Abstract
Coal-fired power generation and agriculture account for more than half of global greenhouse gas emissions, but the coal fly ash (CFA) produced in the former can be a resource for reducing emissions from agriculture to minimise environmental footprints in both industries. Our aim in this study was to test how acidic and alkaline CFA addition could minimise loss of C and N from acidic soil, with or without added manure. We determined composition and structural characteristics of acidic and alkaline CFA for their capacity to adsorb organic carbon, but observed poor adsorption because of low concentrations of cenospheres and unburnt carbon as the primary absorbents in the ash. Addition of CFA had no impact on the loss of carbon or nitrogen from unmanured soil in which concentrations of these nutrients were low. Loss of carbon from manured soil was reduced by 36 % with alkaline ashes and by 3-fold with acidic ashes; while loss of N was 30–50 % lower with acidic ashes, but 28 % higher with alkaline ashes, compared with no ash treatment. The increases in C sparing with CFA addition were achieved not by direct C absorption but by restraining microbial population and respiration, and potentially emissions. Alkaline CFA increased soil pH and if used to substitute just 10 % of lime for ameliorating soil acidity would reduce CO2 emission associated with the mining of the lime and its eventual dissolution in soil by ~ 2.66 Tg or 2.8 % of Australia’s annual agricultural emissions. High concentrations of oxides of phosphorus, silicon, titanium and clay particles in acidic ashes, and oxides of cations in alkaline ashes, were associated with potential for promoting C storage and acidity amelioration in soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Power generation and agriculture both generate substantial environmental footprints that pose significant risk to the long-term viability of these two industrial sectors. Greenhouse gas (GHG) emission is a major component of the environmental footprint for which the two industries account for ~80 % and ~7 %, respectively, of the total global GHG emissions in 2011 (UNFCCC 2014). The majority of the emission in the energy sector arises from burning of fossil fuel. Coal contributes about 40 % of total power generation (IAEA 2012) but accounts for 56 % of the emission from the sector (ASN Bank/Ecofys 2013). A substantial part of the emissions from the agriculture sector emanate from soils which are responsible for up to 54 % of agricultural emissions in the USA, 18 % in Australia, 25 % in China, and 34 % in Brazil (UNFCCC 2014). Most of this is associated with the use of fertilisers and soil amendments (Garnaut 2011).
Between 5 and 15 % of coal fed into boiler remains unburnt forming solid residues that are collectively termed Coal Combustion Products (CCPs). The majority of these residue (>85 %) comprises of fine particles (<0.2 mm) referred to as coal fly ash (Heidrich et al. 2013) of which only about half of the 780 Mg global production is effectively used. The unused CFA stockpiles grow by 370 Mg annually, and along with gaseous emissions, has to be sustainably managed as part of the Clean Coal Technology (Seshadri et al. 2013). Potential use of coal fly ash (CFA) to ameliorate soil constraints on agricultural productivity, along with potential risks, has been extensively reviewed (Seshadri et al. 2013; Shaheen et al. 2014; Yunusa et al. 2012). Protocols for logistical, economic, monitoring and regulatory imperatives for routine agricultural application of CFA have been proposed (Yunusa et al. 2012). However, with the exception of a recent study (Masto et al. 2014), little attention has been given to potential application of CCPs for mitigating GHG emissions from agriculture through promotion of C and N storage in soil proposed earlier (Amonette et al. 2004; Palumbo et al. 2004).
Furthermore, the C in limestone and dolomite used to ameliorate soil acidity on farms is inevitably released as CO2 to the atmosphere (IPCC 2006). This emission accounts for about 9 Tg CO2 yearly in the United States of America (USEPA 2013). Liming contributes up to 7 % of total greenhouse gas (GHG) emission from a typical wheat farm in Australia (Brock et al. 2012) and would account for about 30 % of the total agricultural emissions by 2015 in Germany (Benndorf 2013). These farm emissions could be reduced by substituting liming products with CFA that contains non-oxidisable C and which reportedly increased pH of treated acidic soils by up to 2 units (Manoharan et al. 2010; Yunusa et al. 2013). Soil N is transformed into potentially volatile and/or soluble forms and easily lost from the soil during mineralisation of organic matter, and should be considered in strategies for abating farm emissions.
