Abstract
Total concentrations of heavy metals in soils may not be enough to understand their mobility and bioavailability. It is important to evaluate the degree of association of heavy metals with different chemical forms of soil. The sequential extraction method was applied to evaluate the mobile behavior of Cd, Cr, Cu, Ni, Pb, and Zn in 42 representative soil samples from the Linhuan subsidence of Huaibei Coalfield, Anhui Province, China. The results showed that mean concentrations of heavy metals were higher than background values of Huaibei City surface soil by a factor of 1.16 to 3.21 (Cd, 3.21; Cr, 1.19; Cu, 1.16; Ni, 1.23; Zn, 1.85) except Pb (0.89). Most of the total Cr, Cu, Ni, Pb, and Zn were present in the residual forms (above 70 %), while Cd was dominated by the exchangeable forms (42 %). The correlations analysis showed that the mobility of Cd, Cu, Pb, and Zn in soil was affected by both physicochemical properties and total metal concentrations. In contrast, the moblity of Cr and Ni of soil was mainly affected by their total metal concentrations. According to assessments by the potential ecological risk index (RI) and the risk assessment code (RAC), Cr, Cu, Ni, Pb, and Zn posed no or low risk. However, Cd presents high to very high risk, due to its higher exchangeable and carbonate-bound fractions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The contamination of soil ecosystem by heavy metals is considered as a global environmental issue (Facchinelli et al. 2001; Solgi et al. 2012). Heavy metals in soils are originated from natural and anthropogenic sources (Facchinelli et al. 2001; Li et al. 2014). The major natural sources are parent rock materials and volcanic emissions (Blaser et al. 2000; Sun et al. 2016). The anthropogenic sources include fertilizers and pesticides (Nicholson et al. 2003; Nziguheba and Smolders 2008), agricultural and industrial waste discharges (Chabukdhara and Nema 2013; Jan et al. 2010; Silveira et al. 2003; Solgi et al. 2012), and mining (Bhuiyan et al. 2010; Li et al. 2014; Rey et al. 2013; Šajn et al. 2013). Among anthropogenic sources of heavy metals, coal mining plays a significant role. Many toxic heavy metals are released during coal mining and the burning of coal (Chen et al. 2016; Rout et al. 2013; Sun et al. 2013).
Heavy metals do not vanish in time unlike some organic materials. The soil heavy metals in the environment are relatively stable and are difficult to be removed through natural processes. Although essential for living tissues, they show toxic effect if they exceed the limit values. The most important impact of soil pollution on environmental health is that heavy metals can introduce into the food chain by plants and by their direct use or consumption by animals and human beings (Boussen et al. 2013; Dong et al. 2011; Ji et al. 2013; Li et al. 2014; Park and Choi 2013; Türkdoğan et al. 2003). Heavy metals taken into the human body at doses higher than the limit values proposed by the World Health Organization (WHO) are known to cause carcinogenic, teratogenic, toxic, or cardiovascular problems. Accordingly, the environmental risk of heavy metal pollution in agricultural soils by mining activities has been of great concerns.
Much attention has been focused on the investigation of the total metal concentrations in soils. Previous researches have revealed that, in most cases, soils in the vicinity of mines are contaminated severely by heavy metals. For example, in coal mine-affected agricultural soils in the northern part of Bangladesh, the average concentrations of Ti, Mn, Zn, Pb, As, Fe, Rb, Sr, Nb, and Zr exceeded the world normal averages and, in some cases, Mn, Zn, As, and Pb exceeded their toxic limits (Bhuiyan et al. 2010). In the soils around a lead and zinc smelter, the average concentrations of Pb, Cd, Hg, As, Zn, and Cu are 20, 11, 5.5, 4.6, and 3.2 times higher than the European median values, respectively (Šajn et al. 2013). In agricultural soils in non-ferrous metal mine area, high heavy metal concentrations were found in the agricultural soils closer to mines and metal smelters, and Cd and Cu were two primary pollutants in the soils with concentrations of 0.52–2.55 and 27.87–426.15 mg kg−1, respectively (Liu et al. 2013). The mean concentrations of Pb, Zn, and Cd in the arable land soils around an old Spanish Pb-Zn mine were as high as 393.05, 186.09, and 2.47 mg kg−1, respectively (Rodríguez et al. 2009). Although total metal concentrations may give information about the overall contamination conditions of the soil, the chemical forms of heavy metals determine their ecotoxicity, mobilization capacity, and behavior in the environment. When heavy metals are released into the soil, they are transformed into different geochemical forms through a series of physical, chemical, and biological processes (Kraemer and Hering 2004). Among these different geochemical forms, the water-soluble and carbonate forms are considered as most bioavailable, reducible bounded to Fe/Mn oxides and oxidizable bounded to organic matters may be potentially bioavailable, while the residue bounded to the soil matrix is not bioavailable (Delgado et al. 2011; Rodríguez et al. 2009). Thus, the chemical forms of heavy metals might provide much more useful information regarding the chemical nature or potential mobility and bioavailability of a particular element.
