Abstract
The study investigated the adsorption characteristic of Cd(II), Zn(II) and Cu(II) ions and their mixture on activated sludge immobilized in 1.5 % sodium alginate with 0.5 % polyvinyl alcohol (ASAA), and the desorption of these metal ions from biosorbent using biosurfactants (saponin and JBR 515) and HNO3. Cadmium, zinc and copper were most effectively adsorbed by the immobilized sludge in a pH range of 5.0–6.0. Desorption of metals with saponin was the most effective at pH 1–5, with rhamnolipid JBR 515—at pH 5–6 and with HNO3 at pH 2.0. The results of adsorption and desorption were presented with the use of Freundlich, Langmuir, Redlich–Peterson, Sips and a double Langmuir adsorption isotherm equations. The coefficient of determination R 2 and the average relative error were used to evaluate whether the models fit to experimental data. The best fit of the adsorption isotherm equation to experimental data was demonstrated for the double Langmuir isotherm. The highest adsorption capacity from solutions containing single metals was obtained for Cd (108.83 mg/g dm). For solutions containing metal mixture, the highest adsorption capacity (37.21 mg/g dm) was obtained for Cu. The highest desorption of single metals was received with the use of saponin and HNO3 (over 90 %). Biosurfactants were shown to be the most effective in leaching of metals from their mixture. The desorption efficiency of saponin for each metal was over 99 %.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The generation of vast volumes of industrial wastewater, containing potentially toxic metals that are hazardous to the natural environment, is due to multiple technological processes applied in industrial plants. Wastewater involving metals is produced in mining, paper, photograph and tanning industries as well as during the production of fertilizers, pigments and batteries (Oliveira et al. 2010; Fu and Wang 2011). According to Volesky (2001), metals in wastewater discharged from industrial plants usually occur as a mixture, not as single elements, and pose a serious threat to the environment without appropriate treatment.
The necessity of environmental protection and economic concerns has recently made the removal of metals from industrial wastewaters a practical issue of high significance. Much attention is devoted globally to methods of biological removal of inorganic contaminants, particularly biosorption and bioaccumulation. The methods consist of metals absorption from aqueous solutions by dead or live biological materials (Chen et al. 2005; Bayramoglu and Arica 2008; Mane and Bhosle 2012).
Especially noteworthy is the use of biosorbents, owing to their biological origin and considerably lower acquisition costs compared to ion exchangers or filtration membranes. Biosorbents are readily available as they may include by-products of various industrial processes or natural materials excessively occurring in the environment, e.g., bacteria (Hassimi et al. 2012; Tabaraki et al. 2013), algae (Freitas et al. 2011; Plaza Cazón et al. 2013) or fungi (Cerino Córdova et al. 2011; Khani et al. 2012). An equally effective biosorbent is biomass of microorganisms which is a by-product from wastewater treatment using activated sludge (Khosravan and Lashkari 2011). It is constituted by different types of microorganisms, mainly of bacteria, fungi, yeast, algae and protozoa; it is readily available in wastewater treatment plants and has a high sorption potential (Kasan 1993; Kumar and Gayathri 2009; Ong et al. 2013).
The use of biosorption properties of biomass in a freely suspended form is limited owing to low mechanical resistance and dispersion proceeding during adsorption. Biomass immobilization eliminates these problems and increases biomass applicability. The immobilization of biomass often increases the efficiency of metal removal from solution and enables them to be recovered by the lowering of pH with mineral acids (HCl, H2SO4, HNO3) (Mata et al. 2009; Njikam and Schiewer 2012; Mishra 2014) or the use of complexing agents such as EDTA, DTPA and NTA (Wen et al. 2009; Hararah et al. 2012). The literature shows that the use of acids allows a high efficiency of the process, but can also cause a change in the structure of biosorbent, thereby reducing the amount of metal released. Complexing agents, however, are not biodegradable and often carcinogenic (Dermot et al. 2008). This leads to the search for other substances that will efficiently extract the metals without any detrimental effects on the structure of the biosorbent and the environment, e.g., surfactants and biosurfactants.
Compared to surfactants, the biosurfactants possess a higher capability to reduce surface and interphase tension and a critical micellar concentration in the range of 1.0 to 2000.0 mg/L (Wang and Mulligan 2004). In addition, they are characterized by specificity of action, high stability in a wide temperature range and possibility of multiple-re-application in washing out processes (Kosaric 2001). From the environmental point of view, their low toxicity and susceptibility to biodegradation are of key significance.
Most biosurfactants have anionic or neutral character, but only some of them—those containing amine groups—belong to cationic compounds (Mulligan et al. 2001). They owe their surface-active properties to a characteristic chemical structure. Their hydrophilic phase contains amino acids, peptides, anions, cations, mono-, di- and polysaccharides, whereas their hydrophobic phase is constituted by saturated and unsaturated fatty acids (Singh and Cameotra 2004). Initially, biosurfactants have been used to remove petroleum products from soils. However, a number of scientific reports about the possibility of their application for metal removal have been published recently (Wang and Mulligan 2009; Asci et al. 2010; Chen et al. 2011). Although investigations addressing adsorption and desorption of metals ions from biosorbents are necessary to better understand mobility and bioavailability of metals in the natural environment, studies describing desorption equilibrium are sparse.
