Abstract
This study provides insight into the enhanced in situ bioremediation applied for remediation of groundwater contaminated by petroleum hydrocarbons. Activities prior to the application of this remediation approach included removal of the source of groundwater contamination—an underground storage tank and contaminated sediments—from the unsaturated zone. The hydraulic feasibility of this remediation approach was proved by hydraulic conductivity characterization of the site. Enhanced in situ bioremediation was performed by a combination of biostimulation and bioaugmentation within the closed bipolar system (one extraction and two injection wells). Biostimulation was conducted by addition of nutrients, and stimulation of oxidation processes by injection of H2O2, into the aquifer. Bioaugmentation/re-inoculation was achieved by injection of a zymogenous consortium of hydrocarbon degrading microorganisms isolated from the contaminated groundwater. The average extraction capacity was 0.5 l/s, with an average injection capacity of 0.25 l/s per well. The efficiency of the remediation approach was measured by changes in the content of total petroleum hydrocarbon (TPH), total chemoorganoheterotrophic (TC) and hydrocarbon degrading (HD) microorganisms. After biostimulation and bioaugmentation, the number of TC and HD microorganisms started to increase. This was followed by a decrease of TPH concentration in the groundwater from an initial 6.8–0.5 mg/l at the end of the bioremediation. The applied remediation approach was highly efficient and very effective in reducing TPH to acceptable levels. Together, these facts provide strong evidence of its potential for remediation of groundwater contaminated by petroleum hydrocarbons.
Similar content being viewed by others

Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Groundwater represents about 98 % of the available fresh water of the planet (Alvarez and Illman 2006). The public water supply of most European countries predominantly comprises groundwater. Similarly, about 80 % of the public water supply of the Republic of Serbia comes from groundwater (Polomcic and Ristic Vakanjac 2011). On the other hand, negative effects on groundwater quality are a consequence of many aspects of human activities. Growth and urbanization of the world population (e.g., Saha et al. 2010; Takem et al. 2010), agricultural production (e.g., Jordan and Smith 2005; Jeevanandam et al. 2012), and inadequate disposal of sewage and industrial wastes (e.g., Kelly et al. 1996; Gilchrist et al. 2011; Amin and Alan 2013) are increasing stress upon the groundwater resources. Considering the extensive human use of petroleum hydrocarbons, this group of compounds is among the most common groundwater contaminants. Most contaminated groundwater sites are the result of leaking underground storage tanks, particularly from fuel hydrocarbon storage (Cunningham et al. 2000). Application of the pump-and-treat method for remediation of these sites has proven to be less practical, reliable and economical than first envisioned (Mackay and Cherry 1989; Travis and Doty 1990; National Research Council 1994). Thus, the focus of the remediation approach for groundwater contaminated by petroleum hydrocarbons has been altered to bioremediation. Bioremediation technologies can be broadly classified as ex situ or in situ (Hatzinger et al. 2002; Talley and Sleeper 2006). In situ groundwater bioremediation is a technology that encourages growth and reproduction of indigenous microorganisms to enhance biodegradation of organic constituents in the saturated zone (U.S. EPA 1995). The enhanced process indicates that conditions at the site have been engineered in order to stimulate or accelerate the biological degradation of contaminants. The general aim of this approach is to provide a mechanism for stimulating and maintaining the activity of microorganisms. Successful laboratory biodegradation of petroleum hydrocarbons has been reported by many authors (e.g., Ilić et al. 2011; Gojgic-Cvijovic et al. 2012; Uzochukwu et al. 2014). On the other hand, in situ bioremediation requires understanding and engineering a set of subsurface processes. Optimal remediation performance in field conditions is a result of successful engineering of subsurface processes. The aim of this paper is to provide insight into research and activities to enhance in situ bioremediation of groundwater contaminated by petroleum hydrocarbons. This study was conducted at the Nitex industrial complex from September 19, 2011 to July 01, 2012.
Study area
Serbia is a country situated in southeastern Europe (Fig. 1). The textile industry, Nitex, in the city of Nis (south of Serbia) was active for more than 110 years (1897–2010), and for many decades it was the largest in the country. The total area of the Nitex industrial complex is 82,106 m2. This industrial complex is located on the terrace sediments of Nisava River, with the river bed about 7–8 m below the surface of the research area (Studio Geotecnico dott. Luciano Baratti 2011). The river flows from east to west and represents the southern border of this industrial complex.
