6188-y