In this study on the adsorption–desorption of Cd, Zn and Cu and their mixture, instead of a commonly applied alginate carrier (Bayramoğlu et al. 2006; Bayramoğlu and Arica 2009; Tam et al. 2009; Do and Lee 2013) we used a 3:1 mixture of alginate with polyvinyl alcohol (PVA) in order to increase the mechanical resistance of the applied immobilized activated sludge. Aqueous solutions of biosurfactants: saponin and JBR515 as well as nitric acid were used to test metals recovery from biosorbent.
The study include determination of parameters affecting adsorption and desorption processes of the analyzed metals, i.e., pH value of solution, time necessary to reach the equilibrium state and desorbent dose. The course of adsorption and desorption was characterized with the following adsorption isotherm equations: Freundlich’s, Langmuir’s, Redlich–Peterson’s, Sips’ and double Langmuir’s.
Methods
Materials
To study the adsorption/desorption of cadmium, zinc and copper, immobilized activated sludge in a mixture of sodium alginate and polyvinyl alcohol (ASAA) was used as a biosorbent. It was prepared according to the methodology specified by Kuczajowska-Zadrozna and Filipkowska (2016). Experiments on metals desorption from the immobilized biomass were conducted with the use of three desorbents: saponin (Sigma-Aldrich catalog no. 84510), JBR 515 (Jeneil Biosurfactant Co., LLC, USA) and nitric acid (Sigma-Aldrich). The CMC of the biosurfactants were saponin, 1206 mg/dm3, and JBR, 515—279 mg/dm3 (Kuczajowska-Zadrożna et al. 2015).
Optimization of adsorption conditions
Batch studies of biosorption were carried out with 50 mL of metal solution which contained metal at an initial concentration of 100 mg/L, and 0.29 g dm biosorbent. These studies were performed at 25 °C and pH ranging from 3.0 to 9.0. The pH was adjusted with diluted nitric acid and sodium hydroxide. The effect of contact time was examined with 500 mL of solution containing metal at an initial concentration of 300 mg/L and 2.9 g dm biosorbent, at 25 °C and optimized pH. Metal concentration was measured at 0, 5, 10, 15, 30, 60, 90 and 120 min. The adsorption was carried out on a shaker (Gerhard, Germany) operating at 180 revolutions per minute. Then, samples were centrifuged at 15,000 rpm for 10 min and the concentration of metals remaining in the solution after adsorption was marked. Adsorption experiments were repeated three times under identical conditions, and the final results are presented as the mean value.
Optimization of desorption conditions
Desorption studies were conducted with the biosorbent beads saturated with metals as follows. To the beaker placed on a magnetic stirrer, 5.8 g dm biosorbent (ASAA) was measured off and added 1L of a metal solution at an initial concentration of 100 mg/L (for each metal in mixture) or 300 mg/L (for single metal), and was left on the stirrer for 24 h. After sorption, biosorbent saturated with metals was washed several times with distilled water and then dried with filter paper. Desorbent dosage was optimized using saponin and JBR 515 at initial concentrations of 0–250 g/L, and nitric acid at 0–126 g/L. The effect of dose was studied with 0.29 g dm biosorbent saturated with metals in 50 mL of desorbents at the optimum concentrations at 25 °C for 120 min. The effect of pH was analyzed for saponin at pH ranging from 1 to 12. For the rhamnolipid JBR515, the size of the pH range was reduced to pH 6–12 because rhamnolipid precipitates in an acidic environment. The third desorbent was nitric acid, for which the measured pH ranged from 1.0 to 6.0. The effect of contact time was studied with 2.9 g dm biosorbent saturated with metals in 500 mL of solution at the previously determined desorbent concentration, 25 °C and optimized pH. Metal concentrations were measured at 0, 5, 10, 15, 30, 60, 90 and 120 min. The desorption experiments were repeated three times under identical conditions, and the results are presented as the mean value.
Determination of the maximum adsorption and desorption capacity
To determine the adsorption capacity of the ASAA, solutions of single metals or their mixtures at a 1:1:1 ratio were prepared in concentrations ranging from 1 to 600 mg/L. Then, 100 mL of these prepared solutions was added to 250-mL reaction flasks along with 8 g of the biosorbent. The flasks were shaken with a shaker for 2 h at 180 rpm. Then, the concentration of metals left in the solution after adsorption was determined. Next, the filtrated and rinsed biosorbent (1 g) saturated with metal was placed in a reaction vessel, which was next filled with 50 mL of a desorbent solution. Experiments were carried out with 5 % solutions of saponin and JBR 515, and with 1 M HNO3. The reaction flasks were shaken in a shaker for 2 h. Finally, analyses were conducted to determine the concentration of metals released from the biosorbent to the solution.
Analytical method
For AAS, analytical grade standard solutions of Cd, Cu and Zn (1.000 g/L) obtained from Sigma-Aldrich were used as stock solutions. All working solutions were prepared by diluting the stock solutions with deionized water. Solutions of 0.1 M HNO3 and 0.1 M NaOH were used for pH adjustment. The concentration of metals left in the aqueous solution of each sample was checked with the flame method using an AA 280FS atomic adsorption spectrometer by Varian. The measurements of metal concentration were performed at the following wavelengths (in nm): 228.8 (Cd), 213.9 (Zn) and 324.8 (Cu). Oxidant (air) and fuel (acetylene) flows were 13.5 and 1.5 L/min, respectively. Absorbance was recorded in triplicate to evaluate the reproducibility and the mean values were used for the concentration calculation (average error did not exceed 5 %). The blank experiments were always carried out for each study without biosorbent to check any deviation in the solute concentration.
