Abstract
Concentrations of trace elements (Cd, Co, Cr, Cu, Ni, Pb and Zn) and major elements (Ca, Mg, K, Fe, Al and Mn) as well as pHKCl and total organic carbon (TOC) were measured on 22 profiles located in a 3 km radius of a former ore treatment plant in Wallonia (Belgium). The enrichment factor (EF), vertical impoverishment factor (VIF) and availability ratio (AR) were used as diagnostic tools of contamination and migration of Cd, Pb and Zn in profiles. The data revealed that the soil profiles are significantly contaminated. The ranges of EFs for Cd (17–3570), Pb (1–2883) and Zn (2–309) are very broad with the higher EF in the topsoil of the profiles. VIFs of subhorizons are rarely above 1. In the few cases with VIF > 1, the main factor explaining is the soil type (in particular, Colluvic Regosols, Luvisols and Cambisols with shale load). Cd, Pb and Zn ARs are strongly correlated with TOC and to a lesser extent with pHKCl; Pb and Zn ARs are influenced by soil type, while land use only has an effect on Zn AR. The Cd availability is independent of these two factors. The contents of trace elements measured in soil profiles are very high, especially in topsoil. At present, the Cd, Pb and Zn vertical distribution shows a low migration from topsoil to deeper levels. However, we must not discount the hazard of a future potential transfer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Trace elements (TEs) occur naturally in soils; some are essential micronutrients for plants and animals and are thus important for human health and food production (Hooda 2010). However, as a consequence of industrial activities, accumulation of TEs may occur in the natural environment, and they now pose a range of ecological and health problems.
Unlike slag and waste deposits, visual evidence of historical atmospheric contamination is difficult to detect in soils. However, considering factors such as distance from the potential sources of contamination, wind direction, land use or soil type allow a delineation of the contaminated areas and prediction of the spatial distribution of contaminants in surface layers (Godin et al. 1985; Liénard et al. 2014; Othmani et al. 2015). In addition, once deposited on soils, directly or after rain washing of vegetation leaves, metallic particles are subject to biogeochemical processes and can be physically redistributed in the soil and the landscape (Hernandez et al. 2003; Soubrand-Colin et al. 2007; Sterckeman et al. 2000). Chemical reactions can also affect the mobility and bioavailability of the metals according to the soil characteristics and land use.
Furthermore, the total content of TEs in the topsoil does not always originate from anthropogenic sources but can also result from natural processes. To determine the importance of the lithologic contribution compared with anthropogenic origin, some studies have used the enrichment factor (EF) as a discriminating tool (Blaser et al. 2000; Bourennane et al. 2010; Reimann and De Caritat 2000, 2005; Soubrand-Colin et al. 2007). The EF evaluates for a soil layer the level of enrichment of a given element compared with another one considered as weakly affected by pedogenic processes. The EF is calculated as the ratio of the studied element to the reference element and normalized by the same ratio for the continental crust composition (Reimann and De Caritat 2000, 2005). It allows identification of the origin of contaminants, i.e. air, atmospheric dusts or precipitations, and was used by some authors to detect the level of TEs that are due to anthropogenic inputs (Bourennane et al. 2010; Hernandez et al. 2003; Soubrand-Colin et al. 2007). Regardless of its wide use for estimating topsoil contamination, this method has been criticized for its cut-off values and the assumption of the constancy of traces/reference element ratios in nature (Dung et al. 2013; Reimann and De Caritat 2005). To deal with this argument, many researchers apply a different normalization [e.g. with an uncontaminated natural soil (Manta et al. 2002; Singh et al. 2010)] or they use local background values instead of the average crust composition (Loska et al. 2003, 2004). However, all of these approaches require direct comparison between data on topsoil and on deeper mineral soil layers or the continental crust and neglect any impact of the biosphere or soil formation processes on the geochemistry of the Earth’s surface (Reimann et al. 2015). Other methods of comparison use the reactivity or the solubility of TEs such as availability ratios (Massas et al. 2010, 2013; Ramos-Miras et al. 2011; Zu et al. 2014) or risk assessment code (De Andrade et al. 2011; Dung et al. 2013). The use of these methods based on the reactive pool of TEs reflects that all species of a particular metal do not present an equal impact with regard to the ecosystem (Dung et al. 2013).