The CFA rich in lime CaO (≥3.0 %) can directly adsorb CO2 on to their particles through carbonation. Up to 7 % w/w of CO2 was absorbed through this process under natural conditions over a 20- year period, but the process is enhanced at high temperatures and pressure (60–90 oC; ~4.0 MPa) in the laboratory (Muriithi et al. 2013). Particulate organic C can also be absorbed onto ash particles, the unburnt C and/or cenospheres; the latter are ash particles that burst under high temperatures forming hollow spheres with large surface areas (Amonette et al. 2004; Palumbo et al. 2004). However, CFAs contain low amounts of cenospheres (<4% w/w) and unburnt C (Ngu et al. 2007) so that large amounts of ash would be required to achieve significant absorption of particulate C. Hence both carbonation and adsorption of organic C onto particles in CFA are therefore likely to have negligible short-term capacity for soil C sequestration.
Increased C storage in soil with CFA amendment is more likely to be indirect through alterations of processes and properties in the soil, such as aggregation, pH, salinity, and, where the ash contains significant amounts of unburnt C, the C/N (Palumbo et al. 2004; Amonette et al. 2004). Increases in pH due to ash addition reportedly enhanced humification in analytical organic monomers (Amonette et al. 2004), which along with reduced mineralisation stabilised peroxidase and tryrosinase activities and enhanced humification in ash-amended soil (Masto et al. 2014). Improved soil aggregation with ash amendment (Yunusa et al. 2011) should protect organic C from microbial decomposers (Beare et al 1994; Jastrow et al. 2007; Rabbi et al., 2013; Young and Ritz 2000).
In the current study, we determined the potential of acidic and alkaline CFA for enhancing storage of C and N in acidic soil with or without incorporation of sheep manure. Our objectives were to (1) quantify cenosphere and unburnt C contents of the CFA and their capacity to adsorb particulate organic C, (2) determine depletion of C and N from an acidic soil, (3) identify chemical properties that influence loss of C and N from ash-amended soil, and (4) identify knowledge-gaps in developing CFA technology for enhancing C and N storage in soils.
2 Materials and methods
Five CFAs were obtained from power stations in Western Australia, South Australia, Victoria, New South Wales and Queensland, in Australia. Three of the ashes were alkaline and the other two acidic; henceforth denoted as acidic1, acidic2, alkaline1, alkaline2 and alkaline3, and all five had low salinity except alkaline3 (Table 1) and used in the following experiments.
2.1 Ash characterisation and adsorption of organic carbon
Cenospheres were harvested by dispersing ash samples in distilled water in 1:20 (w/v) ash/water at room temperature, and then followed by a 24-hour sink-float separation. Chemical composition of the cenosphere was determined in a two-step procedure of fusion and analysis prescribed in the Australian Standard AS 1038.14.1–2003 and reported by Yip et al. (2010). A scanning electron microscope (SEM) was used to determine morphological characteristics of cenospheres, while the unburnt C was quantified using thermogravimetric analysis (TGA) following a procedure reported (Ngu et al. 2007).
Adsorption of organic carbon by the CFA cenospheres was determined in a batch adsorption reactor system involving shaking 0.35 g of cenosphere in 200 ml of synthetic water containing methylene blue (10 ppm). The dye concentration in the solutions was determined before and after adsorption using a Spectrophotometer (Spectronic 20 GenesysTM, Thermo Scientific, Melbourne, Australia) to measure absorbance at λmax of 665 nm for methylene blue (Wang et al. 2008).
2.2 Soil incubation experiment
An acidic sandy loam soil (Yellow Chromosol) equivalent to a Haplic Xerosol (FAO 1974) was collected from the UNE research farm, Armidale (30o 29′ 16″ S, 151o 38′ 29″ E); it belongs to the order Alfisol in the USDA classification (Soil Survey Staff 2012) and had been under lightly fertilized mixture of native pastures. It was amended with sheep manure and/or CFA for this study. The sheep manure was collected from a local shearing shed. The soil, manure and CFA were air dried, and the soil and manure were ground to pass a 2 mm sieve, and mixed in plastic containers to form the following factorial treatments:
-
i.
Ash amendment:85 % soil (153 g) +15 % ash (27 g) + 0% manure
-
ii.
Manure amendment: 85 % soil (153 g) + 15 % manure (27 g) + 0 % ash
-
iii.
Ash & manure amendment: 70 % soil (126 g) + 15 % ash (27 g) + 15 % manure (27 g)
-
iv.
Control: 100 % soil (180 g) + 0 % ash + 0 % manure
The soil or mixtures were blended uniformly and then weighed at 180 g into 0.5 L clear plastic containers (80 mm x 110 mm x 90 mm) with each treatment prepared in 4 replicates. The containers were arranged randomly on benches in a glasshouse maintained at 22 oC and humidity at ~50 %. The treatments were gradually wetted to bring their weights to predetermined field capacity and weight recorded. The treatments were covered with a black shade-cloth to minimise evaporation, and brought back to their prescribed weights with addition of water once a week for 3 months.