Identification of functional groups and the type of the bonds was performed by Fourier transform infrared spectroscopy (FTIR) with a spectrometer with a mono-reflective ATR attachment. Before FTIR analysis, the sorbent was dehydrated with a hydraulic press. Dehydrated sample in a compressed form was placed in a diamond crystal of the ATR attachment and then down forced to the crystal with the “pressure screw.” The adsorption of the sample was determined at wavelengths 400–6000 cm−1, with a resolution of 2 cm−1. Each sample was scanned 64 times, and then, the mean value was calculated. The ATR crystal attachment was cleaned with acetone between the analyses of different sorbents.
Theory
The quantity of metal adsorbed from the solution was determined based on the change in the concentration of metal left in the solution and calculated according the following formula:
where Q s mass of metal adsorbed by the biosorbent (mg/g dm); C 0 initial concentration of metal in the solution (mg/L); C s metal concentration in the solution after adsorption (mg/L); m weight of biosorbent used (g dm); V volume of metal containing solution (L). The quantity of desorbed metal was computed from the following equation:
where Q d mass of metal desorbed from the biosorbent (mg/g dm); C d metal concentration in the solution after desorption (mg/L).To fit the equilibrium adsorption data, the Langmuir, Freundlich, Redlich–Peterson, Sips (Foo and Hameed 2010) and double Langmuir (Machida et al. 2004) isotherm equations were examined. They are given, respectively, by Eqs. (3–7):
where q max, b, K F, n, K R a R, q max1 q max2, b 1, b 2 are constants of isotherms and C s,d are metal concentration in the solution after adsorption/desorption, Q s,d mass of metal adsorbed/desorbed by/from the biosorbent.
Program STATISTICA 10.0 determined the degree to which the curves fit (with the determined constant) to the experimental data with the use of nonlinear estimation by the method of least squares, at a significance level of p < 0.05.
The minimization of the average relative error (ARE) was computed as follows in order to show how well the equilibrium models agree with experimental results (Cojocaru et al. 2009):
where, respectively, z is the number of data points; q exp and q calc are the experimental adsorption capacity and the adsorption capacity calculated with the theoretical models.
Results and discussion
FTIR analysis of biosorbent
FTIR spectra were obtained for activated sludge, polyvinyl alcohol (PVA) and sodium alginate, which were the components of the biosorbent shown in Fig. 1. For the activated sludge the spectroscopic FTIR analysis showed a wide adsorption of –OH group from 3660 to 3200 cm−1. The appearance of –OH groups in polyvinyl alcohol is shown in a wide band at 3600–3100 cm−1. Regarding to the sodium alginate, the vibration of –OH groups occur at a spectrum of 3660–3004 cm−1. In consequence, the peak at 2922–2852 cm−1 in case of activated sludge and 2900–2940 cm−1 referring to polyvinyl alcohol may be connected with –CH group. The occurrence of proteins in activated sludge can be observed by the band at 1639 cm−1, which is probably caused by stretching vibrations of COO, C=O and C–N. As a result of stretching vibrations of C=O of the acetate group of polyvinyl alcohol, the peak at 1713 cm−1 was created. The stretching vibrations of C=O of sodium alginate are indicated by the peak at 1602 cm−1. The peak at 1443 cm−1 for activated sludge points out vibrations of C–O and O–H. Carboxyl groups in a molecule of sodium alginate are probable to appear according to vibrations of COO– groups in asymmetric adsorption band at 1403 cm−1. The spectrum of activated sludge also showed a peak at 1004 cm−1 which indicates the occurrence of uronic acids and the peak at 827 cm−1 is the evidence of existence of functional groups containing phosphorus and sulfur. The adsorption band at 1141–1096 cm−1 in polyvinyl alcohol was most likely connected with the occurrence of C–O functional groups. The occurrence of –COC groups in sodium alginate is shown by the vibration band at 1035 cm−1. Others obtained the same functional groups with comparable length of the spectra as in our study (Table 1).
Effect of doses of desorbents
Figure 2 shows the efficiency of metal leaching from biosorbent as a function of the desorbent dosage. The optimal dose of saponin and rhamnolipid JBR 515 was at 5 g/100 mL (≈5 % solution) (Fig. 2a, b). Aşci et al. (2008) investigated washing of Zn from Na-feldspar as soil component using rhamnolipid JBR 425 and showed that the dosage of 3 % was the most effective for Zn recovery (85.3 %).
In case of HNO3, the amount of desorbed metal did not depend on the dose (Fig. 2c) and was the highest among the used desorbents. The optimal dose of nitric acid was 6.3 g/100 mL (1 M solution), at which the amount of removed metals was in the range from 60.7 (Cu) to 66.0 mg/g dm (Zn).
Among the investigated biosurfactants, saponin effectively desorbed more metals than the rhamnolipid JBR 515—in case of copper more than 5 times and more than 3 times regarding other metals. Increase in the saponin and JBR515 dose up to 5 g/100 mL caused rise in the amount of metals desorbed (Fig. 2a, b). Further increase in the dose did not result in an increase in desorbed metal amount. Hong et al. (2002) reported similar correlation in research of Cd, Cu, Zn and Pb desorption from three different types of soils (Andosol: soil A, Cambisol: soil B, Regosol: soil C), which were washed with saponin. Metal removal efficiency in soil C raised successively in the range of 0.1–3 g/100 mL. Further increase in saponin dose up to 10 % did not lead to better removal of metals.