Huaibei Coalfield is one of the biggest coal-mining areas in China. The exploitation of coal resources promoted the development of the economy and society of Huaibei City, but it resulted in a large-scale coal-mining area being subsided or to be subsided, damaging the local ecological environment. In addition, heavy metal releases may increase during coal exploitation, storage, and utilization, the disposal of coal gangue and fly ash. Only one study described As, Cu, and Zn contamination in soil and wheat during coal mining in this area (Shi et al. 2013). In the present study, we investigate the Linhuan subsidence area of Huaibei Coalfield for the first time to (1) determine the chemical forms of heavy metals (Cd, Cr, Cu, Ni, Pb, and Zn) by applying the sequential extraction procedure and (2) to assess the potential ecological risk of heavy metal contamination in the soils.
Materials and methods
Study area
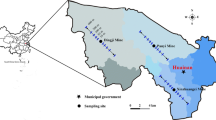
The Linhuan coal mining area is located in warm temperate, semi-humid monsoon climate zone. The average annual temperature, rainfall, evaporation, frost-free period, and relative humidity are 14.8 °C,830 mm, 1815.6 mm, 206.9 days, and 71 %, respectively, and prevailing winds are in an southeast direction in summer, and a northeast direction in winter. Due to the exploitation of coal resources, extensive land subsidence and submergence occurs in the Linhuan subsidence area. The total subsidence areas come to 13.2 km2 with the water area of 3.5 km2 and water storage of 12,188,480 m3. Meanwhile, large amounts of coal gangue and fly ash are produced during mining, washing, and coal consumption, and are usually piled around mines. In addition, in south of the Linhuan subsidence area, there are coal cleaning plant, coking plant, and coal-fired power plant which may also release heavy metals into the surrounding environment.
Sampling
A total of 42 surface soil samples (0–20 cm) were collected in the study area in July (Fig. 1). Three to five subsamples were taken with a stainless steel blade and a plastic scoop, and then mixed thoroughly to get a composite soil sample weighted about 1.0–1.5 kg. The collected soil samples were stored in a polyethylene bag, transported to the laboratory where they were air-dried at ambient temperature, and sieved through a 2-mm mesh to remove coarse debris for measuring pH, electric conductivity (EC), water content (WC), organic matter (OM) content, total N (TN), total C (TC), available potassium (AK), and available phosphorus (AP). For the determination of heavy metals, the soil samples were further ground with the agate mortar and passed through 100-mesh sieve.
Analytical method
The pH and EC were measured using pH meter and electric conductivity (sample/water = 1 g:5 ml). The WC was measured using the oven-drying method and were dried at 105 ± 2 °C for 12 h. Soil OM was determined by the potassium bichromate titrimetric method, while soil TN and TC were determined by the elemental analyzer (Element, Germany). The AP of samples was extracted using 0.5 mol L−1 NaHCO3, and then determined by the molybdate blue spectrophotometric method. AK was extracted using 1 mol L−1 NH4OAc, and then determined by the flame atomic absorption spectroscopy method.
The soil samples were digested with HCl, HNO3, HF, and HClO4. About 0.5 g soil sample was weighted into a cleaned Teflon vessels, 10 mL high-purity HCl was added, after initial decomposition, and 13 mL high-purity acid mixture, i.e., HClO4/HNO3/HF = 3:5:5, was used to digest the samples. After cooling, the solution was adjusted to 50 mL with 5 % HNO3. The sample solutions were analyzed Cd, Cr, Cu, Ni, Pb, and Zn using an inductively coupled plasma atomic emission spectroscopy (ICP-AES) (XSP Intrepid II, USA). Reagent blanks and soil standard reference material (GSS-3) were processed along with the samples, and the recoveries of Cd, Cr, Cu, Ni, Pb, and Zn were 87–115, 95–99, 93–99, 106–128, 96–108, and 110–140 %, respectively.
Chemical forms of metals were measured by a sequential extraction procedure adapted from Tessier et al. (1979). The extraction was carried out in order to obtain the following: F1, exchangeable; F2, bound to carbonate; F3, bound to Fe–Mn oxides; F4, bound to organic matter; and F5, residual. A check of the results of the sequential extraction procedure was conducted by comparing the sum of the five steps (exchangeable + carbonate + Fe–Mn oxide + organic + residual) with the total metal concentration. The recoveries of Cd, Cr, Cu, Ni, Pb, and Zn were 80–121, 75–118, 82–114, 86–130, 80–115, and 80–119 %, respectively, indicating satisfactory results.
