Abstract
Contamination of soils with heavy metals is widespread and poses a long-term risk to ecosystem health. Abandoned and active mining sites contain residues from ore-processing operations that are characterised by high concentrations of heavy metals. The distribution and mobility characteristics of heavy metals (As, Cd, Cu, Pb, and Zn) in paddy soil samples from Kočani Field (Macedonia) using ICP-EAS and a sequential extraction procedure was evaluated. The results indicate that highly elevated concentrations of As, Cd, Cu, Pb, and Zn were detected in the paddy soil sample from location VII-2 in the vicinity of Zletovo mine and Zletovska river in the western part of Kočani Field, which drains the untreated acid mine waters and mine wastes from the active Zletovo mine. The degree of contamination based on index of geoaccumulation (I geo) from strong to weak in the paddy soils samples is Pb > As > Cd > Zn > Cu. The mobility potential of heavy metals in all paddy soil samples increases in the order As < Cu < Pb < Zn < Cd. According to the results of the anthropogenic impact on the paddy soils, a further study on the heavy metal concentrations in rice and other edible crops, the remediation process of the paddy soils and a dietary study of the local population are needed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
High concentrations of heavy metals are usually found in the vicinity of abandoned and active mines due to the discharge and dispersion of the mines’ untreated waste materials into nearby agricultural soils, food crops, riverine water, and stream sediments (Jung 2001; Korre et al. 2002; Liu et al. 2005; Lu and Zhang 2005). Consequently, contamination of soils with heavy metals originating from abandoned and active mines is widespread and may adversely affect environmental quality.
Accurate measurements of the heavy metal content of the neighbouring soils are required to assess the potential risk of pollution. But to evaluate the true short- and long-term environmental impact of heavy metals, one of the most crucial factors to consider is the mobility of heavy metals. The chemical behaviour of heavy metals in soils and thus their mobility is controlled by several factors, including the soil type, pH, cationic exchange capacity, nutrient status, organic matter content, redox potential, texture, the nature of the contamination in terms of origin, and characteristics of the deposition/composition and environmental conditions that may lead to weathering (Ure 1996). The soluble, exchangeable, and chelated metal species in the soils are the only labile fractions available to plants (Kabata-Pendias 1993). Therefore, assessment of the environmental risks requires measurement not only of the total amount of heavy metals in the soils, but also of the heavy metals present in the available fraction. A widely used method for the identification and evaluation of the availability of heavy metals or the binding forms of heavy metals in soils is soil leaching by means of chemical extractants. During recent decades, several leaching/extraction tests have been developed and modified for these purposes in the fields of geochemistry, marine chemistry, and agricultural science (Tack and Verloo 1995). Among the various sequential procedures presented, the most widely applied is that proposed by Tessier et al. (1979).
Numerous studies have investigated the heavy metal concentrations in soils all around the world (Kabata-Pendias 1993). However, in Macedonia studies of heavy metal concentrations in soils derived from the abandoned and active mine wastes are very rare (Dolenec et al. 2007). A little is known about the distribution of heavy metals in soils derived from historical and/or recent base-metal mining, milling, and smelting activities in different parts of Macedonia. Such an example is Kočani Field in eastern Macedonia, which is well known for its thermal waters, long history of base-metal mining, and paddy fields of rice (Oryza sativa L.). The untreated wastes from old abandoned mine sites and the present mining activities in the Pb–Zn Zletovo-Kratovo and Sasa-Toranica ore district, located in the vicinity of the Kočani Field, were spread across the entire region of Kočani Field, comprising a high load of heavy metals. Continuous water sampling in the area of Kočani Field has suggested that the riverine waters from Zletovska river and Bregalnica river used for irrigation of the Kočani paddy fields were contaminated with heavy metals on account of the discharge of acid mine water and untreated effluents derived from the ore-processing facilities from the Zletovo mine and Sasa mine into the abovementioned rivers (Serafimovski et al. 2004). For this reason, it is very likely that the paddy soil in this area contains increased levels of heavy metals.