A detailed field survey within the entire complex was first conducted in order to assess the current state of the environment. Considering the aim of this paper, activities related to groundwater remediation will be discussed here. Preliminary research activities were performed at existing monitoring wells (Fig. 2a). Based on these results, site L2 was identified as the zone of petroleum hydrocarbon contamination. The underground storage tank at this location was assumed to be the source of contamination. Further research activities included drilling ten boreholes (piezometers) within the site L2 (Fig. 2b). This allowed the lithological composition and aquifer properties within the site to be determined. The surface of the terrain is made of heterogeneous building material, from 0.2 to 1.7 m thick. Quaternary clays make up the following unit, which is from 2.6 to 5.6 m thick. These clay sediments overlay the sandy-gravelly deposits, which are from 1.5 to 3.0 m thick. With increasing depth, the silty and sandy component within these sediments decreases. On the other hand, there is an obvious trend of the coarse component decreasing from east to west. Pliocene clays at the bottom of sandy-gravelly deposits represent the lowest drilled lithological unit. The dominant mineralogical constituents of the sandy-gravelly deposits are quartz, mica and limestone material, while within Pliocene sediments kaolinite represents the most abundant clay mineral (Babič 2012). Within the sandy-gravelly deposits, a confined aquifer has formed, with Quaternary clays as the upper, and the Pliocene clays as the lower impermeable boundary (Fig. 3). Recharge and discharge zones of the aquifer extend outside the industrial complex. There is no hydraulic contact between the river and the aquifer within the industrial complex, due to the existence of impermeable clay sediments in this part of the terrain (Dinić and Punišić 2011). The average depth of the water table was 5.5–5.7 m. Water table measurements at piezometers within L2 showed that the direction of groundwater flow movement was northeast–southwest (Fig. 2).
Materials and methods
Site characterization
Within the site L2, ten boreholes (piezometers) were mechanically drilled, capped and cased with 10.1 cm diameter perforated PVC pipes. The bottom of the perforated interval corresponded to the bottom of the sandy-gravelly deposits, while its top was 1–1.5 m above the water table. The piezometers were completed in Pliocene clays. Spatial analysis of the lithological composition of the site L2 was performed by RockWare RockWorks 14. The collected sediment samples were analyzed for grain size (mechanical shaking and sieving using standard sets of sieves) and mineralogical content. Water table measurements were performed using an Eijkelkamp 11.08.07 device with acoustic and light signal.
Chemical and microbiological analyses
Sediment core samples were transferred into glass jars and stored at 4 °C during transport to the laboratory. Total petroleum hydrocarbons (TPH) from sediments were extracted as per method ISO 16703 (2004) and determined gravimetrically in accordance with DIN EN 14345 (2004), also previously described by Beškoski et al. (2011). Groundwater samples were collected in high-density polyethylene containers prewashed with 1:1 HCl and distilled water, and were rinsed three times before sampling (after at least 10 min of pumping). The groundwater samples were transported to the laboratory on the same day and stored at 4 °C. These samples were analyzed for TPH, total organic carbon (TOC), NO3 −, Mn, Fe, SO4 2−, H2S, Cl− and P. TPH from groundwater samples were extracted as per method ISO 9377-2 (2000). Spatial analysis of the TPH content in groundwater and sediments was performed by Golden Software Surfer 8. Gas Chromatography analysis was conducted using an Agilent 4890D GC with FID detector and HP-1MS (30 m × 0.25 mm) column, and hydrogen as a carrier gas. Injector temperature was constant: 250 °C. The following temperature program was used: initial temperature 40 °C for 10 min, then increases of 9 °C per min until the temperature reached 285 °C, and isothermal at 285 °C for 12 min. The chemical analyses were carried out as per the procedures given in APHA (1995). Other measured parameters were: O2, EC, pH, and KMnO4 consumption. The number of microorganisms was determined by plating appropriate serial dilutions on agar plates incubated at 28 °C. The media used were nutrient agar for total chemoorganoheterotrophs (TC) and mineral base medium containing 2 g/l standard D2 diesel fuel for hydrocarbon degraders (HD), according to Bossert et al. (2002).
