Abstract
The adsorption of Cu(II), Cd(II) and Ni(II) ions from aqueous solutions by activated sludge and dried sludge was investigated under laboratory conditions to assess its potential in removing metal ions. The adsorption behavior of metal ions onto activated sludge and dried sludge was analyzed with Weber–Morris intra-particle diffusion model, Lagergren first-order model and pseudo second-order model. The rate constant of intra-particle diffusion on activated sludge and dried sludge increased in the sequence of Cu(II) > Ni(II) > Cd(II). According to the regression coefficients, it was observed that the kinetic adsorption data can fit better by the pseudo second-order model compared to the first-order Lagergren model with R2 > 0.997. The adsorption capacities of metal ions onto activated sludge and dried sludge followed the sequence Ni(II) ≈ Cu(II) > Cd(II) and Cu(II) > Ni(II) > Cd(II).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metal ions such as Cu(II), Ni(II), Zn(II), Cd(II) and Pb(II) are among the toxic inorganic pollutants that may cause environmental problem, although at low concentration in surface or subsurface water. The conventional method for removing these toxic metal ions from aqueous solution is by chemical precipitation and the major drawback of this method is the generation of huge amount of chemical sludge. Adsorption is a simple treatment method for removing metal ions in wastewater, and activated carbon is a powerful adsorbent that is commonly used in industrial wastewater treatment plant. There are a lot of researches on the derivation of novel adsorbents from various materials such as poly (vinyl alcohol)/chitosan bead (Li et al. 2011), ion exchange resin (Rengaraj et al. 2003), aminated and protonated mesoporous alumina (Rengaraj et al. 2004), polyrhodanine-encapsulated magnetic nanoparticles (Song et al. 2011), etc.
The high cost of activated carbon limits its uses as adsorbent and this has led to a wide study on the derivation of adsorbent from low cost and easily available raw materials. Using living and non-living biomass is an innovative and alternative technology for removing these inorganic pollutants (Ahluwalia and Goyal 2007). The metal ions can be retained by some of the microbial biomass through the interaction between the positively charged metal ions and the binding sites on the cellular wall of microbial biomass (Manriquez et al. 1997). The exocellular polymeric substances (EPS) in microbial biomass consist of various organic substances such as proteins, polysaccharides, lipids, nucleic acids, etc. (Wingender et al. 1999; Ras et al. 2008). Removal of the metal ions from the cellular surface of microbial biomass could be attributed to physical adsorption, complexation or chemical adsorption (Ting et al. 1991). Zhou et al. (2007) reported that the maximum adsorption capacity of Cr(VI) from aqueous solution on the dead cells of Bacillus licheniformis was 69 mg/g. The high adsorption capacities of Cd(II) and Cr(VI) ions by Staphylococcus xylosus and Pseudomonas sp. were studied by Ziagova et al. (2007).
In this work, activated sludge and dried sludge were used as adsorbents for removing Cu(II), Cd(II) and Ni(II) from aqueous solution. As we know, activated sludge is a commonly used biological treatment process for removing organic matters present in the wastewater. The excess biological sludge waste from the biological reactor can be used as a low cost adsorbent for metal ion removal. The adsorption behavior of heavy metals on activated sludge and dried sludge was analyzed with Weber–Morris intra-particle diffusion model, Lagergren first-order model and pseudo second-order model.
Materials and methods
Adsorbent preparation
A laboratory scale sequencing batch reactor (SBR) was used to provide activated sludge biomass for heavy metal adsorption. The reactor was operated with FILL, REACT, SETTLE, DRAW, and IDLE periods in the time ratio of 0.5:3.5:1.0:0.75:0.25 for a cycle time of 6 h. The activated sludge seed was obtained from a municipal wastewater treatment plant and then acclimatized in laboratory by feeding it with a synthetic wastewater consisting of a base mixture of peptone, sucrose, nutrients and buffer solution.
In each cycle, 3 l of synthetic wastewater was added in the SBR during FILL period and the same amount of treated effluent was removed during DRAW period to maintain a residual volume of 2 l before the start of a new cycle. The activated sludge used for adsorption study was collected directly from SBR, whereas the dried sludge was prepared by washing the activated sludge with tap water and distilled water, filtered and then dried in oven at 105 °C.