Most authors consider that increase in biosurfactant dosage in cleaning solution above the determined CMC value is required for the micellization process to occur due to adsorption of biosurfactant by sorbent (Liu et al. 1992; Schippers et al. 2000). The previous research performed by Kuczajowska-Zadrożna et al. (2015) has shown that considering the CMC value as criterion to establish the minimal biosurfactant concentration in cleaning solutions (%), the minimal concentration of saponin and JBR 515 rhamnolipid should be 0.071 and 0.0063 %, respectively. Taking the biosurfactant adsorption on the tested biosorbent into account, the solution of saponin and JBR 515 rhamnolipid in the cleaning solution should be at least 0.1 and 0.025 %, respectively. In desorption of Cu and Ni from kaolin Chen et al. (2008) considered adsorption of saponin on kaolin and estimated the dose of the biosurfactant as 2.0 g/100 mL in a pH range of 5–8.
Effect of pH on adsorption and desorption
Adsorption
As the literature shows, each system consisting of an aqueous solution of adsorbent with metal ions is characterized by pH that enables maximum adsorption by the adsorbent (Rajaei et al. 2013; Meitei and Prasad 2013).
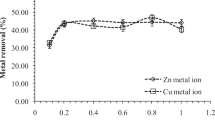
Figure 3 shows that cadmium, zinc and copper were most effectively adsorbed by the immobilized sludge in a pH range from 5.0 to 6.0, reaching 94, 83 and 91 % on average, respectively. Reduction of the pH value to 3.0 diminished the adsorption of the analyzed metals by about 30 % on average. With zinc and copper, adsorption efficiency also decreased as pH increased to 9.0. Therefore, pH values at the optimal range of 5.0–6.0 were used in further experiments in this study.
Desorption
Figure 4 shows how the efficiency of metal desorption changed with pH. Saponin and nitric acid desorbed all three metals (Cd, Zn and Cu) most effectively. Saponin was the most efficient in desorbing the metals at pH 1–5 (over 90 % efficiency). HNO3 was most efficient at pH 2 (over 90 % desorption capability). Increase of pH to 12 caused a large decrease in efficiency of all tested desorbents, especially in desorption of cadmium. At pH 12 saponin reached an efficiency of 6.4 %; HNO3 of 0.4 %. Although JBR 515 could not be used below pH of 5 because it would precipitate from the solution, changes in pH from 5 to 12 had smaller effect on its efficiency than on saponin and HNO3. JBR 515 most efficiently desorbed all three metals in a pH range 5–6. Zn was most efficiently desorbed at all pH values (59–50 %; pH 5–12), whereas desorption ability of Cd decreased from 24 to 3.3 % as pH was increased from 5 to 12.
Analysis of the impact of time and adsorption and desorption
Figure 5 presents the mass of single metal adsorbed and desorbed from the biosorbent (Q and Q d). The time necessary to reach the equilibrium concentration in the adsorption process depended on the type of metal. Copper was the fastest (15 min), with 50 mg/g dm removed. Within the same time span, the adsorption of zinc was similar (51.5 mg/s dm), and that of cadmium was lower (32.9 mg/g dm). Cadmium did not reach the equilibrium after 120 min. When using 1 M HNO3 to remove zinc from the biosorbent, the time to reach equilibrium was 15 min. With other combinations of biosurfactants and metals, the time needed to reach equilibrium ranged from 30 to 120 min (Fig. 5a–c).
Determination of the maximum adsorption and desorption capacity
From the economic point of view, a sorbent should exhibit both good adsorption properties (i.e., be characterized by a high adsorption capacity) and the high recovery of adsorbed substances.
The immobilization of activated sludge in sodium alginate aimed at producing an adsorbent that would be characterized by both: good adsorption properties and high desorption efficiency. The adsorption isotherms of metals were interpreted using five adsorption isotherm equations: Langmuir, Freundlich, Redlich–Peterson, Sips and double Langmuir. The R 2 coefficient and the average relative error (ARE), which was computed according to Cojocaru et al. (2009), were adopted as measures of model fits to the experimental data (with the determined parameters). Based on the obtained R 2 values it was difficult to evaluate the suitability of adsorption isotherm equations for the description of experimental data. Values of R 2 were very high for all adsorption isotherm equations, i.e., higher than 0.99 for Langmuir, Redlich–Petersen, Sips and double Langmuir equations and insignificantly lower though still high (over 0.98) for Freundlich equation (Fig. 6).
Considerably more useful in this respect was shown to be the average relative error (ARE). Its values were more variable that enabled to select the most suitable equation for the description of experimental adsorption isotherms (Fig. 7). The least suitable equations appeared to be those with two adjustable parameters, i.e., Freundlich and Langmuir equations, for which the values of ARE were the highest. The analysis of the ARE values enables concluding that the four-parameter double Langmuir equation was the most suitable for the description of Cd, Zn and Cu adsorption and desorption on the ASAA in the case of both single metals as well as their mixture. The determined ARE values were the lowest irrespective of the type of metals and desorbent applied. Montazer-Rahmatia et al. (2011) studied biosorptive removal of Cd (II), Ni (II) and Pb(II) ions by both intact and pre-treated brown marine algae. The equilibrium experimental data were tested using the most common adsorption isotherms. In the study, the relationship between metal biosorption capacity and metal equilibrium concentration in solution has been described by four two-parameter adsorption isotherm equations: Langmuir, Freundlich, Temkin and Dubinin–Radushkevich and five three-parameter isotherm equations: Toth, Khan, Sips, Redlich–Peterson and Radke–Prausnitz. Adjustment of the equations to experimental data was evaluated on the basis of the constants—residual root mean square error (RMSE), Chi-square (χ 2) and coefficient of determination (R 2). The results were fitted best with the Freundlich equation among two-parameter equations and the Toth, Khan and Radke–Prausnitz equations among three-parameter isotherm equations. Hajahmadi et al. (2015) investigated biosorption of Zn(II), Co(II) and Cd(II) ions onto pre-treated dried Aspergillus niger in batch system. The adsorption data were fitted to the multicomponent Langmuir, Freundlich, Temkin and Sips equations. The experimental data were fitted best with the Temkin adsorption isotherm for Zn(II) and Co(II), and the Langmuir isotherm for Cd(II).