2.2.1 Measurements
Carbon and nitrogen content and their stable isotopes. After three months, half of the sample in each jar was taken by cutting cleanly vertically through the soil; the remaining half was stored in a fridge to be used for microbial analysis. The samples were air-dried and sieved to pass 2.0 mm screen, and used to determine salinity as electrical conductivity (EC) and pH using a portable bench-top pH-EC meter (LabCHEM Model 901-CP, TPS Pty Ltd, Australia) on filtrate of air dried sample mixed into deionised water (1:5). Both total C (TC) and total N (TN) were measured on 5 g samples in a high temperature furnace (LECO®, St Joseph, MI, USA), while δ13C was determined using a mass spectrometer (ANCA-GSL IRMS, Sercon Ltd., Cheshire, UK). The δ13C isotope composition of the samples was computed as (IAEA 2001).
in which R represents the mass ratio of 13C/12C for the sample or 0.0112372 for the standard. The δ13C for the mixtures at the start of incubation was calculated thus:
where ƒ represents fractions of C for either the soil, manure or ash in the mixture. The TC and TN lost during the incubation period were estimated from their quantities (Q) at the start (day 0) and after the 3-month incubation (Q3):
The quantities of TC and TN at the start of the incubation were estimated from the masses of soil, CFA and manure in the mixtures, and the respective concentrations of the two nutrients:
where m i are the masses of the component in the substrates, i, (soil, CFA and manure), and c i are their respective TC or TN concentrations; n is the number of components (1–3) in the mixture. The Q 3 values were obtained from the product of the mass of the incubation substrate and its measured TC or TN values.
Microbial biomass and respiration
These were measured on the stored half of the samples using microplate-based respiration technique (MicroResp™, Craigiebuckler, Aberdeen, UK) as per Campbell et al. (2003). Basal respiration rate (BSR) was monitored over three days after which 0.22 g of glucose was stirred into the soil and monitored for a further 24 hours to determined substrate-induced respiration and the dependent microbial biomass.
2.3 Data analyses
Data on cenosphere and unburnt carbon in the ashes were evaluated in a one-way analysis of variance. All data from the incubation experiment were subjected to two-way analysis of variance (ANOVA); statistical significance was determined when p ≤ 0.05. Two sets of principal component analyses (PCA) were undertaken to examine (i) interrelationships amongst TC and TN, EC, pH and microbial biomass and BSR at the completion of the incubation period, and (ii) whether TC and TN post-incubation could be associated with specific properties of the CFA. Both ANOVA and PCA were carried out using IBM SPSS Statistics, V22.0 (IBM Corp, Armonk, NY).
3 Results
3.1 Physico-chemical properties of the ashes and their cenosphere yields
The five ashes consisted mostly of silt- and sand-size particles, whose mineral composition was dominated by SiO2 and Al2O3 (Table 1). The SiO2 and Al2O3 accounted for >70 % in four ashes, but <20 % in alkaline3 in which Fe2O3 dominated (30 %) Other oxides, especially CaO, Na2O5, MgO, Mn3O4, SO3, were generally higher in alkaline3 than in the other ashes.
Only alkaline1 and alkaline2 yielded any cenospheres with an average of 0.5 % w/w. The cenospheres had smooth round and spherical shapes (Fig. 1) with ~30 - 150 μm diameter. Close-up views of individual cenospheres reveals macro-size (>10 μm) pores and finer spherical ash particles adhered onto the surface. The amount of unburnt C in the cenospheres was generally small, ~1.8 % w/w in alkaline1 and ~0.2 % in alkaline2.
3.2 Carbon adsorption onto cenospheres and unburnt carbon in ash
The adsorption of methylene blue onto the cenospheres of alkaline1 and alkaline2 showed a two-phase trend (Fig. 2a): an initial exponential phase in the first 10 mins, followed by a slow linear phase in the following 4 hours. On the whole, the adsorption capacity of cenosphere after 4 hours was 0.7 mg methylene blue per gram of cenospheres. Over the same duration, the amounts of methylene blue adsorbed by akaline2 ash directly (2.0 mg/g) or by its unburnt carbon (~21 mg/g) were larger than adsorption by its cenospheres alone (Fig. 2b).
