Abstract
The toxicity and availability of heavy metals in soil are determined by modes of occurrence of metals. Therefore, the quantification of associations of heavy metals is more significant than the assessment of their total concentrations. Thirty soil samples that were collected from coal mining areas with different mining durations in Huainan Coalfield were employed to determine the modes of occurrence and potential environmental implications of heavy metals. Sequential chemical extraction procedure was carried out to determine the fractionation profiles of heavy metals. The elevated concentrations of heavy metals in the mining activities areas indicated that the mining activities (mining, transportation, utilization and waste disposal) might be one of the sources for the heavy metal pollution in soil. The fractionation characterizations are various among different heavy metals. According to the international sediment quality guidelines (SQGs) calculations, the adverse biological effects caused by Cd, Cr, Pb and Zn are expected to be negligible. For As and Cu, the occasional adverse biological effects are predicted. The risk assessment code reveals that these heavy metals could pose a medium risk to the ecosystem. Consequently, environmental issues involved in heavy metals in the studied coal mining area soil deserve urgent concern.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The rapid development of the coal mining industry in China over the past 20 years has resulted in serious environmental issues (Zhou et al. 2014a). The heavy metals, which are presented in less than 0.1 % in matrix (coal, coal wastes), are of great concern due to their toxicity, persistence and non-degradability (Dai et al. 2008; Finkelman et al. 2002; Ribeiro et al. 2010; Scott et al. 2009; Zhang et al. 2003). Heavy metal in soil may be derived from both natural source and anthropogenic contributions. Among these, the anthropogenic sources, typically coal mining activities, are regarded as the main sources. The heavy metals in coal (coal wastes) were released and accumulated in soil by various processes (leaching, weathering, combustion and biological reaction) (Gidarakos et al. 2009; Shafer et al. 2012; Singh and Paul 2001; Zhang et al. 2008; Zhou et al. 2014b, c). Therefore, the study on the environmental implications of heavy metals in soil around coal mining areas is of significance.
Evaluation of total concentrations of heavy metals in soil provides important information for contamination levels when compared with the background values. Nevertheless, the assessment of the total contents of heavy metals could not be able to provide information about its toxicity and bioavailability (Gadh 1991; Cabarcas-Montalvo et al. 2012). Consequently, it is important to induce chemical fractionation to investigate the potential environmental implications.
Currently, the associations of heavy metals could be investigated by both theoretical calculations and experimental determination (Jain et al. 2010). Among them, the sequential chemical extraction procedure is regarded as the sophisticated method for the modes of occurrence of heavy metals. Once the associations of heavy metals in soil were obtained, its bioavailability and toxicity could be ascertained (Jain et al. 2010). The exchangeable and carbonate bound are weakly bound and regarded as more rapidly bioavailable. The Fe–Mn oxides and organic matter bound have a scavenging effect and may provide a deposition for heavy metals; the release ratio of heavy metal from these fractions was determined by the pH and redox potential (Burt et al. 2014). The heavy metal in residual was regarded as inert fraction and retained at soil. Therefore, the improved sequential extraction method reported by Tessier et al. (1979) was employed to determine the fractionations of heavy metal in soil.
Huainan Coalfield is the national identified coal base and coal-electricity base, which contains 17 coal mines and 21 coal-fired power plants. Intensive coal mining and long-scale mining duration inevitably led to high retention capacity for heavy metals in soil and eventually resulted in potential environmental and health risks. In spite of the significant environmental issues of heavy metals in coal mining areas’ soil, reports on the heavy metals’ fractionation and ecological risk assessment in Huainan are extremely limited (Li et al. 2010; Wang et al. 2012). Therefore, the objectives of this study are: (1) to determine the contamination levels of heavy metals in coal mining areas’ soil; (2) to investigate the fractionation characteristics of heavy metals; and (3) to evaluate the potential environmental and ecological risks posed by these contaminated soils. This study provides basic information for local environmental management and use for reference for other coal mining activities areas.