Ecological risk assessment
The potential ecological risk index (RI) was introduced by Hakanson (1980), according to the toxicity of elements and the response of the environment, to assess the degree of element pollution in sediments:
\( {C}_f^i \), \( {C}_s^i \), and \( {C}_o^i \) are, respectively, contamination factor, measured concentration, and reference concentration of element i, and the reference concentrations of metals in this study are Cd = 0.081, Cr = 70.8, Cu = 22.1, Ni = 32.3, Pb = 30.9, and Zn = 76.8 (AHEMC 1992). \( {E}_r^i \) is the monomial ecological risk factor, and \( {T}_r^i \) is the biological toxic factor of an individual element, which are defined as Cr = 2, Cu = Pb = Ni = 5, Zn = 1, and Cd = 30 (Yuan et al. 2014a). RI is calculated as the sum of the risk factors of all heavy metals (Cd, Cr, Cu, Ni, Pb, and Zn).
The risk assessment code (RAC) is useful to assess the environmental risk using sequential extraction as a characterization method, and it has been used to assess the environmental risk of heavy metal pollution in sediments and soils (Liu et al. 2013; Nemati et al. 2011; Yuan et al. 2014b). There is no risk when the exchangeable and carbonate fractions are lower than 1 %, percentages of 1–10 % reflect low risk, 11–30 % poses medium risk, 31–50 % poses high risk, and above 50 %, the soil poses a very high risk.
The RI assesses potential ecological and environmental effects based on total heavy metal concentration and their toxicology, whereas RAC considers mobile proportions of metals (i.e., exchangeable and carbonate fractions) (Perin et al. 1985; Singh et al. 2005). Both methods are complementary in evaluating the mobility and toxicity of metals.
Statistical and geostatistical analyses
To identify the relationship among heavy metals in soils and their possible sources, Pearson’s correlation coefficient analysis was performed (Facchinelli et al. 2001) using the commercial statistics software package SPSS version 19.0 for Windows. The correlation coefficient measures the strength of the interrelationship between two heavy metals.
Results and discussions
Spatial distribution and source analysis of heavy metals
The mean concentrations of Cd, Cr, Cu, Ni, Pb, and Zn were 0.26, 84.3, 25.7, 39.6, 27.5, and 142 mg kg−1, respectively. The mean concentrations of heavy metals in soil samples decreased as follows: Zn > Cr > Ni > Pb > Cu > Cd. The mean concentrations of these heavy metals were higher than background values of Huaibei City surface soil by a factor of 1.16 to 3.21 (Cd, 3.21; Cr, 1.19; Cu, 1.16; Ni, 1.23; Zn, 1.85) except Pb (0.89) (AHEMC 1992). In addition, the mean concentrations of these metals were higher than their background values of China soil by a factor of 1.14–3.51 (Cd, 3.51; Cr, 1.38; Cu, 1.14; Ni, 1.69; Pb, 1.17; Zn, 2.1) (CNEMC 1990), and the concentrations of Cd and Zn were higher than the Natural Environmental Quality Standard values for the soils in China (CNEPA 1995), suggesting that heavy metal concentrations in the studied soils are of the danger levels. The coefficient variations of heavy metals were comparatively low, with a decreasing order of Zn (36 %) > Cd (27 %) > Cu (22 %) > Ni (18 %) > Pb (15 %) > Cr (14 %), which suggests that the concentration levels of these metals in soils were less variable.
To identify the relationship among heavy metals in soils and their possible sources, Pearson’s correlation coefficient analysis were performed as shown in Tables 1 and 2.
A significant positive correlation was found among heavy metals (p < 0.01) in surface soils. It can be seen that Cd showed a significant positive relationship with Cu (0.69), Pb (0.54), and Zn (0.43). Cu showed a significant positive relationship with Pb (0.40) and Zn (0.47). The strong correlations among these heavy metals indicate their common origin, possibly sourced from carbonate and sulfide minerals (e.g., sphalerite, strontianite, galena, etc.) that are associated with coal seams (Hower and Robertson 2003; Sakurovs et al. 2007). However, the concentrations of Cr and Ni showed positive relationship with each other but very weak correlations with Cd, Cu, Pb, and Zn. This indicates that Cr and Ni may have different geochemical behaviors and sources from other metals.
Significant correlations between Cd, Cu, Pb, Zn, and soil physicochemical properties were observed, which revealed that the metals may have an anthropogenic source. Significant positive correlations of Cd, Cu, Pb, and Zn with OM, TN, and TC may be due to the fact that soil organic matter is one of the major elemental adsorbents in the soil (Quenea et al. 2009). Humic acid and fulvic acid are two major components of organic matter having strong complexation ability with trace elements (Tang et al. 2014). Comparatively, Cr and Ni showed slight correlations with other heavy metals and physicochemical properties, indicating their pedogenic nature (Chai et al. 2015; Micó et al. 2006).