3.3 Soil incubation
3.3.1 Total carbon and nitrogen concentrations in soil
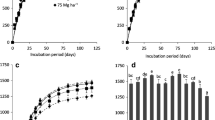
Concentrations of TC and TN at the start of the incubation were larger by factors of 4 and 9, respectively, in the manured soil compared with unmanured soil (Table 2). Addition of CFA to unmanured soil did not alter TC (Fig. 3a) or TN (Fig. 3b) after incubation; however, TC was higher in the manured soil supplied with ash compared with no ash. Post-incubation TN was higher with addition of the acidic CFAs than with the alkaline CFAs. The proportions of TC and TN lost during incubation from the manured soil were generally lower with CFA than without, and also differed with ash type (Table 2). The acidic ashes were more effective than the alkaline ashes in arresting losses of C (28 % vs 43 % or 2.65 g vs 4.37 g) and N (13 % vs 39% or 0.11 g vs 0.36 g). With exception of alkaline2 that reduced N loss only marginally, addition of alkaline1 or alkaline3 increased N loss by 28 and 36 %, or 0.37 g and 0.45 g, respectively, compared with 0.29 g when no ash was added. It was not possible to partition the losses in TC and TN amongst the three sources.
Impact of acidic or alkaline coal fly ash additions on (a) total carbon (TC), (b) total N (TN), (c) basal microbial respiration rate (BSR), and (d) microbial biomass in soil supplied with 0 or 15 % w/w of sheep manure after three-month incubation. The mean (± standard errors) values for 0 and 15 % manure additions are given
The amounts of TC measured in both manured and unmanured soils post-incubation as percentage of their pre-incubation concentrations averaged 57 %; whereas proportionally more TN was lost from the manured (30 %) compared with unmanured soil (2%). The losses of both nutrients from the soils during incubation were coupled:
3.3.2 Microbial respiration and biomass
The BSR in unmanured soil was not affected by addition of acidic CFA, but increased with alkaline CFA (Fig. 3c). However, BSR was at least halved in manured soil with addition of any of the five ashes. Microbial biomass in unmanured soil was unaffected with acidic2 and alkaline1, and significantly with alkaline3 (Fig. 3d). Addition of any of the ashes significantly reduced microbial biomass in the manured soil; acidic1 was the least detrimental to microbial biomass. Both BSR and microbial biomass were 4- and 5- times, respectively, larger in the manured soil compared with unmanured soil.
3.3.3 Basic soil chemistry after incubation
Changes in the soil chemical variables at the end of the incubation are presented in Table 3. With exception of acidic1 and alkaline3, the pH was lower with ash additions in manured soil than in the unmanured soil. The electrical conductivity (EC) in unmanured soil was increased only with addition of alkaline1 or alkaline3. Addition of any ash to the manured soil either had no impact on EC or reduced it, the exception being alkaline3 that increased it significantly. The mean pH was similar for manured and unmanured soil, while the manured soil was more saline, had more depleted δ13C, but higher C/N, than the unmanured soil.
Addition of alkaline3 enriched δ13C in unmanured soil, while addition of other ashes depleted δ13C in unmanured and manured soil (Table 3). Relative to starting conditions, CFA additions depleted δ13C in unmanured soil during incubation, the exception was alkaline3 that enriched δ13C. There was general δ13C enrichment in the manured soil during incubation due to CFA addition, the exception being alkaline2 that caused a slight depletion.
3.3.4 Associations between CFA properties and post incubation total C and TN
Principal component analysis (PCA) showed that most of the total variance (82 %) was accounted by the first two principal axes of variations (Fig. 4a). The first axis (62.8 %) had high negative loadings on TC, TN, microbial biomass and basal respiration, all of which were tightly coupled, and high positive loading on δ13C. There was also a clear separation between manured (suffix _15) and unmanured (suffix _0) soil along the first principal component (PC1) axis, showing manured soil as having high levels of TC, TN, microbial biomass and basal respiration, and depleted 13C. The PC2 (19.5 % of variation) was dominated by pH and EC of the incubation substrate. It showed an inverse correlation between TC, TN and microbial biomass, and also, with BSR and with pH. Four major clusters could be discerned consisting of (i) alkaline1 and alkaline2 with 0 % manure, (ii) acidic ashes with 0 % manure, (iii) alkaline1 and alkaline2 with 0 % manure, and (iv) alkaline3 with either manure treatment.
Biplots from principal component analysis showing vector loadings for relationships amongst (a) coal fly ash characteristics and TC (total C) and TN (total N) measured, and (b) measured soil variables, after 3 month incubation The ash treatments are expressed as either acidic (Acd1, Acd2) or alkaline (Alk1, Alk2, Alk3) applied to either unmaured (_0) or manured (_15) soil; other notations are BSR (basal respiration rate), MB (microbial biomass), and deltaC (δ13C)
3.3.5 Associations between TC, TN, microbial dynamics and chemical attributes
The PCA extracted 78 % of the total variance in the CFA composition and their correlations with post-incubation TC and TN levels (Fig. 4b). The PC1 accounted for 53 % of the total variance with high positive loadings on CaO, Mn3O4, MgO, Fe2O3, SO3, Na2O and associated EC, all of which had high negative correlations with TiO2, P2O5 and SiO2. The PC2 extracted a further 25 % of the total variance, with high positive loadings on pH and negative loadings on clay content, suggesting a strong inverse association between the two. Alkaline1 was the most benign of the ashes in terms of direct impact of its properties on TC and TN.