Figures 8 and 9 present the results as well as adsorption and desorption isotherms for single metal and their mixture for which we achieved the best match of the model (double Langmuir isotherms) to experimental data. The adsorption/desorption constants determined from the double Langmuir equation equations for cadmium, zinc and copper adsorption/desorption from aqueous solutions containing single metals and their mixture are presented in Table 2. The highest adsorption capacity from solutions containing single metal was obtained for Cd (108.83 mg/g dm), followed by Cu (81.23 mg/g dm) and Zn (77.93 mg/g dm). A similar dependence was obtained by Pietrobelli et al. (2009), who studied the adsorption of the same metal on non-living Egeria densa biomass. Designated from Langmuir isotherm equation, adsorption capacity amounted to Cd (140.5 mg/g dm), Cu (90.9 mg/g dm) and Zn (60.8 mg/g dm). On the contrary, Areco et al. (2012) studied adsorption of Cd, Zn and Cu using dead biomasses of green alga Ulva lactuca. The highest adsorption capacity determined from Langmuir isotherm equation obtained for Cu. The adsorption capacities were: 54.0 mg/g dm for Cu, 46.1 mg/g dm for Cd and 22.9 mg/g dm for Zn.
From the solution containing mixture of three metals the highest adsorption capacity of 37.21 mg/g dm was obtained for Cu whereas the adsorbed amounts of Cd and Zn were comparable but lower than for Cu. Studies have also shown that the presence of one metal in the mixture reduces the sorption capacity of the other metal. This may indicate a competence sorption. A similar pattern was concluded by Chong and Volesky (1995) during the biosorption of copper, cadmium and zinc from mixtures at pH 4.5 with Ascophyllum nodosum.
The amount of desorbed metal depended on the used desorbent. The highest amount of desorbed metal from biosorbent after single metal adsorption was achieved for Zn (69.8 mg/g dm) and Cu (81.0 mg/g dm) using saponin. The lowest efficiency of desorption occurred for JBR515 irrespective of the type of metal from 18.6 mg/g dm (Cd) to 38.6 mg/g dm (Zn). Efficiency of desorption for mixture of metals also depended on the type of desorbents. Regardless of the type of metal, the highest efficiency among the tested desorbents was achieved for the saponin desorption (Table 2). Analyzing the desorption constants b 1 and b 2 from the double Langmuir equation for metal mixture, the value of b 1 was always higher than b 2 regardless to the desorbent type. Analogical relationship (b 1 > b 2) for desorption constants was concluded only for zinc when biosorbent was saturated by a single metal.
It may be speculated that owing to the presence of numerous functional groups in the biosorbent that were responsible for metal binding, the adsorption was more complex. For this reason, double Langmuir model was shown be the most useful, which assumed various mechanisms of metal binding in the biosorbent.
Figure 10 presents the desorption from biosorbents determined based on the constants from the double Langmuir equation. Desorption was run in two variants: (1) after adsorption from solutions of single metal (Fig. 10a) and (2) after adsorption from the mixture of three metals (Fig. 10b). Obtained data demonstrate the nitric acid to be the most effective desorbent in the first variant. The efficiency of metal desorption to the solution using HNO3 was the highest for all analyzed metals and ranged from 81.9 to 99.8 %. In the case of the biosurfactants, the highest efficiency of metal desorption in the solution was noted for saponin, i.e., 89.5 and 99.8 % for Zn and Cu, respectively. Different results were obtained in the second variant. Metal desorption from the analyzed biosorbent was very high upon the use of both biosurfactants. For saponin, the efficiency of metal desorption was within the range from 99.6 to 99.9 %, while for JBR 515 from 90.9 to 93.6 % (Fig. 10b). Nitric acid was weaker desorbent than both biosurfactants. In the nitric acid case, the efficiency of desorption did not exceed 90.0 % for any of the analyzed metals and ranged from 63.5 to 89.0 % (Fig. 10b).
Metal bonding occurs as a result of the following mechanisms: chemisorption, complexation, adsorption and absorption, ion exchange, chelation, physical sorption and occlusion in capillaries and the inner network of polysaccharides due to concentration gradients. In our experiment, Cd, Zn and Cu were adsorbed through interactions with functional groups of the sludge immobilized in a mixture of alginate and PVA. Based on FTIR analysis, the biosorbent contained some types of bonds and functional groups such as –OH, C=O–, COO–, –COC, –CH, C=O, C–O, –CH, COO, C–N and acknowledges the occurrence of proteins, uronic acids and functional groups containing phosphorus and sulfur in activated sludge.
The presence of acid groups is particularly important because it enhances the exchangeable properties of the biosorbent. In aqueous solution, these groups were ionized, which led to formation of negatively charged active sites able to adsorb metal cations. In aqueous solution, the metal salt was also ionized and hydrolyzed into free and complex (cationic and anionic) metal forms:
Positively charged H+ ions and metal cations competed for negatively charged active sites on the biosorbent surface.