In the south of Belgium (Wallonia), processing of Zn–Pb ore has occurred along the main rivers for at least 200 years. The smelter activities began with locally mined products and continued with imported material. Following this industrial process, substantial air emissions rich in oxides (SO2, SO3, NO2) and particulates enriched with Cd, Pb and Zn (Graitson 2005) have spread throughout the vicinity and have led to significant contamination of soils and the environment. The combination of high acidity and enrichment by TEs has resulted in environmental toxicity that has caused significant adverse effects to many species, communities and ecosystems (Graitson 2005; Graitson et al. 2005; Rosengarten 2010). The metalliferous sites, which are now protected as natural reserves, present the clearest evidence of the impacts of former industrial activity within the present landscape (Liénard et al. 2011). However, metalliferous sites are usually of limited extent (maximum of 75 ha) and located close to the former plants. Nevertheless, recent studies have shown that atmospheric fallout of contaminants has impacted significantly larger areas around old smelters (Douay et al. 2008; Liénard et al. 2014; Sterckeman et al. 2000; Yuan et al. 2013).
In this study, we have studied the fate of Cd, Pb and Zn in soils affected by atmospheric deposition in the vicinity of old smelters. The importance of vertical redistribution of contaminants in soils is questioned, and the aims of this paper are to (a) characterize the vertical distribution of TEs and soil properties along diverse soil profiles, (b) evaluate enrichment or impoverishment of TEs along the profiles to discriminate anthropogenic or geogenic origin of contaminations and (b) evaluate whether soil type or land use influenced the mobility of contaminants in the selected profiles.
Materials and methods
Study area and soil sampling
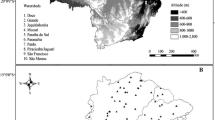
Samples were collected from a contaminated area close to a former Zn–Pb ore treatment site. As shown by Liénard et al. (2014), the main origin of topsoil contamination is the atmospheric fallout of dusts enriched in contaminants, especially Cd, Pb and Zn, from the chimneys of the smelter. Here, the vertical distribution of contaminants has been investigated in 22 soil profiles. They were distributed on both sides of the Meuse valley, 15 on the left bank (LB) and 7 on the right bank (RB), at right angles to the dominant wind direction along a transect (Fig. 1). They were located in the surroundings of the former smelter (maximum 3 km) on various soil types and three land uses (grasslands, crops and forests) (Table 1). The samples were taken according to horizons with a manual auger (maximum 1.2 m); the number of horizons sampled depending on the soil type and depth to bedrock. The identification of horizons was performed according to variations of texture, colour, redox mottles and stone charge.
Analytical methods
Air-dried samples were sieved at 2.0 mm, and a subsample was crushed to 200 µm. Soil pH was measured by creating a slurry in 1 M KCl (pHKCl) (w:v 2:5 ratio). Total organic carbon (TOC) was determined following the Springer–Klee method (Nelson and Sommers 1996). Major (Ca, Mg, K, Fe, Al, Mn) and trace (Cd, Co, Cr, Cu, Ni, Pb, Zn) element concentrations were determined after (a) aqua regia digestion following ISO 11466 (referred to as total metal concentration) and (b) after extraction with CH3COONH4 (0.5 M) and EDTA (0.02 M) at pH 4.65 (w:v 1:5 ratio) and agitation for 30 min (referred to as available metal concentration) (Lakanen and Erviö 1971). Available Al, Fe, Co, Cr and Ni were not measured. The concentrations in the solutions were measured using flame atomic absorption spectrometry (Varian 220, Agilent Technologies, Santa Clara, CA, USA). The detection limits for total/available metals were 0.66/0.10 mg/kg (Cd), 0.99/0.15 mg/kg (Cu), 3.33/0.50 mg/kg (Pb) and 0.33/0.05 mg/kg (Zn). As part of the quality control programme for the study, a standard reference material certified by BIPEA (RCIL no. 2009/2010-463, 15-415-Soils) was used and analysed with each set of samples. The analytical variability, expressed as coefficient of variation (CV), accounted either for less than 20 % for the studied elements or was lower than 1 mg/kg.
Diagnosis of contaminations
Enrichment factor (EF)
Some authors (Baize 1997; Colinet et al. 2004; Myers and Thorbjornsen 2004) have demonstrated that Fe and Al concentrations have frequently been used as indicators of natural occurrence of TEs such as Ni, Cr, Zn, Pb or Cd. The EF is the relation between a reference element (Al) and another chemical element (Cd, Cr, Co, Cu, …) according to their concentrations in the crust (McLennan 2001) and studied sample. The formula used to calculate an EF is (Bourennane et al. 2010; Khalil et al. 2013; Reimann and De Caritat 2000):
where ‘El’ is the element under consideration. The value of the EF is used as an indicator of contamination. Researchers have generally defined enrichment as occurring when EF > 2, the cause of this enrichment being given as anthropogenic contributions (Hernandez et al. 2003; Khalil et al. 2013; Soubrand-Colin et al. 2007). Some authors subdivide EF categories to produce a more detailed indication of the level of contamination (Lu et al. 2009a, b; Sutherland 2000; Yongming et al. 2006): (1) EF < 2 indicates deficiency to minimal enrichment; (2) 2 ≤ EF < 5 indicates moderate enrichment; (3) 5 ≤ EF < 20 indicates significant enrichment; (4) 20 ≤ EF ≤ 40 indicates very high enrichment; and (5) EF > 40 indicates extremely high enrichment. Often, this approach is not discriminative enough to realize a comparison of extremely contaminated samples (EF > 40).