Chemical forms of heavy metals in the agricultural soils
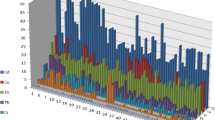
To determine the different forms of heavy metals in the soils, the leachate of each step from the sequential extraction procedure of the soils was analyzed and is shown in Fig. 2.
Different fractions of heavy metals in the soils are not equally available to plants (Morillo et al. 2007; Sundaray et al. 2011). The exchangeable fraction was considered to be easily available to plant, the carbonate fraction was susceptible to pH changes, the Fe–Mn oxide fraction was unstable under low Eh conditions, and the organic fraction could be degraded under oxidizing conditions. In contrast, the residual fraction was inert since it was not expected to be solubilized over a reasonable period of time under natural conditions (Tessier et al. 1979). The order of the bioavailability of metal fractions was exchangeable > carbonate > Fe–Mn oxide > organic > residual, and the potentially mobile fraction was considered as the sum of the first four steps (Guillén et al. 2012; Pérez et al. 2008). In the present study, the percentage of potentially mobile heavy metal fractions in the soils increased in the order of Cr (8 %) < Ni (9 %) < Zn (10 %) < Cu (20 %) < Pb (24 %) < Cd (87 %).
Cd was dominated by the exchangeable fraction, and the mean proportions associated with exchangeable, carbonate, Fe–Mn oxide, organic, and residual fractions were 42, 14, 23, 8, and 13 %, respectively. High percentage of exchangeable and bound to carbonates indicates high solubility and bioavailability, suggesting that the metal was more easily assimilated by crops. Cd in crops can transfer to the human body via the food chain and may pose a serious threat to human health. In the studied area, large amounts of coal gangue and fly ash were dumped on the surface (mine spoil), leachate from mining activities, and weathering of mine spoils may result in high concentration of Cd, as it is weakly adsorbed to the organic matter, clay, and oxide minerals when pH < 6. The results suggest that controlling the mobility of Cd is primary for agricultural soil remediation in the study area. Similar studies also found that Cd was bound in bioavailable fractions (Delgado et al. 2011; Guillén et al. 2012; Liu et al. 2013; Yang et al. 2009). Cu, Zn, and Pb had similar fraction distribution in the soils. The order of various fractions of Cu was residual (80 %) > Fe–Mn oxides (10 %) > organic (9 %) > carbonate (1 %) > exchangeable (0 %). High percentage of Cu in the residual fraction and bound to Fe/Mn oxides and organic matter fractions was also reported by Gibbs (1977). The percentage of Zn in exchangeable, carbonate, Fe–Mn oxide, and organic fractions averaged 0, 0, 5, and 5 %, respectively. Most of the total Zn was present in the residual fraction with an average of 90 %, indicating that Zn is strongly fixed in soils and its mobility is limited. The Pb in soils appeared to be associated with residual and organic fractions, accounting for 77 and 17 %, respectively. The increase of Pb in the organic fraction shows its tendency to be adsorbed by organic matter. The predominant fraction for Cu, Zn, and Pb was the residual (above 70 %); thus, these metals are less likely to harm the environment (Yu et al. 2010).
Cr and Ni were largely retained in the residual fraction (93 % for Cr and 91 % for Ni). The exchangeable proportions of Cr and Ni were very low, with mean percentages of 0 % for Cr and 1 % for Ni. Low exchangeable and high residual proportions of Cr and Ni in soils and sediments were also reported in previous studies (Delgado et al. 2011; Liu et al. 2013; Pérez et al. 2008). As heavy metals in the residual fraction are trapped in the lattice of minerals (Rodríguez et al. 2009; Shi et al. 2008), they are unlikely to be released (Nemati et al. 2011). These results indicate that the environmental risk of Cr and Ni may be low in the study area. High residual proportion of Cr and Ni also indicates that they were from a geological origin (Li et al. 2013).
Behavior of heavy metals in the agricultural soils
To examine the behavior of heavy metals in the studied soils, correlations between different geochemical phases of the sequential extraction and soil physicochemical properties have been established for all soil samples (Table 3).
Significant correlations were found between chemical forms of Cd, Cu, Pb, and Zn and total metal concentrations or soil physicochemical properties. The results suggested that the behavior of Cd, Cu, Pb, and Zn in the different solid phases in soils were controlled by not only their total concentration but also soil physicochemical properties. Significant negative correlations were found between the exchangeable phase of Cd, Pb, Zn, and pH, which indicated that if pH decreased, the concentration of exchangeable phase will be increased. Soil pH has a significant influence on the environmental behavior of Cd in soil (Pérez-Esteban et al. 2014; Wang et al. 2009); a variation of pH can solubilize or immobilize Cd.