The adsorption pH is one of the most important factors affecting the efficiency of metal bonding. This was confirmed in the present study. Cadmium, zinc and copper were most effectively adsorbed by the immobilized sludge in a pH range of 5.0–6.0. Decrease of pH to 3.0 diminished the adsorption of all three metals. With zinc and copper, adsorption efficiency also decreased when pH was increased up to 9.0.
Metal desorption from the biosorbent may also be a consequence of ionic exchange or precipitation–solubilization. Metals may form complexes with biosurfactants on the biosorbent’s surface. As a result of reduced interfacial tension, these complexes detach from the surface and migrate to the solution. This mechanism is confirmed by the high efficiency of Cd, Zn and Cu desorption from the analyzed biosorbent using saponin and JBR 515. The lower efficiency of metals desorption by biosurfactants after the adsorption from solutions of single metals may result from other, stronger binding of metals with the biosorbent.
Industrial wastewaters usually contain a mixture of metals. Biosurfactants, saponin in particular, seem to be effective desorbents. They may be applied in wastewater pre-treatment facilities located at the production plants where wastewaters are treated with the adsorption method. The application of nitric acid for metals recovery is effective only in case of previous adsorption of single metal. For mixtures of metals, the usage of nitric acid is far less effective. Low pH of the solution after desorption would require further processes and actions.
Conclusions
The efficiency of Cd, Cu and Zn adsorption and desorption using three desorbents (saponin, JBR 515 and HNO3) was observed to depend on pH values. In the analyzed pH range of the adsorption process, optimal pH range was from 5.0 to 6.0. Desorption of metals with saponin was the most effective at pH 1–5 and with rhamnolipid JBR 515 at pH 5–6.
The results were analyzed with five adsorption isotherm equations: two di-parameter ones—Freundlich and Langmuir; two three-parameter ones: Redlich–Peterson and Sips; and one four-parameter isotherm—double Langmuir isotherm. The coefficient of determination R 2 and the average relative error ARE were used to evaluate models fit to experimental data. The best fit of the model to experimental data was demonstrated for the double Langmuir isotherm.
The maximum adsorption capacity (q max) of the biosorbent, when the adsorbate contained a single metal, was the highest for cadmium (108.83 mg/g dw). In the case of the metal mixture, the total adsorption capacity for three metals was lower. The highest adsorption capacity was determined for copper (37.38 mg/g dw). For the other two metals, the adsorption capacity reached 16.88 mg/g dm for Cd and 11.91 mg/g dm for Zn.
The high desorption of single metals was obtained with HNO3 and saponin (over 90 % for zinc and cadmium). The most effective, when three metals were adsorbed in the biosorbent, were biosurfactants, i.e., saponin and JBR 515. In the case of saponin, desorption efficiency reached up to 99 % for each metal.
References
Areco MM, Hanela S, Duran J, Afonso MS (2012) Biosorption of Cu(II), Zn(II), Cd(II) and Pb(II) by dead biomasses of green alga Ulva lactuca and the development of a sustainable matrix for adsorption implementation. J Hazard Mater 213–214:123–132. doi:10.1016/j.jhazmat.2012.01.073
Aşci Y, Nurbaş M, Açikel YS (2008) Removal of zinc ions from a soil component Na-feldspar by a rhamnolipid biosurfactant. Desalination 223:361–365. doi:10.1016/j.desal.2007.01.205
Aşci Y, Nurbaş M, Açikel YS (2010) Investigation of sorption/desorption equilibria of heavy metal ions on/from quartz using rhamnolipid biosurfactant. J Environ Manage 91:724–731. doi:10.1016/j.jenvman.2009.09.036
Bayramoğlu G, Arica MY (2009) Construction a hybrid biosorbent using Scenedesmus quadricauda and Ca-alginate for biosorption of Cu(II), Zn(II) and Ni(II): kinetics and equilibrium studies. Bioresour Technol 100:186–193. doi:10.1016/j.biortech.2008.05.050
Bayramoğlu G, Arıca MY (2008) Removal of heavy mercury(II), cadmium(II) and zinc(II) metal ions by live and heat inactivated Lentinus edodes pellets. Chem Eng J 143:133–140. doi:10.1016/j.cej.2008.01.002
Bayramoğlu G, Tuzun I, Celik G, Yilmaz M, Arica MY (2006) Biosorption of mercury(II), cadmium(II) and lead(II) ions from aqueous system by microalgae Chlamydomonas reinhardtii immobilized in alginate beads. Int J Miner Process 81:35–43. doi:10.1016/j.minpro.2006.06.002
Cerino Córdova FJ, García León AM, Garcia Reyes RB, Garza González MT, Soto Regalado E, Sánchez González MN, Quezada López I (2011) Response surface methodology for lead biosorption on Aspergillus terreus. Int J Enviro Sci Technol 8(4):695–704