Vertical impoverishment factor (VIF)
In addition to the EF, we propose to use the VIF, which is a comparison between the surface horizon and another horizon of the profile. The formula used to calculate a VIF is:
The VIF is thus a normalization of element concentration in a subsurface horizon compared with the concentration of this element in the surface horizon of the same soil profile. Here, we intend to evaluate the evolution of the elements with depth compared with the surface that we know is contaminated. Al is again used as the reference element. The value of VIF for the surface horizon is always equal to 1. If VIF < 1, the studied horizon is depleted relative to the surface horizon and a surface contamination is highly probable. When VIF > 1, the studied horizon is enriched compared with the topsoil. Higher contents may depend upon either pedogeochemical conditions of soils or external factors such as anthropogenic inputs. Comparison of VIF should also allow estimation of the vertical extent of contamination within one profile.
Availability ratio (AR)
ARs are metal availability indexes normalized for the total metal concentrations because they represent the percentage of available fraction to total metal concentration in soil (Dung et al. 2013; Massas et al. 2013; Ramos-Miras et al. 2011). The ARs are calculated according to the following formula:
where ‘Ca’ is the available metal concentration in the sample [CH3COONH4 (0.5 M) + EDTA (0.02 M) at pH 4.65 (Lakanen and Erviö 1971)] and ‘Ct’ is the total metal concentration in the same sample (aqua regia digestion, ISO 11466). AR can be considered as a comparative index for mobility of TEs and thus also as an indicator of the recent soil pollution history (Massas et al. 2010) because exogenous metals are more mobile than metals from soil minerals.
Statistical methods
Correlation analyses (Pearson correlation matrix) were performed to identify significant relationships between TEs and other soil characteristics (major elements contents, TOC and pHKCl) and between Cd, Pb and Zn ARs and TOC and pHKCl to identify factors of mobility of TEs along profiles.
Homogeneity of variances within stratified subpopulations (soil type and land use) was verified with Levene’s test, which is robust for small populations. The effects of soil type and land use on ARs were tested using a one-way ANOVA, and differences were considered significant at p ≤ 0.05. All of the statistical analyses were performed with Minitab 16 (Minitab Inc., State College, PA, USA).
Results and discussion
Statistical analysis of soil profiles
Descriptive statistics of pHKCl, TOC, major elements and TEs for all samples are summarized in Tables 2 and 3.
The pHKCl ranges between 3.4 and 7.4 (Table 2). The lowest pH minima in topsoil and deep horizons were related to forests. In contrast, surface horizons of crops and subsoil of grasslands are less acid and present a narrower range of pH, 5.1–6.9 and 5.2–7.3, respectively. Often, pH values diminish from surface to depth. The increase of pH with depth was only met in a sandy soil (LB2) and a loamy colluvial soil (LB10). Concerning the distribution of pH data, it has a particularly flat peak, as shown by the negative kurtosis index (Table 2). This is the only parameter with Mn AR (depth samples) that shows this pattern of distribution. All other parameters studied present a sharply peaked distribution with a positive kurtosis index.
The range of TOC (Table 2) content is large in surface horizons (0.89–13.24 %) and varies between 0.0 and 2.44 % in depth. In topsoil, the variability of TOC content depends mainly on land use: 2.41–13.24, 1.09–5.57 and 0.89–1.65 %, for forests, grasslands and crops, respectively. The values of TOC measured in topsoil of grasslands and crops are in the range of the regional database on soil fertility (Genot et al. 2012).
Concerning major nutrient contents, the range of available concentrations encountered is largest for Ca (0.02–564 mg/100 g), followed by Mg (0.22–97.6 mg/100 g), K (0.09–49.1 mg/100 g) and P (0.06–23.7 mg/100 g) (Table 2). The relative variability for K and Mg available concentrations is always higher than those of total concentrations (Tables 2, 3). For Ca, the variability of available content is slightly less marked in the deep horizons (coefficient of variation (CV) = 194 %) than in topsoil (CV = 223 %). This observation is opposite for total contents with CV equal to 140 and 305 % for topsoil and depth, respectively. This variability may be explained by diversity of soil type, parent material and land use. The variability of Mg total content is medium (70 %) and is mainly linked to land use. Total contents of K as well as Fe and Al are relatively homogeneous; the variability is low (CV < 40 %) (Table 3).