In contrast, only the residual phases of Cr and Ni were significantly positively correlated with their total metal concentrations. Total metal concentrations of Cr and Ni control their residual phases. The exchangeable phase of Ni was negatively correlated with pH and TN, while the exchangeable phase of Cr was not affected by any physical-chemical properties and total Cr concentration. Therefore, behavior of Cr and Ni was mostly affected by their total concentrations in the soil parent material but not the soil physicochemical properties.
Assessment of heavy metal environmental risk
In order to estimate the environmental risk associated with heavy metal pollution in the study area, the soil samples were classified according to the following two methods: the potential ecological risk index (Hakanson 1980) and RAC (Perin et al. 1985) as shown in Table 4.
The assessment of heavy metal ecological risk results (Table 5) indicated that in the mining areas, the ecological risk index for all factors (RI) was between low to moderate risk. The mean \( {E}_r^i \) of Cr, Cu, Ni, Pb, and Zn values were less than 40, which is classified as low ecological risk. However, the \( {E}_r^i \) values of Cd in the studied area were 55.6–188, with a mean of 94, leading to moderate to high risk, suggesting that Cd would bring significant potential ecological risk in the studied area. Also, Cd was the major contributor to the ecological risk index for all factors (RI). The ecological risk Cd was higher than other heavy metals.
The results of RAC (Table 5) indicated that the ecological risk of Cd was highest, with all samples present high or very high risk, whereas the other heavy metals pose no or low ecological risk. Similar results of Cd in soils and sediments were also reported in other studies (Ghrefat et al. 2012; Liu et al. 2013; Yuan et al. 2014b). In our investigated region, coal exploration has been active for decades, and heavy metal emission may increase during coal exploitation, storage, and utilization. Large amounts of coal gangue and fly ash dumped on the surface may contain various heavy metals in which Cd can be long-term reserved in surface soil with high bioavailability (Kuo 1990) and bring strong ecological risk to surrounding environment. The RAC results indicate that high proportions of Cd possibly transfer from agricultural soils to crops, in accordance with the assessment of heavy metal ecological risk results by \( {E}_r^i \)values.
Conclusion
The concentrations, chemical forms, mobility, and environmental risk of Cd, Cr, Cu, Ni, Pb, and Zn in soils from the Linhuan subsidence of Huaibei Coalfield, Anhui Province, China, were investigated in this study. Cd is the major metal pollutant, and the mean concentration of Cd was as high as 3.21 times of background values of Huaibei City. The mean concentrations of other metals were also higher than their background values of Huaibei City surface soil except Pb. Cd and Cu in the soils were mainly from coal mining, washing, and coal consumption, while Cr and Ni were mainly from geogenic source. Both anthropogenic and geogenic sources contributed to the concentrations of Pb and Zn in the soils. Cr, Cu, Ni, Pb, and Zn were mainly present in the residual (above 70 %), while the predominant fraction of Cd was the exchangeable fraction accounting for 42 %. The mobility sequence based on the non-residual fraction decreased in the order of Cd (87 %) > Pb (24 %) > Cu (20 %) > Zn (10 %) > Ni (9 %) > Cr (8 %). The behavior of Cd, Cu, Pb, and Zn in soil was affected by both physicochemical properties and total metal concentrations. In contrast, the behavior of Cr and Ni in the soil was mainly affected by their total metal concentrations. According to assessments by the potential ecological RI and the RAC, Cr, Cu, Ni, Pb, and Zn pose no or low risk. In contrast, the proportions of exchangeable and carbonate fractions of Cd in the soils were very high, which resulted in all samples presenting high or very high risk. More attention is needed by the environmental decision-makers.