In this context, the objectives of the present study were:
-
to detect the total heavy metal concentrations in paddy soil samples from sampling points in Kočani Field,
-
to apply the sequential extraction method (leaching procedure) to determine the available fraction of heavy metals in the investigated soil samples,
-
to evaluate the distribution of heavy metals and their mobility characteristics,
-
to assess the degree of contamination with an index of geoaccumulation (I geo) and
-
to conduct an environmental risk.
Materials and methods
Study area
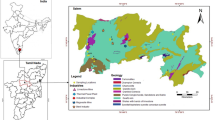
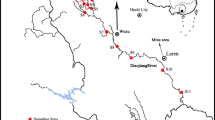
The study area of Kočani Field is located in eastern Macedonia, about 32 km from the city of Stip and 115 km from the capital city, Skopje. With an average length of 35 km and width of 5 km, Kočani Field is situated in the valley of Bregalnica river between the Osogovo Mountains in the north and the Plačkovica Mountains in the south (Fig. 1). The paddy soil of Kočani Field was estimated to originate from the composite material of the sediment derived from igneous, metamorphic, and sedimentary rocks transported by the Bregalnica river and its tributaries and deposited in the Kočani depression (Dolenec et al. 2007).
The broader region has a long history of mining dating to the pre-Middle Ages, with the most recent phase of mining starting after the Second World War.
The Zletovo Pb–Zn deposit is situated along the active continental margin and is intimately associated with the Tertiary volcanism and hydrothermal activity of the area. The Zletovo mine is located 5 km NW from the Zletovo village and about 7 km from the city of Probistip (Fig. 1). Continuous exploitation of the mine started after the Second World War and it has an annual capacity of 300,000 tons (9% Pb and 2% Zn) and significant concentrations of Ag, Bi, Cd, and Cu. The mine is active to date with production of Pb–Zn concentrate. Mineral association comprises galena (principal ore mineral) and sphalerite, with subordinate pyrite, lesser amounts of siderite, chalcopyrite, and occasional pyrrhotine, marcasite, and magnetite. Minor occurrences of U-mineralisation have also been discovered (pitchblende). Ore is concentrated by flotation at Probistip and tailings stored in two impoundments situated in adjacent valleys (Serafimovski et al. 2004).
The Sasa-Toranica ore district lies in the Osogovo Mountains, 10 km from the city of Makedonska Kamenica. It occupies an area of about 200 km (Fig. 1). The formation of the Sasa deposit is related to the late Alpine tectonic, magmatic and mineralisation processes. The Sasa mine has been in production for over 45 years, yielding 90,000 tons of high-quality Pb–Zn concentrate annually. The important Pb and Zn deposits are always accompanied by variable amounts of Cu, Au, Ag, Mo, and Sb. The ore is usually found in quartz–muscovite–graphitic schists and also in greenschists and marbles. Ore is concentrated at the mine by flotation, and tailings are stored in a dam in a narrow valley just below the mine (Serafimovski et al. 2004).
Therefore, the mining activities, old abandoned mine sites, bare tailings, and large amounts of untreated waste material as well as the effluents from the Pb–Zn Zletovo and Sasa mines have caused an increase in heavy metal loads across the entire region and surrounding ecosystem (water, sediment, soil, biota, …).
The Zletovska river drains the central part of the Kratovo-Zletovo volcanic complex and the flotation plant at Probistip from the Pb–Zn Zletovo mine and its ore-processing facilities (Fig. 1). The Bregalnica river, together with its tributaries (Kamenica river), drains not only the igneous, metamorphic, and sedimentary rocks dating from Precambrian to Holocene, but also mine waste, including tailings, mill sewages, and mine effluents from the abandoned and active Pb–Zn mines (Sasa) (Fig. 1).