The total content of Al in soils ranges between 0.41 % in sandy soils and 3.25 % in clayey subsurface horizon of old alluvial deposits. Soils developed from loess deposits (luvisols, colluvic cambisols), shales and sandstones present mean levels between 1.5 and 2.0 %, while sandy soils (arenosols) are quite poor in Al and soils on limestone weathering clay or old alluvial deposits appear richer. These levels of content are consistent with previous studies (Colinet et al. 2004; Liénard et al. 2014). It should be noted also that the value proposed for the upper crust composition is much higher (8.04 %), which implies that EF will be high in our study area even in the absence of contamination.
For the studied TEs (Cd, Cr, Co, Cu, Ni, Pb and Zn), two different shapes of distribution were identified. The first type concerns Co, Cr and Ni, which show a symmetrical distribution and a skewness index between 0 and 1 (Table 3; Fig. 2). The second type of distribution appears clearly left-skewed with a long tail over very highly contaminated points and concerns Cd, Pb and Zn. This is confirmed by the CVs of these three elements (Table 3; Fig. 2). They all are above 100 %, indicating a high variability due to a large data dispersion around the mean and the kind of samples (surface, depth or profiles). Indeed, total Cd, Pb and Zn contents are distributed over wide ranges of concentration, i.e. 0.66–63.5, 6.00–10521 and 42.0–4474 mg/kg, respectively. Total distribution of Cu represents an intermediate situation with a higher variability in topsoil (CV = 123 %) than depth (CV = 26.4 %).
Concerning EF, topsoil of RB1, RB2, LB1, LB2, LB3 and LB4 are extremely contaminated by Cd, Pb and Zn with EF > 40 (Table 4). These six profiles are located near an old source of contamination (maximum 1 km), and the EF values are similar to those calculated by Liénard et al. (2014). Profiles LB1, LB2, LB3 and LB4 are extremely contaminated in Cd, Pb and Zn. There are two forest profiles (LB3 and LB4) located on Carboniferous Dinantian geologic formation. The forest land use is known to present greater TE concentrations than crops or grassland in this area (Liénard et al. 2014). An increase in Pb and Zn EF is observed along the LB3 profile, and this can be attributed to the colluvial origin of the horizons and to the location of the profile directly below the metalliferous site. Among the horizons, 100 % are ‘minimum enriched’ in Cd, Pb or Zn, and 56, 31 and 24 % are extremely enriched in Cd, Pb and Zn, respectively. These proportions confirm the high contamination of soil profiles with the following enrichment order: Cd ≫ Pb > Zn. Even if EF remains a good indicator in the depth, it tends to overstate the levels of contamination compared with the total contents measured, which are close to the background values.
Correlation of trace elements and soil properties
The correlation matrix data set of all parameters from the soil samples is presented in Table 5. High levels of correlation (p < 0.001) were found between Al, Fe and K, Co, Cr and Ni on the one side and between Cd, Cu, Pb, Zn and TOC on the other side (Table 5). The soil concentrations of Al, Fe, Co, Cr, K, Mn and Ni appear to reflect natural geochemical trends driven by the clay and Fe oxides content (Baize and Sterckeman 2001). The geochemical association of Cd, Cu, Pb, Zn and TOC reflects that these metals are deposited on topsoil by anthropogenic sources such as industrial fallout of Cd, Pb, Zn (Bourennane et al. 2010; Liénard et al. 2014) and inflows of livestock manure or fertilizer for Cu deposits (He et al. 2005). The Ca content and pHKCl are not significantly linked to contents of TEs in soils.
Cd, Pb and Zn vertical distribution
The analysis of total concentrations of Cd shows that mean values are much higher than the regional background value, 0.2 mg/kg (Ministère de la Région wallonne 2008). Of the 22 profiles, only 5 present a slightly contaminated surface horizon with a Cd concentration less than 1 mg/kg (RB6, RB7, LB11, LB12, LB15, Table 4). The other soils are contaminated and present a wide range of content ranging from 1.1 to 63.5 mg/kg. LB1, LB3 and LB4 have the most contaminated topsoil with 21.7, 63.5 and 45.0 mg/kg, respectively. In these cases, the Cd content decreases rapidly with depth but remains greater than 10 mg/kg. The contamination is obvious for the entire profile of these soils.
Compared with the regional background value of Pb fixed at 25 mg/kg (Ministère de la Région wallonne 2008), only the topsoil (0–30 cm) of LB15 is non-contaminated with 22.8 mg Pb/kg. The surface horizons of all other profiles are considered as contaminated. Some are very severely contaminated with contents equal to 1888, 2104, 2499 and 5084 mg Pb/kg, respectively, for RB2, LB4, LB2 and LB3. As a general rule, the total Pb concentration decreases with depth. In some cases, the last horizon of the profile reaches a content lower or equivalent to soil pedogeochemical background: RB3, RB7, LB5, LB6, LB7, LB8, LB11, LB13, LB14 and LB15. For profile LB3 (forest colluvial soil), the depth Pb contents double up compared with the surface to reach a level of 10,500 mg/kg.