Preparation of the zymogenous consortium of microorganisms from the contaminated groundwater
A detailed review of zymogenous consortium preparation was given by Beškoski et al. (2011), and this procedure was used to culture indigenous microorganisms from the contaminated groundwater at the Nitex industrial site. Suspensions of the cultured microbial consortium were then used to inoculate four Erlenmeyer flasks (5 l), each containing 2000 ml of the medium containing 23 g of nutrient broth (Torlak, Belgrade, Serbia); 100 ml of soil extract; 20 g of diesel fuel. Commercial non-toxic and readily biodegradable surfactants, BioSolve CLEAR (Westford, MA, USA) were used as surface active agents. The growth conditions were: temperature, 28 °C; 120 rpm; pH 7.0; duration of growth, 96 h. The microbial population from all four flasks was used to inoculate (approx 1 vol. %) a bioreactor originally designed by us (total volume 1000 l) with a working volume of 800 l, producing the microbial consortium. The medium used was: 12 g/l meat peptone (Torlak, Belgrade, Serbia); 0.2 g/1 (NH4)2HPO4; 50 ml/l soil extract; BioSolve CLEAR original solution (1 ml/l); and 10 g/l of diesel fuel. The growth conditions were: non sterile, 25 °C, aeration and agitation 0.70 volume of air/volume of medium with a minimum of 1 l, pH 7.0 (adjusted with 10 M HCl or NaOH), duration 48 h and sunflower oil (1 ml/l) as antifoam.
System for enhanced in situ groundwater bioremediation
The system for enhanced bioremediation installed at the site L2 consisted of one extraction and two injection wells. These wells were mechanically drilled, capped and cased with 10.1 cm diameter PVC pipes, fully screened across the saturated zone.
Results and discussion
Preliminary research activities
Preliminary research activities were performed at existing monitoring wells within the Nitex industrial complex (Fig. 2a; Table 1).
As can be seen, extremely high values of TPH were observed at MW-1 (within site L2), while at MW-2 (outside L2), TPH were not detected (values were below the limit of detection). At the same time, groundwater from MW-1 was characterized by extreme TOC concentrations. Concurrently, depletion of oxygen and nitrate, and increase of iron and manganese concentrations were observed (MW-1 compared to MW-2; in Table 1). On the other hand, sulfate concentrations did not show any obvious trend, while hydrogen sulfide was not detected. After these preliminary research activities, site L2 was noted as contaminated by petroleum hydrocarbons with the assumption of ongoing anaerobic biodegradation processes.
Detailed research activities
Detailed research was then performed in order to assess the current state of groundwater and sediment contamination within site L2. Groundwater and sediment samples were taken from the water table zone (Table 2).
As can be seen from Table 2, concentrations of TPH in the groundwater ranged from 0.84 to 6.8 mg/l, while the spatial distribution can be seen in Fig. 4. The average concentration of 3.6 mg/l was about six times above the maximum allowable concentration (MAC), according to the Regulation for systematic quality monitoring, indicators for risk assessment of soil degradation and remediation methodology (2010) in Serbia.
The highest petroleum hydrocarbon concentrations were observed in piezometers closest to the underground storage tank and those in the direction of groundwater flow movement. Beyond this direction, concentrations were much lower, but still above MAC. TPH concentrations in sediments ranged from 6 to 5060 mg/kg (Table 2), and their spatial distribution can be seen in Fig. 5.
The highest petroleum hydrocarbons concentrations in sediments were also observed in samples closest to the mentioned storage tank (Fig. 5). Compared to Fig. 4, there was no obvious impact of groundwater flow movement on the spatial distribution of TPH in sediments. The number of TC and HD microorganisms in the sediments was determined. On average, the sediments contained 3.6 × 104 CFU/g of TC, and 1.4 × 103 CFU/g of HD microorganisms. The average number of HD microorganisms was slightly above the limit for successful application of in situ bioremediation (1000 CFU/g), according to the U.S. EPA (1995). Prior to in situ groundwater bioremediation, characterization of hydraulic conductivity within site L2 was performed in order to ascertain the technical feasibility of bioremediation. This parameter controls the rate and the distribution of electron acceptors to the bacteria and is an important factor in determining the potential effectiveness of in situ groundwater bioremediation, as shown by U.S. EPA (1995). For successful application of a bioremediation system, hydraulic conductivity values should be higher than 10−4 cm/s (Bedient et al. 1999). Characterization of hydraulic conductivity was performed by sediment-size analysis of samples from piezometers in Fig. 2. Hydraulic conductivity values (Table 3) were calculated by the Hazen formula (Freeze and Cherry 1979):
where K is a hydraulic conductivity (cm/s), A is a coefficient with value 1.0 for K in cm/s and d in mm, is the grain size diameter at which 10 % by weight of the sample particles are finer, while 90 % are coarser.