4 Discussion
4.1 Cenosphere and unburnt carbon yields and their potential for carbon adsorption
The ashes used here generally contained low amounts of cenospheres, and the average yield of 0.5% w/w by the two alkaline ashes was within the range (0.2–3.8%) for a number of CFA in Australia (Ngu et al. 2007). Ngu et al. (2007) reported cenosphere yields of 3.8% w/w for acidic2 contrary to zero yields in the current study. This could be due to variability in the physico-chemical properties of different batches of CFA arising from the operating conditions of the boilers (Raask 1985), the source coal and other factors, including decomposition of carbonates, fusion of silicates and evaporation of pore water (Ngu et al. 2007). For instance, while the 69 % w/w of silicates for acidic2 (Table 1) was comparable to the 65 % reported earlier, the aluminate content of 24 % in the current study was well below the 35 % reported for this ash previously (Ngu et al. 2007). Alkaline3 had low concentrations of silicates and aluminates, suggesting an inherent low potential for cenosphere yield. Similarly low cenosphere production would be expected from acidic1 given its low silicate content. However, both the cenospheres and the unburnt C were poor adsorbents of methylene blue (Fig. 2). The unburnt C had superior C adsorption because its porous structure and associated surface area were preserved, while these were destroyed during melting of the cenospheres (Wang et al. 2005). Thus adsorption by unburnt carbon could be up to 9-fold greater than by CFA particles (Wang et al. 2008), consistent with the findings here (Fig. 2b).
The bulk CFA as a whole therefore has low capacity for physical adsorption of organic carbon, with absorption being only 7–10 mg C/g for Australian CFAs (Wang et al. 2008). The current study, demonstrates that, even accounting for adsorption by the unburnt carbon, CFA is unlikely to have a major impact on soil carbon sequestration since the total C contents of these ashes are generally low (<5.5 % even in acidic1, Table 1). The capacity of unburnt carbon to absorb particulate organic carbon was ≤1 % w/w for the ashes used in this study and demonstrated the poor capacity for direct adsorption of organic carbon by CFA. Therefore, any real benefit for soil C sequestration due to ash addition would accrue only from changes induced in the physico-chemical properties of ash/soil admixtures.
4.2 Changes in total carbon and total nitrogen during incubation
Ash addition altered the properties of the media during incubation and explained the differences in the TC and TN observed afterwards. Addition of ash clearly reduced loss of TC, which along with TN and microbial variables, were strongly and inversely correlated with δ13C (Fig. 4a). This was because the manure was the dominant source of C in the mixture and its loss enriched δ13C towards the soil value of -18.69. The general δ13C enrichment during incubation of manured soil was smaller with ash addition that also increased the C/N, compared with no ash addition (Table 3). Maintenance of high C/N with ash addition would have restrained decomposition and subsequent loss of C (Drinkwater et al. 1998). Minimal loss of C (<40 %) and N (<31 %) were observed with addition of CFA that kept the incubate pH within 6.4–7.6 range (Table 2), well below pH of 9.4 for alkaline3 that lost the most C and N. This was consistent with positive association found between acidic pH conditions and enhanced retention of C and N in soil (Bååth and Anderson 2003; Conyers et al. 2012).
The links between TN, TC and microbial variables, and also pH and the EC, are revealed along the PC-2 axis (Fig. 4a). Both TC and TN were coupled with treatment cluster consisting of acidic ashes and alkaline1 and alkaline2 in the third quadrant of PCA. These clusters could also be partly due to salinity of the ashes, in addition to the pH, that stressed the microbial populations and impaired mineralisation of the organic matter (Rietz and Haynes 2003). The clustering of ashes reflected their chemical composition (Fig. 4b) such that alkaline1 and alkaline 2 were closely associated with K2O, while alkaline3 was associated with a suite of alkali metals (Na2O, MgO, CaO, Mn3O4 and Fe2O3). A third group consisting of the two acidic ashes was closely associated with P2O5 and SiO2, TiO2 and clay particles. These two groups of oxides can then be used for identifying ashes with potential for C conservation in soil.