The background value of Zn, 67 mg/kg, is exceeded for all surface horizons (Ministère de la Région wallonne 2008). Four soils have a heavily contaminated surface with Zn contents greater than 1000 mg/kg, and the content decreases with the depth. This is the case of soils RB1, RB2, LB1 and LB4 with 1150, 1781, 1846 and 3774 mg/kg, respectively. Other soils (RB3, LB3, LB6 and LB13) have a Zn content that increases with depth. For RB3 and LB6, the surface content (250 and 366 mg/kg) is multiplied by factors of 3 and 2 to reach the level of the deepest horizon (820 and 640 mg/kg). The LB3 surface concentration rises slightly in the third horizon to switch from 3428 to 4040 mg/kg.
Generally, our results show that reference values from legislation [Cd = 0.2 mg/kg, Pb = 25 mg/kg, Zn = 67 mg/kg (Ministère de la Région wallonne 2008)] can be used to characterize the local background value of the study area. In addition, a sampling depth higher than 60 cm can be used to characterize the regional background value because under this depth the measured contents are close to reference values.
Vertical impoverishment factor
Most subsurface horizons present a VIF < 1 relative to the surface, which indicates a decrease in contamination with depth (Table 4). The deepest horizons have a VIF that reaches a value close to 0. For Cd, this fact implies that topsoil is usually much more Cd contaminated than deeper horizons. This observation is, however, not made for the profiles LB2, LB6, LB7 and LB14 (see Fig. 3a for LB2 and LB7). These four profiles show a small increase in VIF by a maximum of 0.1 unit. In these cases, the VIF rise is not linked to an increase in Cd concentration in the horizon analysed but to the variation of Al. LB13 (Fig. 3a) and LB15 show a rise of VIF in one subsurface horizon (VIF > 1).
Regarding Pb, a first group of soils with all RB soils and 11 soils of LB show constant impoverishment of Pb (VIF < 1 without rise) under the topsoil (see Fig. 3b for LB1, LB2 and LB7). The second type of profile is represented by LB9, where the decrease is not linear and VIF locally increases: from 0.72 to 0.90 between 50–80 and 80–100 cm layers. However, Pb content (50 and 65 mg/kg) does not indicate a contamination. The last group clusters the soils with all or a part of VIF > 1 (LB3, LB14 and LB15) (Fig. 3b). The whole LB3 profile has a VIF > 1 (1.15, 1.63, 1.48) with a very strong contamination from 6094 to 10521 mg Pb/kg. In contrast, LB14 and LB15 have only their second horizon with a VIF > 1 (1.06 and 1.18) and a low Pb content of 98 and 33 mg/kg, respectively.
The Zn impoverishment in the horizons compared with topsoil (VIF < 1) is noticed for all studied soils except for LB3, LB6, LB10, LB12 and LB13, which have one or more horizons with a Zn VIF > 1 (Fig. 3c). The soils RB7, LB2, LB9 and LB11 all have VIF < 1 with total Zn content >360 mg/kg but VIF increases sometimes along the profile. In spite of a slight increase in VIF, a decrease is generally noted with a change in slope between horizons 2 and 3.
The observation of VIF shows that among the 22 studied profiles only 3 for Cd, 3 for Pb and 5 for Zn present some subsurface horizons with a VIF > 1. The VIF represents the distribution of contaminant along a profile that can be partly explained by properties of soils. Eight profiles that have one or more horizons with VIF > 1 are grouped into three soil types: Colluvic Regosols (LB3, LB10), Luvisols (LB6, LB15) and Cambisols with shale load (LB11, LB12, LB13 and LB14). These soils present a large proportion of clay particles resulting from weathering of parent material or colluvial redistributions. This observation was already noticed for old terrace soils in a previous study (Liénard et al. 2014). The link between clay content and TEs in this context should be viewed as an argument for geogenic contamination (Baize and Sterckeman 2001; Colinet et al. 2004). Land use was not found as a determining factor of differences of evolution TEs with depth. The soil properties associated with the differences of land use are mainly soil reaction (pH) and organic content. In acidic conditions, studied TEs are known to be more mobile, and vertical migrations could be furthered in situations with consequent water drainage. On the other side, organic matter plays a role of retention versus TEs with an importance varying according to their respective affinities. In our study, however, no such evidence of subsurface contamination due to intensive leaching was found.