As previously mentioned, the water table was between 5.5 and 5.7 m below the surface. Sediments, which overlay the aquifer, were characterized by lower hydraulic conductivity values, ranging from 2.0 × 10−6 to 4.9 × 10−5 cm/s (Table 3), and tended to increase with increasing depth. These values corresponded to the finer sediments, which overlay the sandy-gravelly deposits. Hydraulic conductivity values of aquifer material ranged from 4.0 × 10−4 to 3.6 × 10−3 cm/s, with the highest values in the northeastern part of terrain. Based on the results of hydraulic conductivity characterization, it was concluded that bioremediation of the site L2 would be hydraulically feasible.
Enhanced in situ groundwater bioremediation
Remediation of sites contaminated by leakage from underground storage tanks can include a wide range of activities addressing both soil and groundwater contamination (Kehew and Lynch 2011). In the current study, since the highest concentrations of petroleum hydrocarbons both in groundwater and in sediments were observed in the zone of the underground storage tank, its removal was a first remediation step. Then, in order to prevent the further perpetuation of an associated long-term source of groundwater contamination, sediments from the unsaturated zone of L2 were excavated and transported elsewhere for ex situ bioremediation treatment. Excavation of sediments was followed by in-filling with gravel material to 45 MPa according to the construction requirements for this site, as proposed by Dinić and Punišić (2011). After completion of these works, a system for enhanced in situ groundwater bioremediation was established. This system comprised one extraction (B-4) and two injection wells (B-1 and B-3) (Fig. 6b). Based on the results of previous research, bioremediation wells were placed to form a system with zone of the highest groundwater contamination within it. This remediation treatment used biostimulation and bioaugmentation within a closed bipolar system (Fig. 6a). Biostimulation involves injection of growth substrates, co-substrates, and electron acceptors, which are limiting factors in the biodegradation reaction (Knapp and Faison 1997). On the other hand, bioaugmentation refers to the injection of biomass to increase the subsurface microbial population. The cycle of bioremediation consisted of three phases:
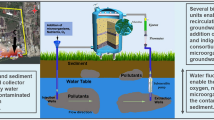
Schematic of the closed bipolar system (a) (1 injection well; 2 extraction well; 3 contaminant plume; 4 direction of groundwater flow movement; 5 unsaturated zone; 6 water table; 7 impermeable boundary; 8 filtration/adsorption column; 9 reservoir) and 3D profile of the system for enhanced groundwater bioremediation (b)
-
Injection of nutrients and chemical oxidant—biostimulation
-
Injection of the zymogenous consortium of microorganisms—bioaugmentation
-
Establishment of recirculation in a closed bipolar system.
This system presupposes detailed understanding of the aquifer and plume properties (3–7). Stimulated in situ bioremediation was started with addition of nutrients from the reservoir (9) through the injection well (1) into the aquifer. Together with nutrients, in order to stimulate chemical oxidation and increase oxygenation of the aquifer, H2O2 was added. Finally, zymogenous HD microbial consortia (previously isolated from contaminated groundwater and laboratory-grown) was added via the same injection well. Recirculation was achieved by extraction of contaminated groundwater using the extraction well (2) followed by filtration through the filtration/adsorption column filled with natural inorganic hydrophobic adsorbents (8) and finally injection to the subsurface through the injection well (1). During water filtration, a biofilm of zymogenous HD microorganisms was formed on the material of the adsorption column. The concentration of TPH in the filtration column was monitored on a daily basis. As a result of intensive microbial activity within the column, TPH concentrations never exceeded remediation values (data not shown). The average extraction capacity was 0.5 l/s, with an average injection capacity of 0.25 l/s per injection well. Remediation treatment was started on May 01, 2012 and it was completed on July 01, 2012. During this period, 1158 m3 of groundwater were treated through the system. The effects of the bioremediation can be seen in Fig. 7.
As can be seen, significant changes of TPH content in groundwater were observed during this enhanced in situ bioremediation. At the end of bioremediation (Fig. 7c), peaks had negligible intensity compared to at the beginning (Fig. 7a). The concentration of TPH decreased from the initial 6.8 mg/l to less than 0.5 mg/l at the end of bioremediation. The efficiency of this bioremediation treatment also can be seen in changes in numbers of TC and HD microorganisms in the groundwater (Fig. 8).