Previous investigations have confirmed that the water from the Zletovska and Bregalnica rivers was contaminated with heavy metals as a result of untreated mining wastes from the Zletovo-Kratovo and Sasa-Toranica ore districts (Serafimovski and Aleksandrov 1995). Repeated water sampling of the Zletovska river has shown marked chemical variations of As (1.7–25 μg l−1), Cd (0.5–5 μg l−1), Cu (6–10 μg l−1), Pb (10–24 μg l−1) and Zn (101–1,250 μg l−1), which can be explained by the water-flow conditions, the varying degrees of mine edits and tailing dam leaching effluent inputs from the Pb–Zn Zletovo mine into the riverine water. The determined concentrations of the above-mentioned heavy metals in the Bregalnica are lower than values from the Zletovska: As (0.53 μg l−1), Cd (0.39 μg l−1), Cu (3 μg l−1), Pb (2.4 μg l−1) and Zn (67 μg l−1) (Serafimovski et al. 2004).
The Kamenica, one of the most severely polluted tributaries of the Bregalnica, drains untreated mine effluents from the Pb–Zn polymetallic ore deposit at Sasa directly into the artificial Lake Kalimanci. Upon mixing with the lake water, the concentrations of the pollutants decline. For that reason, the Bregalnica, when it flows out from Lake Kalimanci, is less polluted than the Kamenica and Zletovska rivers. The pollution of the Zletovska is also easily recognisable in the field. The bed sediments are coated with Fe and Mn oxides/hydroxides, which are the major sink for contamination with several trace elements (Dolenec et al. 2007).
Both the Zletovska and Bregalnica waters are used for irrigation purposes by local farmers for the surrounding paddy fields. Thus, the contaminated riverine waters of the Zletovska and Bregalnica represent a serious pollution source that could affect the soil as well as the food crops of Kočani Field.
Soil sampling and analysis
The sampling of the soil took place in autumn 2005 in order to determine concentrations of the potentially toxic heavy metals, including As, Cd, Cu, Pb, and Zn, that originate from base metal mining activities in the region. The soil was collected from five locations across the Kočani paddy fields (Fig. 2). Near-surface soils were sampled (0–20 cm in depth), because in the agricultural soil it is not possible to distinguish the A, B, and C horizons. The samples were obtained using a plastic spade to avoid any heavy metal contamination. Each soil sample comprised a composite of five sub-samples taken within a 1 × 1 m square. After air-drying at 25°C for a week, the soil samples were disaggregated and sieved through a 2 mm polyethylene sieve to remove plant debris, pebbles, and stones. Soil samples were then ground in a mechanical agate grinder to a fine powder for subsequent geochemical analysis.
All paddy soil samples were analysed for As, Cd, Cu, Pb, and Zn concentrations in a certified commercial Canadian laboratory (Acme Analytical Laboratories Ltd.) after extraction for 1 h with 2-2-2-HCl-HNO3-H2O at 95°C by inductively coupled plasma mass spectrometry (ICP-MS). The accuracy and precision of the soil analyses were assessed using international reference material such as CCRMR SO-1 (soil) and USGS G-1 (granite). The analytical precision and accuracy were better than ±5% for the analysed elements.
Sequential extraction of heavy metals
The binding forms of heavy metals in soil samples were established according to the sequential chemical extraction method (Tessier et al. 1979; Li et al. 1995). The soil samples, weighing 1 g, were placed in screw-top test tubes. To each sample was added 10 ml of leaching solution, the caps were screwed on, and the tubes were subjected to the appropriate extraction procedure depending on the stage of the leach. For sequential leaching, the sample was leached, centrifuged, decanted, and washed and then the residue was leached again in a five-step process from the weakest to strongest solution or chemical reagent: water → ammonium acetate → sodium pyrophosphate → cold hydroxylamine hydrochloride → hot hydroxylamine hydrochloride. Thus, the sequential extraction method operationally defines heavy metals in five chemical fractions:
-
1.
water soluble fraction (distilled water),
-
2.
exchangeable and carbonate bound fraction (1 M ammonium acetate),
-
3.
organic (oxidisable) fraction (0.1 M sodium pyrophosphate),
-
4.