References
AHEMC (AnHui Environmental Monitoring Centre) (1992) The research report of backgrounds of Soil Environment in AnHui Province:78
Bhuiyan MAH, Parvez L, Islam MA, Dampare SB, Suzuki S (2010) Heavy metal pollution of coal mine-affected agricultural soils in the northern part of Bangladesh. J Hazard Mater 173:384–392. doi:10.1016/j.jhazmat.2009.08.085
Blaser P, Zimmermann S, Luster J, Shotyk W (2000) Critical examination of trace element enrichments and depletions in soils: As, Cr, Cu, Ni, Pb, and Zn in Swiss forest soils. Sci Total Environ 249:257–280. doi:10.1016/S0048-9697(99)00522-7
Boussen S, Soubrand M, Bril H, Ouerfelli K, Abdeljaouad S (2013) Transfer of lead, zinc and cadmium from mine tailings to wheat (Triticum aestivum) in carbonated Mediterranean (Northern Tunisia) soils. Geoderma 192:227–236. doi:10.1016/j.geoderma.2012.08.029
Chabukdhara M, Nema AK (2013) Heavy metals assessment in urban soil around industrial clusters in Ghaziabad, India: probabilistic health risk approach. Ecotoxicol Environ Saf 87:57–64. doi:10.1016/j.ecoenv.2012.08.032
Chai Y, Guo J, Chai S, Cai J, Xue L, Zhang Q (2015) Source identification of eight heavy metals in grassland soils by multivariate analysis from the Baicheng–Songyuan area, Jilin Province, Northeast China. Chemosphere 134:67–75. doi:10.1016/j.chemosphere.2015.04.008
Chen B, Liu G, Sun R (2016) Distribution and fate of mercury in pulverized bituminous coal-fired power plants in coal energy-dominant Huainan City, China. Arch Environ Contam Toxicol 70:724–733. doi:10.1007/s00244-016-0267-7
CNEMC (The Chinese Environmental Monitoring Centre) (1990) The background values of soil elements in China. Chinese Environment Science Press, Beijing
CNEPA (National Environmental Protection Agency of China) (1995) Environmental quality standard for soils (GB 15618–1995)
Delgado J, Barba-Brioso C, Nieto JM, Boski T (2011) Speciation and ecological risk of toxic elements in estuarine sediments affected by multiple anthropogenic contributions (Guadiana saltmarshes, SW Iberian Peninsula): I. Surficial sediments. Sci Total Environ 409:3666–3679. doi:10.1016/j.scitotenv.2011.06.013
Dong J, Yang QW, Sun LN, Zeng Q, Liu SJ, Pan J, Liu XL (2011) Assessing the concentration and potential dietary risk of heavy metals in vegetables at a Pb/Zn mine site, China. Environmental Earth Sciences 64:1317–1321. doi:10.1007/s12665-011-0992-1
Facchinelli A, Sacchi E, Mallen L (2001) Multivariate statistical and GIS-based approach to identify heavy metal sources in soils. Environ Pollut 114:313–324. doi:10.1016/S0269-7491(00)00243-8
Ghrefat HA, Yusuf N, Jamarh A, Nazzal J (2012) Fractionation and risk assessment of heavy metals in soil samples collected along Zerqa River, Jordan. Environmental Earth Sciences 66:199–208. doi:10.1007/s12665-011-1222-6
Gibbs RJ (1977) Transport phases of transition metals in the Amazon and Yukon rivers. Geol Soc Am Bull 88:829–843. doi:10.1130/0016-7606(1977)88<829:TPOTMI>2.0.CO;2
Guillén MT, Delgado J, Albanese S, Nieto JM, Lima A, De Vivo B (2012) Heavy metals fractionation and multivariate statistical techniques to evaluate the environmental risk in soils of Huelva Township (SW Iberian Peninsula). J Geochem Explor 119–120:32–43. doi:10.1016/j.gexplo.2012.06.009
Hakanson L (1980) An ecological risk index for aquatic pollution control. A sedimentological approach. Water Research 14:975–1001. doi:10.1016/0043-1354(80)90143-8
Hower JC, Robertson JD (2003) Clausthalite in coal. Int J Coal Geol 53:219–225. doi:10.1016/S0166-5162(03)00022-3
Jan FA, Ishaq M, Ihsanullah I, Asim SM (2010) Multivariate statistical analysis of heavy metals pollution in industrial area and its comparison with relatively less polluted area: a case study from the City of Peshawar and district Dir Lower. J Hazard Mater 176:609–616. doi:10.1016/j.jhazmat.2009.11.073
Ji K et al. (2013) Assessment of exposure to heavy metals and health risks among residents near abandoned metal mines in Goseong, Korea. Environ Pollut 178:322–328. doi:10.1016/j.envpol.2013.03.031
Kraemer SM, Hering JG (2004) Biogeochemical controls on the mobility and bioavailability of metals in soils and groundwater—preface. Aquat Sci 66:1–2. doi:10.1007/s00027-004-0004-6
Kuo S (1990) Cadmium buffering capacity and accumulation in Swiss chard in some sludge-amended soils. Soil Sci Soc Am J 54:86–91. doi:10.2136/sssaj1990.03615995005400010013x