Materials and methods
Study area and sampling
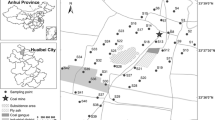
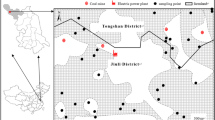
Huainan Coalfield (N32°32′45′′–N33°0′24′′, E116°21′21′′–E117°11′59′′) is located in central China. The climate of this area is a typical seasonal temperature semi-humid monsoon. The average temperature and annual rainfall are 15 °C and 921 mm, respectively. According to local perennial wind direction (southeast wind and northwest wind), samples were collected from the southeast and northwest directions at distance of 10, 50, 100, 300 and 600 m from the coal mining areas. A total of 30 soil samples for Xinzhuangzi Coal Mine (n = 10), Panyi Coal Mine (n = 10) and Dingji Coal Mine (n = 10) were collected (Fig. 1). The mining histories of the selected coal mines are decreased as Xinzhuangzi Coal Mine (68 years old) > Panyi Coal Mine (22 years old) > Dingji Coal Mine (8 years old). These samples were collected from the upper 20 cm of soil in farmland that has not been disturbed for several months using a stainless steel spade, and stored in a plastic bag immediately. In order to obtain a representative sample, subsamples were scraped from five separated level areas randomly around each soil site and then pooled to an integrated sample. When taken back to the laboratory, the soil samples were air-dried and crushed to pass through a 2-mm mesh sieve to homogenize for the subsequent chemical analysis.
Chemical analysis
The homogenous soil samples and standard reference material (GBW07406 (GSS-6)) were extracted by an acid mixture solution (HCl/HNO3/HF) with the ratio of 3:3:2 in a microwave oven. The digested procedure was carried out at 120 °C for 20 min and then heated at 180 °C for 25 min (Zhou et al. 2014b). After extraction, the digested solution was heated at 210 °C to disperse the excess acid mixture solution. Finally, the treated solution was quantified to 25 ml by colorimetric tube for the subsequent analysis. The concentrations of heavy metals (As, Cd, Cr, Cu, Pb and Zn) in the selected soil samples were determined using an inductively coupled plasma mass spectrometry (ICP-MS), with the limit of detection (LOD) and limit of quantity (LOQ) of 0.01 μg/L, 0.001 μg/L, 0.007 μg/L, 0.009 μg/L, 0.001 μg/L and 0.047 μg/L, and 0.03 μg/L, 0.004 μg/L, 0.024 μg/L, 0.03 μg/L, 0.005 μg/L and 0.16 μg/L, respectively. The quality control was subjected to standard references materials GBW07406 (GSS-6). The certified values of As, Cd, Cr, Cu, Pb and Zn in the standard reference materials GBW07406 (GSS-6) are 220 ± 14, 0.13 ± 0.03, 75 ± 6, 390 ± 14, 314 ± 13 and 220 ± 14 mg/kg, respectively. The calibrating solution (contains As, Cd, Cr, Cu, Pb and Zn) with a value of 10 mg/L was employed to calibrate the instrument. Meanwhile, reagent blanks were paralleled in the whole extraction processes to minimize background interference. The acceptable precision is within ±5 wt% for all the heavy metals. In addition, once the difference between the standards and the calibrated values was more than 5 %, the apparatus was required to recalibrate. In order to guarantee the precision and accuracy of the experimental data, all the measurements for each sample were taken in triplicates. The relative standard deviations of the three times analysis were ranged from −1.73 to 2.69 % and below the control level of ±5 wt%.
The sequential chemical extraction method reported by Tessier et al. (1979) was employed to provide information about the modes of occurrence of heavy metals in soil. The selected samples were subjected to specific solvents in five-step sequential chemical extraction procedure in the following order:
-
1.
Exchangeable: 1.5 g powdered sample was digested with 15 ml 1.0 M MgCl2 (pH 7.0) for 1 h under room temperature, and supernatant was obtained by centrifugation at 3800 rpm for 15 min.
-
2.
Carbonate bound: The residues from step (1) were extracted by 15 ml 1 M CH3COONa (pH 5.0) and agitated continuously for 5 h, and suspension was obtained by centrifugation at 3800 rpm for 15 min.
-
3.
Fe–Mn oxides bound: The residues from step (2) were extracted by 30 ml 0.04 M NH2OH·HCl in 25 % (v/v) and agitated continuously for 6 h, and suspension was obtained by centrifugation at 3800 rpm for 15 min.
-
4.
Organic matter bound: The residues from step (3) were extracted by 4.5 ml 0.02 M HNO3 and 7.5 ml 30 % H2O2 (pH 2.0) for 2 h under 85 °C. A 4.5 ml 30 % H2O2 (pH 2.0 with HNO3) was added under 85 °C and agitated for 3 h again. Then, 7.5 ml 3.2 M NH4OAc in 20 % (v/v) HNO3 was added and diluted to 100 ml.