Chen J, Tao X, Xu J, Zhang T, Liu Z (2005) Biosorption of lead, cadmium and mercury by immobilized Microcystis aeruginosa in a column. Process Biochem 40:3675–3679. doi:10.1016/j.procbio.2005.03.066
Chen W-J, Hsiao L-Ch, Chen KK-Y (2008) Metal desorption from copper(II)/nickel(II)-spiked kaolin as a soil component using plant-derived saponin biosurfactant. Process Biochem 43:488–498. doi:10.1016/j.procbio.2007.11.017
Chen H, Chen C, Reddy AS, Chen C, Li WR, Tseng M, Liu H, Pan W, Maity JP, Atla SB (2011) Removal of mercury by foam fractionation using surfactin, a biosurfactant. Inter J Mol Sci 12(11):8245–8258. doi:10.3390/ijms12118245
Chong KH, Volesky B (1995) Description of two-metal biosorption equilibria by langmuir-type models. BiotechBioeng 47:451–460
Cojocaru C, Diaconu M, Cretescua I, Savić J, Vasić V (2009) Biosorption of copper(II) ions from aqua solutions using dried yeast biomass. Colloids Surf A 335:181–188. doi:10.1016/j.colsurfa.2008.11.003
Dermot G, Bergeron M, Mercier G, Richer-Laflèche M (2008) Soil washing for metal removal: a review of phisical/chemical technologies and field applications. J Hazard Mater 152:1–31. doi:10.1016/j.jhazmat.2007.10.043
Do X, Lee B (2013) Removal of Pb2+ using a biochar-alginate capsule in aqueous solution and capsule regeneration. J Environ Manage 131:375–382. doi:10.1016/j.jenvman.2013.09.045
Foo KY, Hameed BH (2010) Insights into the modeling of adsorption isotherm systems. Chem Eng J 156:2–10. doi:10.1016/j.cej.2009.09.013
Freitas APP, Schneider IAH, Schwartz Bold A (2011) Biosorption of heavy metals by algal communities in water streams affected by the acid mine drainage in the coal-mining region of Santa Catarina state, Brazil. Min Eng 24(11):1215–1218. doi:10.1016/j.mineng.2011.04.013
Fu F, Wang Q (2011) Removal of heavy metal ions from wastewaters: a review. J Environ Manage 92:407–418. doi:10.1016/j.jenvman.2010.11.011
Gulnaz O, Kaya A, Dincer S (2006) The reuse of dried activated sludge for adsorption of reactive dye. J Hazard Mater 134(1):190–196. doi:10.1016/j.jhazmat.2005.10.050
Hajahmadi Z, Younesi H, Bahramifar N, Khakpour H, Pirzadeh K (2015) Multicomponent isotherm for biosorption of Zn(II), CO(II) andCd(II) from ternary mixture on to pretreated dried Aspergillus niger biomass. Water Res Ind 11:71–80. doi:10.1016/j.wri.2015.07.003
Hararah AM, Al-Nasir F, El-Hasan T, Al-Muhtaseb AH (2012) Zinc adsorption–desorption isotherms: possible effects on the calcareous vertisol soils from Jordan. Environ Earth Sci 65:2079–2085. doi:10.1007/s12665-011-1188-4
Hassimi HA, Siti RSA, Noorhisham TK, Siti KK (2012) Isotherm equilibria of Mn+2 biosorption in drinking water treatment by locally isolated Bacillus species and sewage activated sludge. J Environ Manage 111:34–43. doi:10.1016/j.jenvman.2012.06.027
Hong KJ, Tokunaga S, Kajiuchi T (2002) Evaluation of remediation process with plant-derived biosurfactant for recovery of heavy metals from contaminated soils. Chemosphere 49:379–387. doi:10.1016/S0045-6535(02)00321-1
Kasan HC (1993) The role of waste activated sludge and bacteria in metal-ion removal from solution. Crit Rev Environ Sci Technol 23:79–117. doi:10.1080/10643389309388442
Khani MH, Pahlavanzadeh H, Alizadeh K (2012) Biosorption of strontium from aqueous solution by fungus Aspergillus terreus. Environ Sci Pollut Res 19:2408–2418. doi:10.1007/s11356-012-0753-z
Khosravan A, Lashkari B (2011) Adsorption of Cd(II) by Dried Activated Sludge. Iranian J Chem Eng 8(2):41–56
Kosaric N (2001) Biosurfactant their application for soil bioremediation. Food Technol Biotechnol 39(4):295–301
Kuczajowska-Zadrożna M, Filipkowska U (2016) Kinetics of desorption of heavy metals and their mixtures from immobilized activated sludge. Desalin Water Treat 57:9396–9408. doi:10.1080/19443994.2015.1031708
Kuczajowska-Zadrożna M, Filipkowska U, Jóźwiak T (2015) Application of biosurfactants for heavy metals leaching from immobilized activated sludge. Arch Environ Prot 41(1):43–52. doi:10.1515/acp-2015-0006
Kumar PS, Gayathri R (2009) Adsorption of Pb2+ ions from aqueous solutions onto bael tree leaf powder: isotherms kinetics and thermodynamics study. J Eng Sci Technol 4(4):381–399
Liu Z, Edwards DA, Luthy RG (1992) Sorption of non-ionic surfactants onto soil. Water Res 26:1337–1345. doi:10.1016/0043-1354(92)90128-Q
Machida M, Kikuchi Y, Aikawa M, Tatsumoto H (2004) Kinetics of adsorption and desorption of Pb(II) in aqueous solution on activated carbon by two-site adsorption model. Colloids Surf A 240(1–3):179–186. doi:10.1016/j.colsurfa.2004.04.046
Mane PC, Bhosle AB (2012) Bioremoval of some metals by living algae Spirogyra sp. and Spirullina sp. from aqueous solution. Int J Environ Res 6(2):571–576