Losses of the two nutrients were tightly coupled (eq. 4), although on average about 30 % TN was lost compared with 42 % of TC (Table 2) possibly due to mineralisation of N lagging behind that of C, with the former reportedly commencing only after the microbes become C-starved (Weintraub and Schimel 2003). Differences in the labile fractions of the two nutrients in the manure, similarity in C/N notwithstanding, could affect their mineralisation (Blair et al. 2005), because mineralisation of C is dependent more on pH, while that of N is more on C/N (Tian et al. 2013).
The reductions of 40–70 % in the loss of C with both ash types and N with acidic ashes demonstrated the capacity of CFA to mitigate GHG emission. Although we were unable to directly quantify the fluxes, negligible methane emissions from manure-rich arable soils, such as that used here, have been reported (Hütsch 1998). Despite the neutral impacts of CFA in unmanured soils, the capacity of alkaline ashes in raising soil pH (Table 3) as found in earlier studies (Manoharan et al. 2012; Yunusa et al. 2012) means these ashes can substitute lime for treating soil acidity and thereby avoid associated emissions. For example, applying just 2 Mg/ha of lime to the 1.7 M ha of agricultural land limed annually in Australia (ABS 2014), mining the required 3.4 Tg lime would generate ~ 2.66 Tg CO2 using the lime characteristics (CaO, MgO, water content) in Approach1 of the Greenhouse Gas Protocol (WRI 2008). This emission would be avoided as would the expected annual emission of 0.02 Tg CO2, i.e. 0.059 Mg C/Mg lime (West and McBride 2005), from eventual dissolution of the soil applied lime. This 2.66 Tg emission abatement is equivalent to 2.8 % of the ~98 Tg annual emission from the Australian agricultural sector. Acidic ash additions would also significantly mitigate N emissions from the manure treated soil by as much as two-thirds (Table 2). Aerobic decomposition of soil applied manure disposes of at least half of the N as NH3 or N2O within the first year (Dorahy et al. 2010). Therefore, applying the manure with 3.39 % N w/w (Table 1) at 10 Mg/ha over 100 ha would add 3.4 Mg N to the soil and generate 1.7 Mg of gaseous N emissions; this amount would, however, be cut by between 50 and 67% with the acidic ashes. This potential emission mitigation would be even larger in countries such as the United Kingdom where up to 42 Mg/ha of manures are prescribed routine farm applications (DEFRA 2009). Application of an acidic CFA should cut N emission from such practice in the first year to <10 Mg/ha compared with the expected 21 Mg/ha.
In conclusion, use of CFA for promoting storage of carbon and nitrogen in cropping soils is a viable technology for reducing GHG emissions in agriculture and environmental foot prints in this and the coal-power generation by minimising the CFA disposed in landfill. In selecting ashes for mitigating the loss of these nutrients from soil, acidic ashes are preferred over alkaline ashes that were effective in minimising only C loss. High concentrations of P2O5 and SiO2, TiO2 and clay particles in acidic ashes, and alkali/alkaline-earth metals (Na2O, MgO, CaO, Mn3O4 and Fe2O3) and sulphur in alkaline ashes, provided initial selection criteria for identifying CFAs for soil carbon sequestration. Knowledge-gaps to be addressed in developing this technology further include: (1) characterisation of the enzymatic processes in organic C mineralisation in ash amended soil, (2) major forms of N loss from the system, (3) whether similar C sparing is possible with plant residues, and (4) optimum rates and mode of ash additions.
References
Amonette JE, Kim J, Russell CK (2004) Enhancement of soil carbon sequestration: a catalytic approach. Ame Chem Soc Div Fuel Chem 49:366–367
Australian Bureau of Statistics (2014). Soil management in 2011-12: National and State/Territory. www.abs.gov.au/ausstats/abs@.nsf/Lookup/4630.0main+features112011-12. (Accessed: February 17, 2015)
ASN Bank/Ecofys (2013) World GHG Emissions Flow Chart (2010) http://www.ecofys.com/files/files/asn-ecofys-2013-world-ghg-emissions-flow-chart-2010.pdf. (Accessed: March 29, 2015)
Bååth E, Anderson TH (2003) Comparison of soil fungal/bacterial ratios in a pH gradient using physiological and PLFA-based techniques. Soil Biol Biochem 35:955–963