-
5.
Residual: The residues from step (4) were treated by an acid mixture of (3:3:2) HCl/HNO3/HF mixture in a microwave oven.
The modes of occurrence of heavy metals could be clustered into five parts as: exchangeable, carbonate bound, Fe–Mn oxides bound, organic matter bound and residual. After extracted, the fractions obtained by each extraction step were measured for As, Cd, Cr, Cu, Pb and Zn by ICP-MS. In order to assess the potential mobility of heavy metals, the compliance leaching test RN 12457 Part 2 procedure was implemented (EN 12457-2 2002) in this study. Briefly, the selected soil samples were treated by deionized water at a solid-to-liquid ratio of 1:10 kg/L and agitated for 24 h. Each sample was analyzed in triplicate. The concentrations of heavy metals in leachates are measured by ICP-MS, and the anion concentrations (F−, SO4 2− and Cl−) in leachates were determined by high-performance ion chromatography (HPIC). The quality control and reagent blanks were consistent with the procedure used for the aforementioned powered samples.
Results and discussion
The concentration of heavy metal
The contents of heavy metals in the selected soil samples were consistent with the results presented by other previous studies (Table 1) (Komnitsas and Modis 2006; Qiao et al. 2011). When compared with the background values of the Huainan historical data (Yang et al. 1997), the concentrations of As, Cd, Cu (except Panyi Mine) and Pb (except Panyi Mine and Dingji Mine) were higher in the selected soil samples. Typically for As and Cd, the concentrations of As and Cd in Xinzhuangzi Mine, Panyi Mine and Dingji Mine are about 3.4-, 2.2-, 1.4- and 2.9-, 2.1-, 1.7-fold higher than that of Huainan soil, respectively. In comparison with the Chinese soil (Wei et al. 1991), As, Cd and Cu are higher in the Xinzhuangzi Mine, Panyi Mine and Dingji Mine. The elevated concentrations of heavy metals in the mining activities areas indicated that the mining activities (mining, transportation, utilization and waste disposal) may be one of the sources for the heavy metal pollution in soil (Gidarakos et al. 2009; Ribeiro et al. 2010; Zhang et al. 2003). The concentrations of heavy metals increase gradually with increasing mining duration, which indicated that the coal mining activities might lead to potential environmental implications.
The concentrations of heavy metals in soil are attributed to the host mineral composition, soil physicochemical properties, soil formation mechanism and the pollution sources (Al-Khashman 2012; Candeias et al. 2014; Chen et al. 2014). Arsenic, Cd, Cu, Pb and Zn are known as chalcophile elements. There have been many investigations into the modes of occurrence of these elements in coal and reported that these elements are mainly associated with sulfide minerals (pyrite, siderite) and clay minerals (Finkelman et al. 2002; Dai et al. 2008). Under natural weathering processes, the chemical bonds of the sulfide minerals could be broken and decomposed easily through oxidation. As a consequence, these elements (As, Cd, Cu, Pb and Zn) that existed in the sulfide minerals would release from coal/coal gangue under natural weathering and dispose into the soil. Heavy metal in soil may be derived from both natural source and anthropogenic contributions. The correlation coefficients among the selected heavy metals in soils are analyzed and summarized in Table 2. The positive correlation coefficients of As with Zn (r = 0.664), Cd with Cu (r = 0.675), Cd with Zn (r = 0.647), Cu with Pb (0.623) and Cu with Zn (r = 0.583) indicate that these heavy metals may be derived from similar pollution sources (coal mining activities) and associated with sulfide minerals. For Cr, various studies demonstrated that Cr mainly existed in association with clays and organic matter (Querol et al. 1996; Finkelman et al. 2002), while Cr in the selected samples is mainly associated with residual and exited in clay minerals. Therefore, the correlations were not observed between Cr and other heavy metals.