After biostimulation and inoculation of the zymogenous consortium of microorganisms, the number of TC and HD microorganisms started to increase. This was, as expected, followed by a decrease in the TPH concentration in groundwater. The concentration of HD microorganisms reached its maximum 30 days after the beginning of bioremediation. At the end of bioremediation, the concentration of HD microorganisms fell to a minimum, due to the residual and minimal concentration of TPH remaining in the system.
Conclusion
A detailed field survey within the textile industry complex Nitex revealed the urgent necessity of an active remediation approach for the site L2. Both groundwater and sediments within this site had been contaminated by the leakage of petroleum hydrocarbons from an underground storage tank. Concentrations of TPH several times above the MAC were found both in groundwater and sediments. Based on the results of the hydraulic conductivity characterization, it was concluded that bioremediation of the site L2 was hydraulically feasible. In order to avoid the perpetuation of the long term source of groundwater contamination, the underground storage tank responsible for the spread of pollution was first removed from the site. Sediments from unsaturated zone were then excavated (followed by in-filling with gravel material) and transported elsewhere for ex situ bioremediation treatment. Groundwater bioremediation was performed in situ by biostimulation and bioaugmentation within a closed bipolar system (one extraction and two injection wells). Biostimulation was conducted by injection of nutrients and H2O2 into the aquifer. Bioaugmentation/re-inoculation was achieved by injection of a zymogenous consortium of HD microorganisms, which we had previously isolated from the contaminated groundwater. Recirculation was established by an average extraction capacity of 0.5 l/s and an average injection capacity of 0.25 l/s per injection well. The efficiency of the remediation approach was measured by changes in the concentration of TPH, and in numbers of TC and HD microorganisms. After biostimulation and re-inoculation, the number of TC and HD microorganisms started to increase. Levels of HD bacteria reached their maximum 30 days after the start of treatment. The initial concentration of TPH decreased from the initial level of 6.8–0.5 mg/l at the end of the bioremediation. At the same time, the concentration of HD microorganisms reduced to their minimum. These are results of the application of groundwater bioremediation in field conditions. The applied remediation approach was highly efficient (the actual process took just 60 days in total) and very effective in reducing TPH to acceptable levels. Together, these facts provide strong evidence of its potential for remediation of groundwater contaminated by petroleum hydrocarbons.
References
Alvarez JP, Illman AW (2006) Bioremediation and natural attenuation: process fundamentals and mathematical models. John Willey & Sons, New York
Amin EI, Alan Jacobs M (2013) A study of the contaminated banks of the Mahoning River, Northeastern Ohio, USA: characterization of the contaminated bank sediments and river water–groundwater interactions. Environ Earth Sci 70:3237–3244
APHA (1995) Standard methods for the examination of water and wastewater, 17th edn. APHA, Washington, DC
Babič D (2012) Report on the grain size and mineralogical analyzes of alluvial clastic sediments of locality Nitex in Nis. Faculty of Mining and Geology, Belgrade
Bedient P, Rifai H, Newell C (1999) Ground water contamination-transport and remediation, 2nd edn. Prentice Hall, Upper Saddle
Beškoski VP, Gojgić-Cvijović G, Milić J, Ilić M, Miletić S, Šolević T, Vrvić MM (2011) Ex situ bioremediation of a soil contaminated by mazut (heavy residual fuel oil)—a field experiment. Chemosphere 83:34–40
Bossert ID, Shor LM, Kosson DS (2002) Methods for measuring hydrocarbon biodegradation in soils. Manual of environmental microbiology, 2nd edn. ASM Press, Washington, pp 934–943
Cunningham AJ, Hopkins DG, Lebron AC, Reinhard M (2000) Enhanced anaerobic bioremediation of groundwater contaminated by fuel hydrocarbons at Seal Beach, California. Biodegradation 11:159–170
DIN EN 14345 (2004) International standard: characterization of waste. Determination of hydrocarbon content by gravimetry. DIN, Berlin
Dinić S, Punišić Z (2011) Study on performed geotechnical works for the purpose of soil remediation at the site Nitex in Nis. Geoengineering, Nis