Benndorf R (2013) Germany 2050 - A Greenhouse Gas-Neutral Country. http://www.umweltbundesamt.de/en/publikationen/germany-2050-a-greenhouse-gas-neutral-country. (Accessed: January 20, 2015)
Blair N, Faulkner RD, Till AR, Sanchez P (2005) Decomposition of 13C and 15N labelled plant residue materials in two different soil types and its impact on soil carbon nitrogen aggregate stability and aggregate formation. Soil Res 43:873–886
Brock P, Madden P, Schwenke G, Herridge D (2012) Greenhouse gas emissions profile for 1 tonne of wheat produced in Central Zone (East) New South Wales: a life cycle assessment approach. Crop Pasture Sci 63:319–329
Campbell CD, Chapman SJ, Cameron CM, Davidson MS, Potts JM (2003) A rapid microtiter plate method to measure carbon dioxide evolved from carbon substrate amendments so as to determine the physiological profiles of soil microbial communities by using whole soil. Appl Environ Microbiol 69:3593–3599
Conyers M, Newton P, Condon J, Poile G, Mele P, Ash G (2012) Three long-term trials end with a quasi-equilibrium between soil C N and pH: an implication for C sequestration. Soil Res 50:527–535
DEFRA (2009). Nitrate Vulnerable Zones (NVZs)—Guidance for farmers. Summary of the guidance for farmers in NVZs (Leaflet 1 - PB12736a). Department for Environment Food and Rural Affairs, UK. http://adlib.everysite.co.uk/adlib/defra/content.aspx?doc=251225&id=251489. (Accessed: February 25, 2015)
Dorahy C, Dougherty W, Chan Y, Waters D (2010) Using recycled organics and manures in grain cropping systems. PrimeFact 1008. http://www.dpi.nsw.gov.au/__data/assets/pdf_file/0005/341762/Using-recycled-organics-and-manures-in-grain-cropping-systems.pdf. (Accessed: February 24, 2015)
Drinkwater LE, Wagoner P, Sarrantonio M (1998) Legume-based cropping systems have reduced carbon and nitrogen losses. Nature 396:262–265
FAO (1974) Key to the FAO Soil Units http://www.fao.org/soils-portal/soil-survey/soil-classification/fao-legend/key-to-the-fao-soil-units/en/ (Accessed: September 23, 2013)
Garnaut R (2011) Australia’s emissions in a global context Chapter 7. http://www.garnautreview.org.au/pdf/Garnaut_Chapter7.pdf (Accessed: February 4 2014)
Heidrich C, Feuerborn HJ, Weir A (2013) Coal Combustion Products: a Global Perspective. http://www.flyash.info/2013/171-Heidrich-Plenary-2013.pdf (Accessed: February 4 2014)
Hütsch BW (1998) Methane oxidation in arable soil as inhibited by ammonium, nitrite, and organic manure with respect to soil pH. Biol Fert Soils 28:27–35
IAEA (2001) Use of Isotope and Radiation Methods in Soil and Water Management and Crop. International Atomic Energy Agency Nutrition Training Course Series 14. http://www-naweb.iaea.org/nafa/swmn/public/TCS14-forward-contents.pdf . (Accessed: January 20, 2015)
IAEA (2012) Coal’s share of global energy mix to continue rising with coal closing in on oil as world’s top energy source by 2017. http://www.iea.org/newsroomandevents/pressreleases/2012/december/name,34441,en.html (Accessed: January 20, 2015)
IPCC (2006) N2O emissions from managed soils and CO2emissions from lime and urea application IPCC Guidelines for National Greenhouse Gas Inventories. http://www.ipcc-nggip.iges.or.jp/public/2006gl/pdf/4_Volume4/V4_11_Ch11_N2O&CO2.pdf (Accessed: February 4, 2014)
Jastrow JD, Amonette JE, Bailey VL (2007) Mechanisms controlling soil carbon turnover and their potential application for enhancing carbon sequestration. Clim Chang 80:5–23
Manoharan V, Yunusa IAM, Loganathan P, Lawrie R, Skilbeck CG, Burchett MD, Murray BR, Eamus D (2010) Assessments of Class F coal fly ashes for amelioration of soil acidity and their influence on growth and uptake of Mo and Se by canola. Fuel 89:3498–3504
Masto RE, Sengupta T, George J, Ram LC, Sunar KK, Selvi VA, Sinha AK (2014) The Impact of Fly Ash Amendment on Soil Carbon. Energ Sour Part A: Recov Util Environ Effect 36:554–562
Muriithi GN, Petrik LF, Fatoba O, Gitari WM, Doucet FJ, Nel J, Nyale SM, Chuks PE (2013) Comparison of CO2 capture by ex-situ accelerated carbonation and in in-situ naturally weathered coal fly ash. J Environ Manag 127:212–220
Ngu LN, Wu H, Zhang DK (2007) Characterization of ash cenospheres in fly ash from Australian power stations. Energ Fuels 21:3437–3445
Palumbo AV, Mccarthy JF, Amonette JE, Fisher LS, Wullschleger SD, Daniels WL (2004) Prospects for enhancing carbon sequestration and reclamation of degraded lands with fossil-fuel combustion by-products. Adv Environ Res 8:425–438