Partition behavior of heavy metal
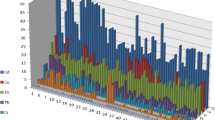
The partition behaviors of heavy metal in soil from different coal mining activities areas are presented in Fig. 2. The speciation characterization of arsenic shows that it was mostly associated with residual, and moderate contributions were composed by Fe–Mn oxides bound, carbonate bound and organic matter/sulfides bound. The above results are in accordance with previous studies reported by Jain (2004) and Rath et al. (2009). Ward (2002) and Zhou et al. (2014b) study the association of As in coal/coal gangue and reported that As in coal/coal gangue mainly existed in residual, organic matter/sulfides bound and Fe–Mn oxides bound (Ward 2002; Zhou et al. 2014b). The proportions of residual, organic matter/sulfides bound and exchangeable As increase with the increase in mining duration. The variation of associations of As among different coal mines may be attributed to the mining activities. It is well known that there are large amounts of sulfide minerals associated in coal/coal waste; these sulfide minerals may be decomposed under various reactions (physical, chemical and biological) (Finkelman et al. 2002; Gidarakos et al. 2009). The obvious negative correlations of exchangeable, organic matter bound and sulfide with carbonate and Fe–Mn oxides bound are given in Table 3. With the decomposition of sulfide minerals, the pH and redox potential are changed and result in the increase in exchangeable and organic matter/sulfide bound and the decrease in carbonate and Fe–Mn oxides bound.
Cadmium mainly existed in residual, organic matter/sulfides bound and Fe–Mn oxides, the rest being associated with carbonate bound and exchangeable (Fig. 2). Tessier et al. (1979) reported that large amounts of Cd could be presented in carbonate bound in Yamaska and St. Francois area. The low rate of carbonate bound in this study may be due to the low pH in the mining activities areas. The negative correlations of exchangeable, organic matter bound and sulfide with carbonate and Fe–Mn oxides bound are given in Table 4. The rates of residual, organic matter bound and exchangeable Cd increase with increasing mining duration, which is consistent with the partition behavior of arsenic.
The fractionation behavior of chromium in Huainan coal mines is predominantly presented in residual and carbonate bound (Fig. 2). There are also appropriate concentrations associated with organic matter bound, Fe–Mn oxides bound and exchangeable. The results are consistent with the previous studies reported by Pardo et al. (1990) and Rath et al. (2009). The negative correlations of exchangeable and sulfide with carbonate and Fe–Mn oxides bound are given in Table 5. The residual and exchangeable fractions increase gradually with increasing mining duration.
Speciation behavior of copper shows that it is distributed among residual, organic matter/sulfide bound and Fe–Mn oxides bound (Fig. 2). Gibbs (1977) reported that Cu mainly existed in residual, while the concentration bound to Fe–Mn oxides and organic/sulfide is much lower. The different tendency of Cu in this study area may be attributed to the effect of mining activities. The negative correlations of exchangeable and organic matter bound with carbonate and Fe–Mn oxides bound are given in Table 6. The organic matter/sulfide bound and exchangeable increase, while the carbonate bound decreases with increasing mining duration.
The fractionation characterization of lead suggests that the major portion of Pb is existed in residual and moderate contributions are consisted by organic matter/sulfide bound and Fe–Mn oxides bound. Jha et al. (1990) studied Pb association in Delhi soil and reported that Pb is mainly focused on Fe–Mn oxides bound and the different modes of occurrence of Pb may be due to the different soil physicochemical properties, depositional environmental and contamination sources. The obvious negative correlations of exchangeable and organic matter bound with carbonate bound are given in Table 7. The exchangeable increases rapidly with increasing mining duration.
For zinc, the major portion is associated with residual. There are also appropriate ratios of the existence of organic matter/sulfide bound, Fe–Mn oxides bound, carbonate bound and exchangeable, which is consistent with the previous studies reported by Tessier et al. (1979), Jain (2004) and Pardo et al. (1990). The negative correlations of exchangeable, organic matter bound and sulfide with carbonate and Fe–Mn oxides bound are given in Table 8.
Potential leaching behavior and ecological risk assessment
The concentrations of heavy metals and ion in soil leachates were employed to evaluate the heavy metal mobility under natural conditions. The contents of heavy metals and waste acceptance criteria for landfilling according to Annex 2 of the 2003/33/CE Council Decision are given in Table 9. The leaching rate (Lr) of heavy metal is also applied to assess the degree of the heavy metal mobility in the soil during leaching, which is defined as:
According to Table 9, the leaching rates of all the selected heavy metal are less than 1 %. When compared with the contents of heavy metals in the leachates with the waste acceptance criteria for landfilling stated in the Annex 2 of the Council Decision (2003/33/CE), the concentrations of the selected heavy metals were lower than the limit values.