Freeze R, Cherry J (1979) Groundwater. Prentice Hall, Upper Saddle River
Gilchrist S, Gates A, Gorring M, Elzinga EJ (2011) Metal contamination and filtering in soil from an iron (magnetite) minesmelter complex in the critical Hudson Highlands watershed, New York. Environ Earth Sci 63(5):1029–1041
Gojgic-Cvijovic DG, Milic SJ, Solevic MT, Beskoski PV, Ilic VM, Djokic SL, Narancic MT, Vrvic MM (2012) Biodegradation of petroleum sludge and petroleum polluted soil by a bacterial consortium: a laboratory study. Biodegradation 23(1):1–14
Hatzinger PB, Whittier MC, Arkins MD, Bryan CW, Guarini WJ (2002) In situ and ex situ bioremediation options for treating perchlorate in groundwater. Remed J 12:69–86
Ilić M, Antić M, Antić V, Schwarzbauer J, Vrvić M, Jovančićević B (2011) Investigation of bioremediation potential of zymogenous bacteria and fungi for crude oil degradation. Environ Chem Lett 9:133–140
ISO 16703 (2004) Soil quality—determination of content of hydrocarbon in the range C10–C40 by gas chromatography. ISO, Geneva
ISO 9377–2 (2000) International standard: water quality—determination of hydrocarbon oil index—Part 2: method using solvent extraction and gas chromatography, First edition 2000-10-15. ISO, Geneva
Jeevanandam M, Nagarajan R, Manikandan M, Senthilkumar M, Srinivasalu S, Prasanna MV (2012) Hydrogeochemistry and microbial contamination of groundwater from lower Ponnaiyar Basin, Cuddalore District, Tamil Nadu, India. Environ Earth Sci 67:867–887
Jordan C, Smith RV (2005) Methods to predict the agricultural contribution to catchment nitrate loads: designation of nitrate vulnerable zones in Northern Ireland. J Hydrol 304:316–329
Kehew AE, Lynch PM (2011) Concentration trends and water-level fluctuations at underground storage tank sites. Environ Earth Sci 62:985–998
Kelly J, Thornton I, Simpson PR (1996) Urban geochemistry: a study of the influence of anthropogenic activity on the heavy metal content of soils in traditionally industrial and nonindustrial areas of Britain. Appl Geochem 11:363–370
Knapp RB, Faison BD (1997) A bioengineering system for in situ bioremediation of contaminated groundwater. J Ind Microbiol Biotechnol 18:189–197
Mackay DM, Cherry JA (1989) Groundwater contamination: pump-and-treat remediation. Environ Sci Technol 23:630–636
National Research Council (1994) Alternatives for ground water cleanup. National Academy Press, Washington, DC
Polomčić D, Ristic Vakanjac V (2011) Groundwater in water supply of Serbia—current state and perspectives. Faculty of Mining and Geology, Beograd, pp 45–77
Regulation for systematic quality monitoring, indicators for risk assessment of soil degradation and remediation methodology (2010) Official Gazette, no. 88, Belgrade, p 8 (in Serbian)
Saha D, Sarangam SS, Dwived SN, Bhartariya KG (2010) Evaluation of hydrogeochemical processes in arsenic-contaminated alluvial aquifers in parts of Mid-Ganga Basin, Bihar, Eastern India. Environ Earth Sci 61(4):799–811
Studio Geotecnico dott. Luciano Baratti (2011) Report on the preliminary assessment of soil and aquifer pollution in the industrial complex of Nitex in Nis, MACE, Serbia
Takem GE, Chandrasekharam D, Ayonghe SN, Thambidurai P (2010) Pollution characteristics of alluvial groundwater from springs and bore wells in semi-urban informal settlements of Douala, Cameroon, Western Africa. Environ Earth Sci 61(2):287–298
Talley WF, Sleeper PM (2006) Roadblocks to the implementation of biotreatment strategies. Annu NY Acad Sci 829:16–29
Travis CC, Doty CB (1990) Can contaminated aquifers at superfund sites be remediated? Environ Sci Technol 24:1464–1466
U.S. EPA (1995) How to evaluate alternative cleanup technologies for underground storage tank sites: a guide for corrective action plan reviewers. (EPA 510-B-94-003; EPA 510-B-95-007; and EPA 510-R-04-002)
Uzochukwu CU, Jones DM, Head MI, Manning ACD, Fialips IC (2014) Biodegradation of crude oil saturated fraction supported on clays. Biodegradation 25. Issue 1:153–165
Acknowledgments
This study was supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia under Grant No. 43004.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marić, N., Ilić, M., Miletić, S. et al. Enhanced in situ bioremediation of groundwater contaminated by petroleum hydrocarbons at the location of the Nitex textiles, Serbia. Environ Earth Sci 74, 5211–5219 (2015). https://doi.org/10.1007/s12665-015-4531-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-015-4531-3