Availability ratio
As illustrated in Table 6, the AR values for Cd, Pb and Zn are significantly correlated. These three TEs do not have the same mobility in the soil with the following AR order: Cd ≫ Pb > Zn (see Fig. 4a; Table 4). Zn AR is similar to those presented by Massas et al. (2013). However, Pb AR seems high considering that Pb is not a highly mobile element. ARs of topsoil are systematically higher than all other horizons named as subhorizons; this indicates that the availability of TEs is greater in the surface. Moreover, the significant correlations between Cd, Pb and Zn ARs and TOC indicate that the availability of these metals in the studied soils is at least partly influenced by TOC variations (Clemente et al. 2008; Linde et al. 2007). This observation cannot be explained by the impact of land use (forest, crop or grassland, Fig. 4b) or soil type. These factors are not statistically considered as influencing Cd, Pb and Zn ARs in the topsoil.
Concerning the profile (all horizons), Cd AR was not affected by soil type or land use. Regarding the Pb relative mobility in the profile, AR was influenced by soil type (F 6,84 = 8.79, p = 0.000) because a large range of Pb AR is encountered from 18.5 to 90.1, a Dystric Cambisol (loamic, ruptic) and a topsoil of an Arenosol, respectively. In profiles, Zn AR is influenced by land use (F 2,84 = 4.92, p = 0.010) but only crops have a really different AR range than forests or grasslands. The soil type is already an influencing factor for Zn AR (F 6,84 = 13.50, p = 0.000) with the Arenosol (Zn ratio = 30.2), which still has the highest AR.
Conclusions
We evaluated the vertical distribution of concentrations of Cd, Pb and Zn in soil profiles collected within 3 km of a former Zn–Pb ore treatment plant. The contamination of soils was investigated through the use of the EF, the vertical distribution of total contents, the evaluation of migration along the profile through the VIF and the mobility of contaminants with the AR.
The study of contaminant concentrations and their EF shows a strong contamination of topsoil and also very high levels deeper in some profiles. The most significant contamination is observed for profiles sampled on the left bank (LB) up to 1 km from the former smelter in the following order Cd > Pb > Zn. For all profiles, the major explanation of topsoil contamination is anthropogenic considering (a) that EFs are all greater than 5 which means significant topsoil enrichment and (b) that left bank is located under dominant wind.
Regarding the contaminant transfer in the profiles, no systematic migration was found. However, an accumulation of contaminants is recognized in some horizons. This local enrichment depends on soil properties and soil type (colluvic soil, loamy soil and loamy–stony soil with shale stone). By chance, these horizons do not show a large AR, so a migration risk is unlikely.
All results described above suggest that Cd, Pb and Zn contents in profiles are due to past human activities and that the redistribution along profiles through lixiviation of contaminants is not an important phenomenon. However, although the mobility of contaminants is not excessive, the measured contents are very high, especially in the topsoil. Therefore, we cannot exclude risks to human health and the environment.
References
Baize D (1997) Un point sur… teneurs totales en éléments traces métalliques dans les sols (France). INRA edn, Paris
Baize D, Sterckeman T (2001) Of the necessity of knowledge of the natural pedo-geochemical background content in the evaluation of the contamination of soils by trace elements. Sci Total Environ 264(1–2):127–139
Blaser P, Zimmermann S, Luster J, Shotyk W (2000) Critical examination of trace element enrichments and depletions in soils: As, Cr, Cu, Ni, Pb, and Zn in Swiss forest soils. Sci Total Environ 249(1–3):257–280
Bock L, Bah B, Veron P, Lejeune P (2006) Carte des Principaux Types de Sols de Wallonie à 1/250 000. Unité Sol-Ecologie-Territoire (Laboratoire de Géopédologie) et Unité de Gestion des Ressources forestières et des Milieux naturels, Faculté universitaire des Sciences agronomiques de Gembloux, Gembloux, Belgium
Bourennane H, Douay F, Sterckeman T, Villanneau E, Ciesielski H, King D, Baize D (2010) Mapping of anthropogenic trace elements inputs in agricultural topsoil from Northern France using enrichment factors. Geoderma 157(3–4):165–174. doi:10.1016/j.geoderma.2010.04.009