International sediment quality guidelines (SQGs) calculations, such as effects range low (ERL) and effects range median (ERM), were applied to assess the biological toxicity of heavy metals (As, Cd, Cr, Cu, Pb and Zn) in soil. The ERM values are chemical concentrations above which adverse effects are likely to occur, while the ERL values represent the contents lower than the potential adverse effect concentrations (Long et al. 1995). The incidence of toxicity is determined among samples in which none of the samples is higher than the ERL values (<ERL values), in which one or increasing numbers of chemicals exceed ERL values, but none is lower than ERM values (>ERL values and <ERM values), and in which one or increasing numbers of substances exceed ERM concentration (>ERM values) (Pekey et al. 2004). The ERL and ERM values for the heavy metals are given in Table 1. The concentrations of Cd, Cr, Pb and Zn in all the selected samples are lower than ERL, which suggested that the adverse biological effects are expected to be negligible. For As and Cu, 65 and 46 %, respectively, those are in the middle range, which indicated that occasional adverse biological effects are predicted. None of the samples exceed the ERM values.
According to the results of the sequential chemical extraction procedures, heavy metals in soil are associated with different species. The partition behaviors of heavy metals could be employed as an indication to evaluate the potential ecological risk (Dellantonio et al. 2008; Rath et al. 2009). The risk assessment code (RAC) classified that heavy metals could be regarded as safe (no risk) to the ecosystem when the exchangeable and carbonate bound are less than 1 % of the total values. In contrast, once the fractions of exchangeable and carbonate bound of any of these heavy metals are more than 50 % of the totals values, these heavy metals could be resulted in a strong environmental impacts (very high risk). Meanwhile, 1–10, 11–30 and 31–50 % of RAC values could be regarded as low risk, medium risk and high risk, respectively (Perin et al. 1985). According to the fractionation profiles of the selected heavy metals (Fig. 2), the RAC values of the selected heavy metals reveal that these heavy metals could pose a medium risk to the ecosystem. Therefore, the environmental impacts induced by heavy metals in mining activities areas soil deserve further concern.
Conclusions
The concentrations of heavy metals increase with increasing mining duration, which indicated that the mining activities are the important sources for the heavy metal pollution in soil. The fractionation profiles are various among different heavy metals. For As, Cd, Cr and Zn, the proportions of residual bound, organic matter bound and exchangeable bound increase with increasing mining duration. Based on the leaching experiment, the concentrations of the selected heavy metals were lower than the limit values. According to the international sediment quality guidelines (SQGs) calculations, the adverse biological effects caused by Cd, Cr, Pb and Zn of all samples are expected to be negligible. For As and Cu, occasional adverse biological effects are predicted. Based on the risk assessment code (RAC), the RAC values of the selected heavy metals reveal that these heavy metals could pose a medium risk to the ecosystem. Therefore, the environmental impacts induced by heavy metals in the selected mining activities areas soil may be caused if no countermeasures are adopted.
References
Al-Khashman OA (2012) Assessment of heavy metal accumulation in urban soil around potash industrial site in the East of the Dead Sea and their environmental risks. Soil Sediment Contam 21:276–290. doi:10.1080/15320383.2011.609358
Burt R, Hernandez L, Shaw R, Tunstead R, Ferguson R, Peaslee S (2014) Trace element concentration and speciation in selected urban soils in New York City. Environ Monit Assess 186:195–215. doi:10.1007/s10661-013-3366-1
Cabarcas-Montalvo M, Olivero-Verbel J, Corrales-Aldana H (2012) Genotoxic effects in blood cells of Mus musculus and Iguana iguana living near coal mining areas in Colombia. Sci Total Environ 416:208–214. doi:10.1016/J.Scitotenv.2011.11.080