Mansur HS, Costa HS (2008) Nanostructured poly(vinyl alcohol)/bioactive glass and poly (vinyl alcohol)/chitosan/bioactive glass hybrid scaffolds for biomedical applications. Chem Eng J 137(1):72–83. doi:10.1016/j.cej.2007.09.036
Mata YN, Blázquez ML, Ballester A, González F, Muñoz JA (2009) Sugar-beet pulp pectin gels as biosorbent for heavy metals: preparation and determination of biosorption and desorption characteristics. Chem Eng J 150:289–301. doi:10.1016/j.cej.2009.01.001
Meitei MD, Prasad MNV (2013) Lead (II) and cadmium (II) biosorption on Spirodela polyrhiza (L.) Schleiden biomass. J Environ Chem Eng 1:200–207. doi:10.1016/j.jece.2013.04.016
Mishra SP (2014) Adsorption–desorption of heavy metal ions. Curr Sci 107(4):601–612
Montazer-Rahmatia MM, Rabbania P, Abdolalia A, Keshtkarb AR (2011) Kinetics and equilibrium studies on biosorption of cadmium, lead, and nickel ions from aqueous solutions by intact and chemically modified brown algae. J Hazard Mater 185:401–407. doi:10.1016/j.jhazmat.2010.09.047
Mulligan CN, Yong RN, Gibbs BF (2001) Surfactant-enhanced remediation of contaminated soil: a review. Eng Geol 60:371–380
Njikam E, Schiewer S (2012) Optimization and kinetic modeling of cadmium desorption from citrus peels: a process for biosorbent regeneration. J Hazard Mater. doi:10.1016/j.jhazmat.2012.01.084
Oliveira FD, Soares AC, Freitas M, Figueiredo AS (2010) Copper, nickel and zinc removal by peanut hulls: batch and column studies in mono, tri-component systems and with real effluent. Global NEST J 12(2):206–214
Ong S, Toorisaka E, Hirata M, Hano T (2013) Comparative study on kinetic adsorption of Cu(II), Cd(II) and Ni(II) ions from aqueous solutions using activated sludge and dried sludge. Appl Water Sci 3:321–325
Pietrobelli JMTA, Módenes AN, Fagundes-Klen MR, Espinoza-Quiñones FR (2009) Cadmium, copper and zinc biosorption study by non-living Egeria densa biomass. Water Air Soil Pollut 202:385–392. doi:10.1007/s11270-009-9987-x
Plaza Cazón J, Viera M, Donati E, Guibal E (2013) Zinc and cadmium removal by biosorption on Undaria pinnatifida in batch and continuous processes. J Environ Manage 129:423–434. doi:10.1016/j.jenvman.2013.07.011
Rajaei GE, Aghaie H, Zare K, Aghaie M (2013) Adsorption of Cu(II) and Zn(II) ions from aqueous solutions onto fine powder of Typha latifolia L. root: kinetics and isotherm studies. Res Chem Intermed 39:3579–3594. doi:10.1007/s11164-012-0864-7
Schippers C, Geßner K, Muller T, Scheper T (2000) Microbial degradation of phenenthrene by addition of a sophorolipid mixture. J Biotech 83:189–198. doi:10.1016/SO168-1656(00)00304-7
Singh P, Cameotra SS (2004) Enhancement of metal bioremediation by use of microbial surfactants. Biochem Biophys Res Commun 319:291–297
Song WJ, Pan X, Zhang D (2012) Lead complexation of soluble and bound extracellular polymeric substances from activated sludge: characterized with fluorescence spectroscopy and FTIR spectroscopy. Biotechnol Biotechnol Equip 26(6):3371–3377
Swamy BY, Chang JH, Ahn H, Lee WK, Chung I (2013) Thermoresponsive N-vinyl caprolactam grafted sodium alginate hydrogel beads for the controlled release of an anticancer drug. Cellulose 20(3):1261–1273. doi:10.1007/s10570-013-9897-3
Tabaraki R, Ahmady-Asbchin S, Abdi O (2013) Biosorption of Zn(II) from aqueous solutions by Acinetobacter sp. isolated from petroleum spilled soil. J Environ Chem Eng 1:604–608. doi:10.1016/j.jece.2013.06.024
Tam NFY, Wong YS, Wong MH (2009) Novel technology in pollutant removal at source and bioremediation. Ocean Coast Manag 7:368–373. doi:10.1016/j.ocecoaman.2009.04.009
Volesky B (2001) Detoxification of metal-bearing effluents: biosorption for the next century. Hydrometallurgy 59:203–216
Wang S, Mulligan CN (2004) An evaluation of surfactant foam technology in remediation of contaminated soil. Chemosphere 57:1079–1089
Wang S, Mulligan CN (2009) Rhamnolipid biosurfactant-enhanced soil flushing for the removal of arsenic and heavy metals from mine tailings. Process Biochem. doi:10.1016/j.procbio.2008.11.006
Wen J, Stacey SP, McLaughlin MJ, Kirby JK (2009) Biodegradation of rhamnolipid, EDTA and citric acid in cadmium and zinc contaminated soils. Soil Biol Biochem 41(10):2214–2221. doi:10.1016/j.soilbio.2009.08.006
Acknowledgments
This research was financially supported by the Grant: KBN NN523 452936 (Poland).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Filipkowska, U., Kuczajowska-Zadrożna, M. Investigation of the adsorption/desorption equilibria of Cd(II), Zn(II) and Cu(II) ions on/from immobilized digested sludge using biosurfactants. Environ Earth Sci 75, 814 (2016). https://doi.org/10.1007/s12665-016-5674-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-016-5674-6