Adsorption study
The adsorption experiment was conducted in a similar manner as described in our earlier report (Ong et al. 2010). This study was conducted to determine the adsorption of Cu(II), Cd(II) and Ni(II) on activated sludge and dried sludge. 0.05 g of adsorbent was mixed with 100 ml of metal solutions in plastic bottles. The plastic bottles were agitated on a thermostat shaker bath and sampled at different time intervals. Then, the solution was centrifuged at the speed of 240 rpm, filtrated, and then the supernatant was analyzed for heavy metals concentration using ICPS-7000 (Shimadzu).
Results and discussion
Adsorption time
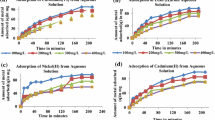
The adsorption time of Cu(II), Cd(II) and Ni(II) ions onto the activated sludge and dried sludge was obtained by monitoring the decrease of metal ion concentration in aqueous solution with time. As shown in Fig. 1, the concentration of Cu(II), Ni(II) and Cd(II) ions decreased rapidly during the first 1 h and then remained nearly constant after 3 h of adsorption. After this equilibrium time, the amount of heavy metals adsorbed did not significantly change with time. The adsorption behavior of Cu(II), Cd(II) and Ni(II) by activated sludge and dried sludge followed the two-step reaction model proposed by Cheng et al. (1975). The drastic removal of metal ions in the first 1 h was ascribed to the adsorption of large quantity metal ions onto the cell surface, followed by slower second phase which may extend over many hours and is dependent on the viability of the sludge (Cheng et al. 1975; Ong et al. 2004, 2005).
Weber–Morris intra-particle diffusion model
The Weber–Morris (1963) kinetic model is used to evaluate the intra-particle diffusion and to determine the rate determining step in adsorption process.
where %R is the percentage amount of adsorbed substance, t is the contact time between adsorbate and adsorbent, K is the adsorption rate constant, and m is a constant related to intra-particle diffusion.
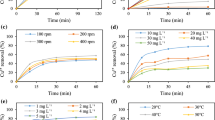
As shown in Fig. 2, the plot of Weber–Morris model given a straight line to the data obtained with R2 greater than 0.8. This shows the adsorption of Cu(II), Ni(II) and Cd(II) ions onto the activated sludge and dried sludge was through the intra-particle diffusion process. If m = 0.5, then intra-particle diffusion would be the rate determining step. As shown in Table 1, all of the values of m are much lower than 0.5 indicating that the intra-particle diffusion was not the rate determining step for the adsorption of Cu(II), Ni(II) and Cd(II) onto the activated sludge and dried sludge. The adsorption rate constant, log K, can be used as a relative measure of adsorption rate. The rate constant of intra-particle diffusion on activated sludge and dried sludge followed the sequence Cu(II) > Ni(II) > Cd(II), which is agrees with the order obtained in adsorption capacity of the adsorbents for the metal ions according to Langmuir and Freundlich isotherms (Ong et al. 2010). In our previous report, the maximum adsorption capacity of Cu(II), Ni(II) and Cd(II) ions onto activated sludge and dried sludge were 44 > 30 > 24 mg/g and 20 > 13 > 11 mg/g, respectively (Ong et al. 2010).
First-order Lagergren model
Another importance of adsorption study is to predict the order of adsorption. The Lagergren (1898) equation is the first-order rate equation developed for adsorption in liquid–solid systems. The model has been widely used by researchers on kinetic study of metal ions adsorption onto various derived adsorbents (Rengaraj et al. 2003, 2004; Li et al. 2011; Argun and Dursun 2008). The first-order Lagergren model has the following form:
where q t is the amount of metal ions adsorbed onto adsorbent (mg/g) at time t (min), Kad the Lagergren rate constant (l/min), and qe the amount of adsorption equilibrium (mg/g). Figure 3 shows the linear plots of log (q e − q t ) against t. Table 2 shows the amount of adsorption equilibrium (qe), Lagergren rate constant (Kad), calculated amount of adsorption equilibrium (qe,c) and the correlation coefficient (R2) which are derived from the Lagergren equation.