Candeias C, Melo R, Avila PF, da Silva EF, Salgueiro AR, Teixeira JP (2014) Heavy metal pollution in mine–soil–plant system in S. Francisco de Assis–Panasqueira mine (Portugal). Appl Geochem 44:12–26. doi:10.1016/j.apgeochem.2013.07.009
Chen ZQ, Ai YW, Fang C, Wang KX, Li W, Liu S, Li CL, Xiao JY, Huang ZY (2014) Distribution and phytoavailability of heavy metal chemical fractions in artificial soil on rock cut slopes alongside railways. J Hazard Mater 273:165–173. doi:10.1016/J.Jhazmat.2014.03.042
Dai SF, Li D, Chou CL, Zhao L, Zhang Y, Ren D, Ma YW, Sun YY (2008) Mineralogy and geochemistry of boehmite-rich coals: new insights from the Haerwusu Surface Mine, Jungar Coalfield, Inner Mongolia, China. Int J Coal Geol 74:185–202. doi:10.1016/J.Coal.2008.01.001
Dellantonio A, Fitz WJ, Custovic H, Repmann F, Schneider BU, Grunewald H, Gruber V, Zgorelec Z, Zerem N, Carter C, Markovic M, Puschenreiter M, Wenzel WW (2008) Environmental risks of farmed and barren alkaline coal ash landfills in Tuzla, Bosnia and Herzegovina. Environ Pollut 153:677–686. doi:10.1016/J.Envpol.2007.08.032
EN 12457-2 (2002) Characterization of water-leaching: compliance test for leaching of granular waste materials and sludges—part 2: One stage batch test at a liquid to solid ratio of 10 L/kg for materials with particle size below 4 mm (without or with size reduction)
Finkelman RB, Orem W, Castranova V, Tatu CA, Belkin HE, Zheng BS, Lerch HE, Maharaj SV, Bates AL (2002) Health impacts of coal and coal use: possible solutions. Int J Coal Geol 50:425–443. doi:10.1016/S0166-5162(02)00125-8
Gadh R (1991) Metal speciation in the Yamuna River water, sediments. PhD thesis, University of Roorke, Roorkee
Gibbs HC (1977) Spring rise in fecal nematode egg counts in sheep in Maine. Am J Vet Res 38:533–534
Gidarakos E, Petrantonaki M, Anastasiadou K, Schramm KW (2009) Characterization and hazard evaluation of bottom ash produced from incinerated hospital waste. J Hazard Mater 172:935–942. doi:10.1016/J.Jhazmat.2009.07.080
Jain CK (2004) Metal fractionation study on bed sediments of River Yamuna, India. Water Res 38:569–578. doi:10.1016/j.watres.2003.10.042
Jain CK, Rao VVSG, Prakash BA, Kumar KM, Yoshida M (2010) Metal fractionation study on bed sediments of Hussainsagar Lake, Hyderabad, India. Environ Monit Assess 166:57–67. doi:10.1007/s10661-009-0984-8
Jha PK, Subramanian V, Sitasawad R, Vangrieken R (1990) Heavy-metals in sediments of the Yamuna River (a tributary of the Ganges), India. Sci Total Environ 95:7–27. doi:10.1016/0048-9697(90)90049-Z
Komnitsas K, Modis K (2006) Soil risk assessment of As and Zn contamination in a coal mining region using geostatisretics. Sci Total Environ 371:190-196. doi:10.1016/j.scitotenv.2006.08.047
Li HW, Yan SL, Cui LP (2010) Preliminary study on soil heavy metal fractions in Huainan mining area. In: Proceedings of 2010 international workshop on diffuse pollution-management measures and control technique, pp 196–199
Long ER, Macdonald DD, Smith SL, Calder FD (1995) Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuarine sediments. Environ Manag 19:81–97. doi:10.1007/Bf02472006
Pardo R, Barrado E, Perez L, Vega M (1990) Determination and association of heavy metals in sediments of the Pisuerga River. Water Res 24:373–379
Pekey H, Karakas D, Ayberk S, Tolun L, Bakoglu M (2004) Ecological risk assessment using trace elements from surface sediments of Izmit Bay (Northeastern Marmara Sea) Turkey. Mar Pollut Bull 48:946–953. doi:10.1016/J.Marpolbul.2003.11.023
Perin G, Craboledda L, Lucchese M, Cirillo R, Dotta L, Zanette M, Orio A (1985) Heavy metal speciation in the sediments of Northern Adriatic Sra—a new approach for environmental toxicity determination. In: Lekkas TD (ed) Heavy metal in the environment, vol 2, New York, pp 454–456
Qiao M, Cai C, Huang YZ, Liu YX, Lin AJ, Zheng YM (2011) Characterization of soil heavy metal contamination and potential health risk in metropolitan region of northern China. Environ Monit Assess 172:353-365. doi:10.1007/s10661-010-1339-1