Clemente R, Dickinson NM, Lepp NW (2008) Mobility of metals and metalloids in a multi-element contaminated soil 20 years after cessation of the pollution source activity. Environ Pollut 155(2):254–261. doi:10.1016/j.envpol.2007.11.024
Colinet G, Laroche J, Etienne M, Lacroix D, Bock L (2004) Intérêt d’une stratification pédologique pour la constitution de référentiels régionaux sur les teneurs en éléments traces métalliques dans les sols de Wallonie. Biotechnol Agron Soc Environ 8(2):83–94
De Andrade Passos E, Alves JDPH, Garcia CAB, Costa ACS (2011) Metal fractionation in sediments of the Sergipe River, Northeast, Brazil. J Braz Chem Soc 22(5):828–835
Douay F, Pruvot C, Roussel H, Ciesielski H, Fourrier H, Proix N, Waterlot C (2008) Contamination of urban soils in an area of Northern France polluted by dust emissions of two smelters. Water Air Soil Pollut 188(1–4):247–260
Dung TTT, Cappuyns V, Swennen R, Phung NK (2013) From geochemical background determination to pollution assessment of heavy metals in sediments and soils. Rev Environ Sci Bio/Technol 12(4):335–353. doi:10.1007/s11157-013-9315-1
Genot V, Renneson M, Colinet G, Goffaux M-J, Cugnon T, Toussaint B, Buffet D, Oger R (2012) Base de données sols de REQUASUD: 3ème synthèse. Gembloux
Godin PM, Feinberg MH, Ducauze CJ (1985) Modelling of soil contamination by airborne lead and cadmium around several emission sources. Environ Pollut 10(2):97–114
Graitson E (2005) Inventaire et caractérisation des sites calaminaires en Région wallonne. Nat Mosana 58(4):83–124
Graitson E, San Martin G, Goffart P (2005) Intérêt et particularités des haldes calaminaires wallonnes pour l’entomofaune: le cas des Lépidoptères Rhopalocères et des Orthoptères. Notes faunistiques de Gembloux 57:49–57
He ZLL, Yang XE, Stoffella PJ (2005) Trace elements in agroecosystems and impacts on the environment. J Trace Elem Med Biol 19(2–3):125–140. doi:10.1016/j.jtemb.2005.02.010
Hernandez L, Probst A, Probst JL, Ulrich E (2003) Heavy metal distribution in some French forest soils: evidence for atmospheric contamination. Sci Total Environ 312(1–3):195–219
Hooda P (2010) Trace elements in soils. Wiley, New York
Khalil A, Hanich L, Bannari A, Zouhri L, Pourret O, Hakkou R (2013) Assessment of soil contamination around an abandoned mine in a semi-arid environment using geochemistry and geostatistics: pre-work of geochemical process modeling with numerical models. J Geochem Explor 125:117–129. doi:10.1016/j.gexplo.2012.11.018
Lakanen E, Erviö R (1971) A comparison of eight extractants for the determination of plant available micronutrients in soils. Acta Agralia Fennica 123:223–232
Liénard A, Bock L, Colinet G (2011) Intérêt des cartes des sols pour l’élaboration d’une stratégie d’échantillonnage en sols contaminés par retombées atmosphériques: application à l’étude de l’effet sol sur le devenir des éléments traces métalliques. Biotechnol Agron Soc Environ 15(2):669–682
Liénard A, Brostaux Y, Colinet G (2014) Soil contamination near a former Zn–Pb ore-treatment plant: evaluation of deterministic factors and spatial structures at the landscape scale. J Geochem Explor 147:107–116. doi:10.1016/j.gexplo.2014.07.014
Linde M, Öborn I, Gustafsson JP (2007) Effects of changed soil conditions on the mobility of trace metals in moderately contaminated urban soils. Water Air Soil Pollut 183(1–4):69–83. doi:10.1007/s11270-007-9357-5
Loska K, Wiechula D, Barska B, Cebula E, Chojnecka A (2003) Assessment of arsenic enrichment of cultivated soils in Southern Poland. Pol J Environ Stud 12(2):187–192
Loska K, Wiechulła D, Korus I (2004) Metal contamination of farming soils affected by industry. Environ Int 30(2):159–165. doi:10.1016/S0160-4120(03)00157-0
Lu X, Li LY, Wang L, Lei K, Huang J, Zhai Y (2009a) Contamination assessment of mercury and arsenic in roadway dust from Baoji, China. Atmos Environ 43(15):2489–2496
Lu X, Wang L, Lei K, Huang J, Zhai Y (2009b) Contamination assessment of copper, lead, zinc, manganese and nickel in street dust of Baoji, NW China. J Hazard Mater 161(2–3):1058–1062
Manta DS, Angelone M, Bellanca A, Neri R, Sprovieri M (2002) Heavy metals in urban soils: a case study from the city of Palermo (Sicily), Italy. Sci Total Environ 300(1–3):229–243. doi:10.1016/S0048-9697(02)00273-5
Massas I, Ehaliotis C, Kalivas D, Panagopoulou G (2010) Concentrations and availability indicators of soil heavy metals; The case of children’s playgrounds in the city of Athens (Greece). Water Air Soil Pollut 212(1–4):51–63. doi:10.1007/s11270-009-0321-4