Li H, Qian X, Hu W, Wang Y, Gao H (2013) Chemical speciation and human health risk of trace metals in urban street dusts from a metropolitan city, Nanjing, SE China. Sci Total Environ 456–457:212–221. doi:10.1016/j.scitotenv.2013.03.094
Li Z, Ma Z, van der Kuijp TJ, Yuan Z, Huang L (2014) A review of soil heavy metal pollution from mines in China: pollution and health risk assessment. Sci Total Environ 468–469:843–853. doi:10.1016/j.scitotenv.2013.08.090
Liu G, Tao L, Liu X, Hou J, Wang A, Li R (2013) Heavy metal speciation and pollution of agricultural soils along Jishui River in non-ferrous metal mine area in Jiangxi Province, China. J Geochem Explor 132:156–163. doi:10.1016/j.gexplo.2013.06.017
Micó C, Recatalá L, Peris M, Sánchez J (2006) Assessing heavy metal sources in agricultural soils of an European Mediterranean area by multivariate analysis. Chemosphere 65:863–872. doi:10.1016/j.chemosphere.2006.03.016
Morillo J, Usero J, Gracia I (2007) Potential mobility of metals in polluted coastal sediments in two bays of southern Spain. J Coast Res:352–361. doi:10.2112/04-0246.1
Nemati K, Bakar NKA, Abas MR, Sobhanzadeh E (2011) Speciation of heavy metals by modified BCR sequential extraction procedure in different depths of sediments from Sungai Buloh, Selangor, Malaysia. J Hazard Mater 192:402–410. doi:10.1016/j.jhazmat.2011.05.039
Nicholson FA, Smith SR, Alloway BJ, Carlton-Smith C, Chambers BJ (2003) An inventory of heavy metals inputs to agricultural soils in England and Wales. Sci Total Environ 311:205–219. doi:10.1016/S0048-9697(03)00139-6
Nziguheba G, Smolders E (2008) Inputs of trace elements in agricultural soils via phosphate fertilizers in European countries. Sci Total Environ 390:53–57. doi:10.1016/j.scitotenv.2007.09.031
Park J-H, Choi K-K (2013) Risk assessment of soil, water and crops in abandoned Geumryeong mine in South Korea. J Geochem Explor 128:117–123. doi:10.1016/j.gexplo.2013.02.004
Pérez G, López-Mesas M, Valiente M (2008) Assessment of heavy metals remobilization by fractionation: comparison of leaching tests applied to roadside sediments. Environmental science & technology 42:2309–2315. doi:10.1021/es0712975
Pérez-Esteban J, Escolástico C, Masaguer A, Vargas C, Moliner A (2014) Soluble organic carbon and pH of organic amendments affect metal mobility and chemical speciation in mine soils. Chemosphere 103:164–171. doi:10.1016/j.chemosphere.2013.11.055
Perin G, Craboledda L, Lucchese M, Cirillo R, Dotta L, Zanette M, Orio A (1985) Heavy metal speciation in the sediments of northern Adriatic Sea. A new approach for environmental toxicity determination. In: Lekkas TD (ed) Heavy metals in the environment, vol 2, pp. 454–456
Quenea K, Lamy I, Winterton P, Bermond A, Dumat C (2009) Interactions between metals and soil organic matter in various particle size fractions of soil contaminated with waste water. Geoderma 149:217–223. doi:10.1016/j.geoderma.2008.11.037
Rey J, Martínez J, Hidalgo MC, Rojas D (2013) Heavy metal pollution in the Quaternary Garza basin: a multidisciplinary study of the environmental risks posed by mining (Linares, southern Spain). Catena 110:234–242. doi:10.1016/j.catena.2013.06.023
Rodríguez L, Ruiz E, Alonso-Azcárate J, Rincón J (2009) Heavy metal distribution and chemical speciation in tailings and soils around a Pb–Zn mine in Spain. J Environ Manag 90:1106–1116. doi:10.1016/j.jenvman.2008.04.007
Rout TK, Masto RE, Ram LC, George J, Padhy PK (2013) Assessment of human health risks from heavy metals in outdoor dust samples in a coal mining area. Environ Geochem Health 35:347–356. doi:10.1007/s10653-012-9499-2
Šajn R, Aliu M, Stafilov T, Alijagić J (2013) Heavy metal contamination of topsoil around a lead and zinc smelter in Kosovska Mitrovica/Mitrovicë, Kosovo/Kosovë. J Geochem Explor 134:1–16. doi:10.1016/j.gexplo.2013.06.018
Sakurovs R, French D, Grigore M (2007) Quantification of mineral matter in commercial cokes and their parent coals. Int J Coal Geol 72:81–88. doi:10.1016/j.coal.2006.12.009
Shi G, Chen Z, Xu S, Zhang J, Wang L, Bi C, Teng J (2008) Potentially toxic metal contamination of urban soils and roadside dust in Shanghai, China. Environ Pollut 156:251–260. doi:10.1016/j.envpol.2008.02.027