Mn hydroxide (reducible) fraction (cold 0.1 M hydroxylamine hydrochloride), and
-
5.
Fe hydroxide (reducible) plus crystalline Mn hydroxide (residual) fraction (hot 0.25 M hydroxylamine hydrochloride).
The concentration of heavy metals in the solution was then measured using a Perkin Elan 6000 ICP-MS for the determination of 60 or more elements. QA/QC protocol incorporated a sample duplicate to monitor the analytical precision, a reagent blanks measured background, and an aliquot of in-house reference material to monitor accuracy.
Results and discussion
Total heavy metal concentration
The total concentrations of heavy metals (As, Cd, Cu, Pb, and Zn) in all paddy soil samples from Kočani Field together with the assumed permissible levels of heavy metals adopted by the National Environmental Protection Agency of Slovenia (Uradni List RS 1996) and the maximum allowable concentrations of trace elements in agricultural soil proposed by the German Federal Ministry of the Environment (1992), as well as the critical total soil heavy metal concentration ranges defined by Kabata-Pendias and Pendias (1984), are given in Table 1. The critical total soil heavy metal concentration is determined as the range of values above which toxicity is considered to be possible.
The data clearly show that the paddy soil samples from locations I-3, II-6, III-5, and VI-4 contain slightly elevated heavy metal concentrations but the paddy soil sample from section VII-2 in the vicinity of the Zletovska River is highly impacted by all the investigated heavy metals: As, Cd, Cu, Pb, and Zn. The increased concentrations of As (42.0 mg/kg), Cd (5.6 mg/kg), Cu (99 mg/kg), and Pb (892 mg/kg) and very high concentration of Zn (1,134 mg/kg) were detected in the paddy soil sample from section VII-2 (Table 1). The concentration values of heavy metals in paddy soil sample VII-2 exceeded the emission limit values reported by Kabata-Pendias and Pendias (1984), and the national environmental protection agencies of Slovenia and Germany (Tables 1, 2).
Many areas throughout the world have been affected by heavy metal contamination of soil, plants, waters, and sediments as a result of metalliferous mining activities (Bird et al. 2003; Jung 2001; Korre et al. 2002; Lee et al. 2001; Witte et al. 2004; Wong et al. 2002). All the heavy metals studied are important ore-forming elements and are paragenetically linked to the Pb–Zn polymetallic mineralisation of the Zletovo-Kratovo ore district (Zletovo mine) and the Sasa-Toranica ore district (Sasa) (Dolenec et al. 2007). The paddy soil sample VII-2, located in the vicinity of the Zletovska river and Zletovo mine, received a comparatively higher input of anthropogenically derived heavy metals than other sampling locations. This pollution is undoubtedly related to the irrigation of the paddy fields with riverine water from the Zletovska, which drains acidic mine waters and untreated wastes from Zletovo mine and is considered to be moderately to highly polluted compared with the less polluted Bregalnica river (Serafimovski et al. 2004). The soil samples from other sampling locations are irrigated with Bregalnica riverine water.
Index of geoaccumulation
The index of geoaccumulation (I geo), introduced by Müller (1969, 1979), can be used to assess metal pollution in soils. I geo is expressed as follows I geo = log2 C N/1.5B N, where C N is measured concentration of examined metal N in the soils and B N is the geochemical background concentration of the metal N. In our calculation of I geo, B N is the concentration of studied metals in the earth’s crust (Taylor and Mclennan 1995). The factor 1.5 is applied because of the possible variations in the background values due to lithological variations.
The Müller Index of Geoaccumulation, I geo, is divided into seven grades ranging from unpolluted to very seriously polluted (Table 3). Grade 6 indicates a 64-fold enrichment over the background values (Singh et al. 1997).