Massas I, Kalivas D, Ehaliotis C, Gasparatos D (2013) Total and available heavy metal concentrations in soils of the Thriassio plain (Greece) and assessment of soil pollution indexes. Environ Monit Assess 185(8):6751–6766. doi:10.1007/s10661-013-3062-1
McLennan SM (2001) Relationships between the trace element composition of sedimentary rocks and upper continental crust. Geochem Geophys Geosyst 2(4). doi:10.1029/2000GC000109
Ministère de la Région wallonne (2008) Décret du 5 décembre 2008 relatif à la gestion des sols. Moniteur belge du 18 février 2009 et 6 mars 2009
Myers J, Thorbjornsen K (2004) Identifying metals contamination in soil: a geochemical approach. Soil Sediment Contam 13(1):1–16. doi:10.1080/10588330490269732
Nelson DW, Sommers LE (1996) Total carbon, organic carbon, and organic matter. In: Sparks DL, Page AL, Helmke PA et al (eds) Methods of soil analysis part 3—chemical methods, vol sssabookseries, vol methodsofsoilan3. Soil Science Society of America Inc., Madison, pp 961–1010. doi:10.2136/sssabookser5.3.c34
Othmani MA, Souissi F, Durães N, Abdelkader M, da Silva EF (2015) Assessment of metal pollution in a former mining area in the NW Tunisia: spatial distribution and fraction of Cd, Pb and Zn in soil. Environ Monit Assess. doi:10.1007/s10661-015-4734-9
Ramos-Miras JJ, Roca-Perez L, Guzmán-Palomino M, Boluda R, Gil C (2011) Background levels and baseline values of available heavy metals in Mediterranean greenhouse soils (Spain). J Geochem Explor 110(2):186–192. doi:10.1016/j.gexplo.2011.05.009
Reimann C, De Caritat P (2000) Intrinsic flaws of element enrichment factors (EFs) in environmental geochemistry. Environ Sci Technol 34(24):5084–5091
Reimann C, De Caritat P (2005) Distinguishing between natural and anthropogenic sources for elements in the environment: regional geochemical surveys versus enrichment factors. Sci Total Environ 337(1–3):91–107
Reimann C, Fabian K, Schilling J, Roberts D, Englmaier P (2015) A strong enrichment of potentially toxic elements (PTEs) in Nord-Trøndelag (central Norway) forest soil. Sci Total Environ 536:130–141. doi:10.1016/j.scitotenv.2015.07.032
Rosengarten D (2010) Les milieux calaminaires, la biodiversité au service du patrimoine. L’Erable 2:2–9
Singh R, Singh DP, Kumar N, Bhargava SK, Barman SC (2010) Accumulation and translocation of heavy metals in soil and plants from fly ash contaminated area. J Environ Biol 31(4):421–430
Soubrand-Colin M, Neel C, Bril H, Grosbois C, Caner L (2007) Geochernical behaviour of Ni, Cr, Cu, Zn and Pb in an Andosol–Cambisol climosequence on basaltic rocks in the French Massif Central. Geoderma 137(3–4):340–351. doi:10.1016/j.geoderma.2006.08.017
Sterckeman T, Douay F, Proix N, Fourrier H (2000) Vertical distribution of Cd, Pb and Zn in soils near smelters in the North of France. Environ Pollut 107(3):377–389
Sutherland RA (2000) Bed sediment-associated trace metals in an urban stream, Oahu, Hawaii. Environ Geol 39(6):611–627
WRB (2014) World reference base for soil resources 2014. International soil classification system for naming soils and creating legends for soil maps. World soil resources reports no. 106. FAO, Rome
Yongming H, Peixuan D, Junji C, Posmentier ES (2006) Multivariate analysis of heavy metal contamination in urban dusts of Xi’an, Central China. Sci Total Environ 355(1–3):176–186. doi:10.1016/j.scitotenv.2005.02.026
Yuan G-L, Sun T-H, Han P, Li J (2013) Environmental geochemical mapping and multivariate geostatistical analysis of heavy metals in topsoils of a closed steel smelter: Capital Iron & Steel Factory, Beijing, China. J Geochem Explor 130:15–21. doi:10.1016/j.gexplo.2013.02.010
Zu Y, Bock L, Schvartz C, Colinet G, Li Y (2014) Mobility and distribution of lead, cadmium, copper and zinc in soil profiles in the peri-urban market garden of Kunming, Yunnan Province, China. Arch Agron Soil Sci 60(1):133–149
Acknowledgments
Elodie Boutique, Aziz Silini and the technical team of the Laboratory of Soil Science participated either in fieldwork, sample preparation or soil analyses and are thanked for their contribution to the project. We also extend our thanks to all of the farmers who allowed us to collect samples in their fields.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liénard, A., Colinet, G. Assessment of vertical contamination of Cd, Pb and Zn in soils around a former ore smelter in Wallonia, Belgium. Environ Earth Sci 75, 1322 (2016). https://doi.org/10.1007/s12665-016-6137-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-016-6137-9