Shi GL, Lou LQ, Zhang S, Xia XW, Cai QS (2013) Arsenic, copper, and zinc contamination in soil and wheat during coal mining, with assessment of health risks for the inhabitants of Huaibei, China. Environ Sci Pollut Res 20:8435–8445. doi:10.1007/s11356-013-1842-3
Silveira MLA, Alleoni LRF, Guilherme LRG (2003) Biosolids and heavy metals in soils. Sci Agric 60:793–806. doi:10.1590/S0103-90162003000400029
Singh KP, Mohan D, Singh VK, Malik A (2005) Studies on distribution and fractionation of heavy metals in Gomti river sediments-a tributary of the Ganges, India. J Hydrol 312:14–27. doi:10.1016/j.jhydrol.2005.01.021
Solgi E, Esmaili-Sari A, Riyahi-Bakhtiari A, Hadipour M (2012) Soil contamination of metals in the three industrial estates, Arak, Iran. Bull Environ Contam Toxicol 88:634–638. doi:10.1007/s00128-012-0553-7
Sun R, Heimbürger L-E, Sonke JE, Liu G, Amouroux D, Berail S (2013) Mercury stable isotope fractionation in six utility boilers of two large coal-fired power plants. Chem Geol 336:103–111. doi:10.1016/j.chemgeo.2012.10.055
Sun R, Streets DG, Horowitz HM, Amos HM, Liu G, Perrot V, Toutain J-P, Hintelmann H, Sunderland EM, Sonke JE (2016) Historical (1850–2010) mercury stable isotope inventory from anthropogenic sources to the atmosphere. Elementa: Science of the Anthropocene 4:000091. doi:10.12952/journal.elementa.000091
Sundaray SK, Nayak BB, Lin S, Bhatta D (2011) Geochemical speciation and risk assessment of heavy metals in the river estuarine sediments—a case study: Mahanadi basin, India. J Hazard Mater 186:1837–1846. doi:10.1016/j.jhazmat.2010.12.081
Tang W-W, Zeng G-M, Gong J-L, Liang J, Xu P, Zhang C, Huang B-B (2014) Impact of humic/fulvic acid on the removal of heavy metals from aqueous solutions using nanomaterials: a review. Sci Total Environ:468–469 . doi:10.1016/j.scitotenv.2013.09.0441014-1027
Tessier A, Campbell PG, Bisson M (1979) Sequential extraction procedure for the speciation of particulate trace metals. Anal Chem 51:844–851. doi:10.1021/ac50043a017
Türkdoğan MK, Kilicel F, Kara K, Tuncer I, Uygan I (2003) Heavy metals in soil, vegetables and fruits in the endemic upper gastrointestinal cancer region of Turkey. Environ Toxicol Pharmacol 13:175–179. doi:10.1016/S1382-6689(02)00156-4
Wang D-Z, Jiang X, Rao W, He J-Z (2009) Kinetics of soil cadmium desorption under simulated acid rain. Ecol Complex 6:432–437. doi:10.1016/j.ecocom.2009.03.010
Yang Z, Wang Y, Shen Z, Niu J, Tang Z (2009) Distribution and speciation of heavy metals in sediments from the mainstream, tributaries, and lakes of the Yangtze River catchment of Wuhan, China. J Hazard Mater 166:1186–1194. doi:10.1016/j.jhazmat.2008.12.034
Yu R, Hu G, Wang L (2010) Speciation and ecological risk of heavy metals in intertidal sediments of Quanzhou Bay, China. Environ Monit Assess 163:241–252. doi:10.1007/s10661-009-0830-z
Yuan G-L, Sun T-H, Han P, Li J, Lang X-X (2014a) Source identification and ecological risk assessment of heavy metals in topsoil using environmental geochemical mapping: typical urban renewal area in Beijing, China. J Geochem Explor 136:40–47. doi:10.1016/j.gexplo.2013.10.002
Yuan X, Zhang L, Li J, Wang C, Ji J (2014b) Sediment properties and heavy metal pollution assessment in the river, estuary and lake environments of a fluvial plain. China CATENA 119:52–60. doi:10.1016/j.catena.2014.03.008
Acknowledgments
The work was financially supported by the National Natural Science Foundation of China (41373108), Science and Technology Support Program of Anhui Provinces (1608085QD79), the Science and Technology Project of Land and Resources of Anhui Province (2013-K-07), and the Key Scientific and Technological Project of Huaibei Mining Industry (Group) Co. Ltd. (HBKY-2014-01). We acknowledge editors and reviewers for polishing the language of the paper and for in-depth discussion.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Stuart Simpson
Electronic supplementary material
ESM. 1
(DOCX 28 kb)
Rights and permissions
About this article
Cite this article
Shang, W., Tang, Q., Zheng, L. et al. Chemical forms of heavy metals in agricultural soils affected by coal mining in the Linhuan subsidence of Huaibei Coalfield, Anhui Province, China. Environ Sci Pollut Res 23, 23683–23693 (2016). https://doi.org/10.1007/s11356-016-7599-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-7599-8