The I geo values of As, Cd, Cu, Pb, and Zn in the studied soils are shown in Table 3. According to the defined I geo classes, the soil sample from location VII-2 was highly to very highly polluted with As, very seriously polluted with Cd and Pb, moderately polluted with Cu and highly polluted with Zn. The soil samples from other locations were moderately to highly polluted with As and Pb, moderately polluted with Cd, uncontaminated to moderately polluted with Cu and uncontaminated to moderately contaminated with Zn. Consequently, the degree of contamination from strong to weak in the paddy soils samples was Pb > As > Cd > Zn > Cu.
From an environmental point of view, it is notable that the paddy soil sample from location VII-2 with highly elevated heavy metal concentrations and values of index of geoaccumulation represents a serious risk for surrounding ecosystems.
Sequential extraction procedure (heavy metal-binding forms)
When the sequential leaching procedure is applied to the chemical partitioning of heavy metals in soil samples, extractants such as electrolytes, weak acids, and chelating agents release metals from coordination sites, while strong acids and redox agents are capable of releasing additional quantities of metals as a result of the decomposition of the solid matrix.
In the sequential extraction procedure the labile/residual fractions considered were water soluble fraction (1), exchangeable and carbonate bound fraction (2), bound to organic matter—oxidisable fraction (3), bound to amorphous Mn hydroxide—reducible fraction (4), and bound to amorphous Fe hydroxide and crystalline Mn hydroxide—reducible and residual fraction (5).
Figure 3 presents the results of the sequential extraction procedure (heavy metal-binding forms).
The water soluble fraction (1) includes metal species, which are easily soluble and thus highly mobile and potentially bioavailable in the environment. The leaching of metals in this fraction is a major environmental concern (Filgueiras et al. 2002). The most leachable portions in soil samples were observed for Cu (3.8%, Fig. 3c).
The exchangeable fraction (2) contains (electrostatically) weakly bound heavy metal species, which can be released through ion-exchange processes, and metals, which are precipitated with carbonates (Filgueiras et al. 2002). Changes in ionic composition, influencing adsorption–desorption reactions, or lowering the pH could cause the remobilisation of metals from this fraction. Metals in the exchangeable fraction are the most readily available for plant uptake and therefore very labile. In all paddy soil samples only, Cd showed a strong tendency to be extracted in this fraction (49.21%, Fig. 3b).
The oxidisable fraction (3) corresponds to elements occurring as oxidisable minerals and organically bound metals. Under oxidising conditions, this fraction releases metals linked to organic matter within the soil matrix into solution. The extraction of Cu (70%, Fig. 3c) was predominantly connected only with this fraction in all paddy soil samples. The element As was also bound to the oxidisable fraction (42.45%, Fig. 3a).
The reducible fraction (4) comprises unstable metal forms connected with amorphous Mn hydroxides. The elements strongly bound to these oxides are thermodynamically very unstable under reducing conditions and therefore easily discharged and available for the surrounding biota. According to Fig. 3, three of the heavy metals studied exhibited a clear tendency to be leachable in the reducible fraction. Cd (68%, Fig. 3b), Zn (49.19%, Fig. 3e), and Pb (38.96%, Fig. 3d) are to a considerable extent leachable and thus potentially bioavailable in all paddy soil samples.
In the reducible plus residual fraction (5) the metals are linked to amorphous Fe hydroxides (reducible part) and under reducing conditions are expected to be released in nature. The residual fraction contains naturally occurring crystalline Mn hydroxide minerals which may hold heavy metals within their crystalline matrix. Heavy metals in residual are not likely to be discharged under normal environmental conditions. Therefore, the metals associated with this fraction can only be mobilised as a result of weathering (Dean 2007; Filgueiras et al. 2002; Fuentes et al. 2004; Kazi et al. 2002). The distinct inclination of the potentially toxic metals As (64%, Fig. 3a), Pb (55.3%, Fig. 3d), and Zn (36.9%, Fig. 3e) to be partitioned in the residual fraction is especially worth mentioning.