As shown in Table 2, the values of R2 for the metal ions adsorption onto activated sludge are larger than 0.85 and higher compared to the adsorption of the metal ions onto dried sludge. This shows that the adsorption of Cu(II), Ni(II) and Cd(II) onto activated sludge was well fitted with the first-order Lagergren model compare to the adsorption onto dried sludge. However, the calculated amount of adsorption equilibrium (q e,c ) is far different from the actual amount of adsorption equilibrium (q e ) for both the cases.
Pseudo second-order model
In 1995, Ho presented an adsorption pseudo second-order rate law expression that showed how the rate depends on the adsorption capacity on solid phase, but not the concentration of adsorbate (Ho 1995; Ho and Mckay 2000).
where K′ is the pseudo second-order rate constant (g/mg/min), q t the amount of adsorption (mg/g) at time t (min) and q e the amount of adsorption equilibrium (mg/g). In many cases, the kinetics of adsorption by biomass is described by the first-order Lagergren equation. However, it has been shown that the pseudo second-order model provides better fit for the adsorption data obtained than the first-order Lagergren model in some cases (Ho et al. 1996; Banquella and Benaissa 2002; Cheung et al. 2000).
The pseudo second-order plots for adsorption of Cu(II), Ni(II) and Cd(II) ions onto activated sludge and dried sludge are shown in Fig. 4. Table 3 shows the amount of adsorption equilibrium (qe), pseudo second-order rate constant (K′), calculated amount of adsorption equilibrium (qe,c) and the correlation coefficient (R2) which are derived from the pseudo second-order equation. As shown in Table 3, the values of R2 for adsorption of the metal ions onto activated sludge and dried sludge are larger than 0.99 which showed the pseudo second-order model provided better description on the data obtained when compared to the first-order Lagergren model. Moreover, the calculated amount of adsorption equilibrium (q e,c ) is comparable to the actual amount of adsorption equilibrium (q e ). As studied by Bulgariu and Bulgariu (2012), the biosorption of Pb(II), Cd(II), and Co(II) ions onto green algae waste biomass was well-fitted with the pseudo-second order kinetic model. This shows the pseudo second-order model is considered more suitable to represent the kinetic data in biosorption process (Febrianto et al. 2009).
The better description of biosorption data by the pseudo second-order model indicating the rate limiting step in biosorption of metal ions could be ascribed to the chemical interactions between functional groups of biosorbent and metal ions (Febrianto et al. 2009).
Conclusion
The adsorption behavior of Cu(II), Ni(II) and Cd(II) ions onto the activated sludge and dried sludge was analyzed with Weber–Morris intra-particle diffusion model, Lagergren first-order model and pseudo second-order model. The kinetic adsorption data can fit better by the pseudo second-order model compared to the first-order Lagergren model indicating the rate limiting step in the biosorption of Cu(II), Ni(II) and Cd(II) ions could be ascribed to the chemical interaction between the metal ions and the functional groups at the surface of microbial biomass. The adsorption capacities of Cu, Ni and Cd ions onto the activated sludge were significantly greater than dried sludge according to the kinetic models.