Querol X, Juan R, Lopez-Soler A, Fernandez-Turiel JL, Ruiz C (1996) Mobility of trace elements from coal and combustion wastes. Fuel 75:821–838. doi:10.1016/0016-2361(96)00027-0
Rath P, Panda UC, Bhatta D, Sahu KC (2009) Use of sequential leaching, mineralogy, morphology and multivariate statistical technique for quantifying metal pollution in highly polluted aquatic sediments—A case study: Brahmani and Nandira Rivers, India. J Hazard Mater 163:632–644. doi:10.1016/J.Jhazmat.2008.07.048
Ribeiro J, da Silva EF, Li Z, Ward C, Flores D (2010) Petrographic, mineralogical and geochemical characterization of the Serrinha coal waste pile (Douro Coalfield, Portugal) and the potential environmental impacts on soil, sediments and surface waters. Int J Coal Geol 83:456–466. doi:10.1016/J.Coal.2010.06.006
Scott AC, Whittal RM, Fedorak PM (2009) Coal is a potential source of naphthenic acids in groundwater. Sci Total Environ 407:2451–2459. doi:10.1016/J.Scitotenv.2008.12.028
Shafer MM, Toner BM, Oyerdier JT, Schauer JJ, Fakra SC, Hu SH, Herner JD, Ayala A (2012) Chemical speciation of vanadium in particulate matter emitted from diesel vehicles and urban atmospheric aerosols. Environ Sci Technol 46:189–195. doi:10.1021/Es200463c
Singh G, Paul BC (2001) Assessment of groundwater quality impacts due to use of coal combustion byproducts to control subsidence from underground mines. Environ Int 26:567–571. doi:10.1016/S0160-4120(01)00042-3
Tessier A, Campbell PGC, Bisson M (1979) Sequential extraction procedure for the speciation of particulate trace-metals. Anal Chem 51:844–851. doi:10.1021/Ac50043a017
Wang XM, Chu ZX, Yao J, Ji MJ, Liu GJ, Dong ZB (2012) A survey of Zn, Pb, Cd, Cr and Cu in earthworms and soil from subsidence area of Xieyi Coal Mine in Huainan, China. Asian J Chem 24:4241–4242
Ward CR (2002) Analysis and significance of mineral matter in coal seams. Int J Coal Geol 50:135–168. doi:10.1016/S0166-5162(02)00117-9
Wei FS, Chen JS, Wu YY, Zheng CJ (1991) Study on the background contents on 61 elements of soils in China. China J Environ Sci 12:12–19 (in Chinese with English abstract)
Yang XY, Sun LG, Zhang ZF, Xie ZQ, Cai ZY, Li MZ (1997) The soil element background values and assessment on the soil environmental quality in Huainan area. Acta Pedol Sin 34:344–347 (in Chinese with English abstract)
Zhang JY, Ren DY, Zheng CU, Zeng RS, Chou CL, Liu J (2003) Trace element abundances in major minerals of Late Permian coals from southwestern Guizhou Province, China. Int J Coal Geol 53:243. doi:10.1016/S0166-5162(03)00003-X
Zhang H, He PJ, Shao LM (2008) Flow analysis of heavy metals in MSW incinerators for investigating contamination of hazardous components. Environ Sci Technol 42:6211–6217. doi:10.1021/Es800548w
Zhou CC, Liu GJ, Wu D, Fang T, Wang RW, Fan X (2014a) Mobility behavior and environmental implications of trace elements associated with coal gangue: a case study at the Huainan Coalfield in China. Chemosphere 95:193–199. doi:10.1016/J.Chemosphere.2013.08.065
Zhou CC, Liu GJ, Wu SC, Lam PKS (2014b) The environmental characteristics of usage of coal gangue in bricking-making: a case study at Huainan, China. Chemosphere 95:274–280. doi:10.1016/J.Chemosphere.2013.09.004
Zhou CC, Liu GJ, Fang T, Sun RY, Wu D (2014c) Leaching characteristic and environmental implication of rejection rocks from Huainan Coalfield, Anhui Province, China. J Geochem Explor 143:54–61. doi:10.1016/J.Gexplo.2014.03.010
Acknowledgments
This work was financially supported by the Science and Technology Plan Projects of Huainan (KJ201356), Research Project of Huainan Union University (LCY1404) and Science and Technology Projects of Huainan (2013A4203), The Key Program for Natural Science Foundation of Anhui Universities (KJ2015A349, KJ2015A230). We acknowledge editors and reviewers for polishing the language of the paper and for in-depth discussion.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
You, M., Huang, Y., Lu, J. et al. Fractionation characterizations and environmental implications of heavy metal in soil from coal mine in Huainan, China. Environ Earth Sci 75, 78 (2016). https://doi.org/10.1007/s12665-015-4815-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-015-4815-7