Raask E (1985) Mineral impurities in coal combustion: behavior problems and remedial measures. Taylor & Francis, Washington, DC, 484p
Rietz DN, Haynes RJ (2003) Effects of irrigation-induced salinity and sodicity on soil microbial activity. Soil Biol Biochem 35:845–854
Seshadri B, Bolan NS, Naidu R, Wang H, Sajwan K (2013) Clean coal technology combustion products: properties agricultural and environmental applications and risk management. Adv Agron 119:310–370
Shaheen SM, Hooda PS, Tsadilas CD (2014) Opportunities and challenges in the use of coal fly ash for soil improvements–a review. J Environ Manag 145:249–267
Soil Survey Staff (2012) Soil Taxonomy http://www.nrcs.usda.gov/wps/portal/nrcs/main/soils/survey/class/taxonomy/ (Accessed: February 24, 2014)
Tian Y, Takanashi K, Toda H, Haibara K, Ding F (2013) pH and substrate regulation of nitrogen and carbon dynamics in forest soils in a karst region of the upper Yangtze River basin China. J For Res 18:228–237
UNFCCC (2014) Greenhouse gas inventory data—detailed data by party. United Nations framework convention for climate change. http://unfccc.int/di/DetailedByParty.do. (Accessed: February 27 2014)
US Environment Protection Agency (2013) Inventory of US greenhouse gas emissions and sinks: 1990–2011. http://www.epa.gov/climatechange/Downloads/ghgemissions/US-GHG-Inventory-2013-Main-Text.pdf. (Accessed: February 26, 2014)
Wang S, Li L, Wu H, Zhu ZH (2005) Unburned carbon as a low-cost adsorbent for treatment of methylene blue-containing wastewater. J Colloid Interface Sci 292:336–343
Wang S, Ma Q, Zhu ZH (2008) Characteristics of coal fly ash and adsorption application. Fuel 87:3469–3473
Weintraub MN, Schimel JP (2003) Interactions between carbon and nitrogen mineralization and soil organic matter chemistry in arctic tundra soils. Ecosystems 6:129–143
West TO, McBride AC (2005) The contribution of agricultural lime to carbon dioxide emissions in the United States: dissolution, transport, and net emissions. Agric Ecosyst Environ 108:145–154
World Resource Institute (2008) Greenhouse Gas Protocol: CO2 emissions from the production of lime. http://www.ghgprotocol.org/calculation-tools/lime-sector. (accessed: February 14, 2015)
Yip K, Tian F, Hayashi JI, Wu H (2010) Effect of alkali and alkaline earth metallic species on biochar reactivity and syngas compositions during steam gasification. Energ Fuels 24:173–181
Young IM, Ritz K (2000) Tillage habitat space and function of soil microbes. Soil Tillage Res 53:201–213
Yunusa IAM, Manoharan V, Odeh IO, Shrestha S, Skilbeck CG, Eamus D (2011) Structural and hydrological alterations of soil due to addition of coal fly ash. J Soils Sediments 11:423–431
Yunusa IAM, Loganathan P, Nissanka SP, Manoharan V, Burchett MD, Skilbeck CG, Eamus D (2012) Application of coal fly ash in agriculture: a strategic perspective. Crit Rev Environ Sci Technol 42:559–600
Yunusa IAM, Manoharan V, Harris R, Lawrie R, Pal Y, Quiton JT, Bell R, Eamus D (2013) Differential growth and yield by canola (Brassica napus L) and wheat (Triticum aestivum L) arising from alterations in chemical properties of sandy soils due to additions of fly ash. J Sci Food Agric 93:995–1002
Acknowledgments
Cenosphere analyses and adsorption experiments were undertaken by Prof Hongwei Hu, Dr Xiangpeng Gao, Prof Shaobin Wang and their teams at Curtin University; Prof Hu also commented on the manuscript. We acknowledge the assistance of Leanne Leslie, Michael Faint, Jan Carruthers, Gabrielle Ray and Ewan Paterson, and methodology advice by Drs S.M. Rabbi, Rebecca Hailing and V. Manoharan, and Assoc Prof Brian Wilson. We thank the reviewers for their helpful comments, and the power stations and the Ash Development Association of Australia (ADAA) for their support. This study was supported with UNE Seed Grant, and evolved from an earlier project funded by ADAA and the Australian Research Council (LP0455110).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yunusa, I.A.M., Blair, G., Zerihun, A. et al. Enhancing carbon sequestration in soil with coal combustion products: a technology for minimising carbon footprints in coal-power generation and agriculture. Climatic Change 131, 559–573 (2015). https://doi.org/10.1007/s10584-015-1388-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10584-015-1388-0