References
Ahluwalia SS, Goyal D (2007) Microbial and plant derived biomass for removal of heavy metals from wastewater. Bioresour Technol 98(12):2243–2257
Argun E, Dursun S (2008) A new approach to modification of natural adsorbent for heavy metal adsorption. Bioresour Technol 99(7):2516–2527
Banquella B, Benaissa H (2002) Cadmium removal from aqueous solutions by chitin: kinetic and equilibrium studies. Water Res 36:2463–2474
Bulgariu D, Bulgariu L (2012) Equilibrium and kinetics studies of heavy metal ions biosorption on green algae waste biomass. Bioresour Technol 103(1):489–493
Cheng MH, Patterson JW, Minear RA (1975) Heavy metals uptake by activated sludge. J Water Pollut Control Fed 47:362–376
Cheung CW, Porter JF, Mckay G (2000) Sorption kinetics for the removal of copper and zinc from effluents using bne char. Sep Purif Technol 19:55–64
Febrianto J, Kosasih AN, Sunarso J, Ju YH, Indraswati N, Ismadji S (2009) Equilibrium and kinetic studies in adsorption of heavy metals using biosorbent: a summary of recent studies. J Hazard Mater 162(2–3):616–645
Ho YS (1995) Adsorption of heavy metals from waste streams by peat. PhD Thesis. Birmingham, UK, University of Birmingham
Ho YS, Mckay G (2000) The kinetic of sorption of divalent metal ions onto sphagnum moss peat. Water Res 34:735–742
Ho YS, Wase DAJ, Forster CF (1996) Kinetic studies of competitive heavy metals adsorption by sphagnum moss peat. Environ Technol 17:71–77
Lagergren S (1898) Zur Theorie der sogenannten Adsorption gelostn. Stoffe. Stcok. Ak. Handl. Bihay. 24 (Afd. 1): 39
Li X, Li Y, Ye Z (2011) Preparation of macroporous bead adsorbents based on poly(vinyl alcohol)/chitosan and their adsorption properties for heavy metals from aqueous solution. Chem Eng J 178(15):60–68
Manriquez RA, Magana PI, Lopez V, Guzman R (1997) Biosorption of Cu by Thiobacillus ferrooxidans. Process Biochem 34:725–730
Ong SA, Toorisaka E, Hirata M, Hano T (2004) Effects of nickel(II) addition on the activity of activated sludge microorganisms and activated sludge process. J Hazard Mater 113(1–3):111–121
Ong SA, Toorisaka E, Hirata M, Hano T (2005) The behavior of Ni(II), Cr(III) and Zn(II) in biological wastewater treatment process. Acta Hydrochimca et Hydrobiologica 33(2):95–103
Ong SA, Toorisaka E, Hirata M, Hano T (2010) Adsorption and toxicity of heavy metal on activated sludge. ScienceAsia 36(3):204–209
Ras M, Girbal-Neuhauser E, Etienne P, Lefebvre D (2008) A high yield multi-method extraction protocol for protein quantification in activated sludge. Bioresour Technol 99:7464–7471
Rengaraj S, Joo CY, Kim Y, Yi J (2003) Kinetics of removal of chromium from water and electronic process wastewater by ion exchange resins: 1200H, 1500H and IRN97H. J Hazard Mater 102(2–3):257–275
Rengaraj S, Kim Y, Joo CK, Yi J (2004) Removal of copper from aqueous solution by aminated and protonated mesoporous aluminas: kinetics and equilibrium. J Colloid Interface Sci 273(1):14–21
Song J, Kong H, Jang J (2011) Adsorption of heavy metal ions from aqueous solution by polyrhodanine-encapsulated magnetic nanoparticles. J Colloid Interface Sci 359(2):505–511
Ting YP, Lawson F, Prince IG (1991) Uptake of cadmium and zinc by the alga Chlorella vulgaris: II multi-ion situation. Biotechnol Bioeng 37(5):445–455
Wingender J, Neu TR, Flemming HC (1999) What are bacterial extracellular polymeric substances? In: Wingender J, Neu TR, Flemming HC (eds) microbial extracellular polymeric substances. Springer, Berlin, pp 1–20
Zhou M, Liu YG, Zeng GM, Li X, Xu WH, Fan T (2007) Kinetic and equilibrium studies of Cr(VI) biosorption by dead Bacillus licheniformis biomass. World J Microbiol Biotechnol 23:43–48
Ziagova M, Dimitriadis G, Aslanidou D, Papaioannou X, Tzannetaki EL, Liakopoulou-Kyriakides M (2007) Comparative study of Cd(II) and Cr(VI) biosorption on Staphylococcus xylosus and Pseudomonas sp. in single and binary mixtures. Bioresour Technol 98:2859–2865
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under license to BioMed Central Ltd. Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Ong, SA., Toorisaka, E., Hirata, M. et al. Comparative study on kinetic adsorption of Cu(II), Cd(II) and Ni(II) ions from aqueous solutions using activated sludge and dried sludge. Appl Water Sci 3, 321–325 (2013). https://doi.org/10.1007/s13201-013-0084-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13201-013-0084-3