Mobility potential of heavy metals
The mobility, immobility, and consequently the toxicity of heavy metals in paddy soils depend most of all on their types of binding forms. Table 4 displays the mobility potential of heavy metals in different forms. All the heavy metals investigated were extracted in the water-soluble fraction in very low percentages (Fig. 3). The Cu has the highest percentage mobility in the water-soluble fraction, which means that it should be the most readily available element from the environmental point of view. Because the percentage of Cu in the extraction solution was very low, Cu does not represent a serious environmental risk. Cd has the highest ability, susceptibility, and mobility potential to be released from the soil by a simple ion-exchange mechanism. However, Cu was found to be relatively insensitive to ion-exchange processes. Cu and As absorbed into the organic matter appear to be very labile under aerobic conditions and stable under anaerobic conditions. Under varied reducing conditions, mobilisation and release of Zn and Cd from the soils are expected. Pb and As represent the highest proportions in the reducible and residual fractions, which indicates that these two elements are the most non-mobile and thus potentially the least harmful. The residual concentration of any heavy metal is considered to be the non-mobile fraction and is an important part influencing the nature of the heavy metal mobility. Consequently, Cd is the most mobile and As the least mobile element, and the mobility potential of all heavy metals increases in the order As < Pb < Zn < Cu < Cd in the paddy soil samples studied.
Given the results of the anthropogenic impact on the paddy soils, a further study on the heavy metal concentrations in rice and other edible crops and the remediation of the paddy soils by the immobilisation of heavy metals are an unavoidable necessity. A project for diminishing heavy metal content in contaminated soils by adding materials which have a high capacity to bind metals in possibly slightly mobile fractions (phosphorites, zeolites, montmorillonites, and humic organic matter) is in preparation.
A dietary study of the local population is also needed to assess the possible health risk.
Conclusions
In this study, the distribution and mobility characteristics of heavy metals (As, Cd, Cu, Pb, and Zn) in paddy soil samples from Kočani Field, Macedonia, were investigated. The total concentrations of heavy metals were detected using ICP-EAS analysis and heavy metal-binding forms were determined using a sequential extraction procedure. The results showed that the paddy soil sample (section VII-2) from the western part of Kočani Field in the vicinity of the Zletovska river exhibited very high concentrations of As, Cd, Cu, Pb, and Zn which significantly exceeded the limits proposed by the Slovenian and German environmental agencies and critical total soil concentration ranges given by Kabata-Pendias and Pendias 1984. The elevated concentrations of As, Cd, Cu, Pb, and Zn in paddy soil sample VII-2 undoubtedly indicated heavy metal contamination related to the irrigation of paddy fields with riverine water of the Zletovska River which drains the untreated wastes from Zletovo mine. According to the heavy metal concentrations and I geo values described the paddy soil sample from location VII-2 represents a serious potential environmental risk to the surrounding ecosystems.
The most exchangeable, labile, and available element for plant uptake and furthermore for possible contamination of the surrounding ecosystem in all paddy soil samples is Cd. The elements Pb and Zn are mostly associated with amorphous Mn hydroxides, and with amorphous Fe hydroxides in the reducible fraction. For that reason they are very unstable and mobile under reducing conditions. Cu is predominantly bound to organic matter and consequently released under oxidising conditions into the environment. The element As is also weakly linked to organic matter and in larger proportions connected to amorphous Fe hydroxides, which are usually very leachable, and to more crystalline Mn hydroxides, which are not expected to escape under normal environmental conditions. The mobility potential of heavy metals in paddy soil samples increases in the order As < Cu < Pb < Zn < Cd.
Given the results of the anthropogenic impact on the paddy soils, a further study on the heavy metal concentrations in rice and other edible crop, the remediation process of the paddy soils and a dietary study of the local population are an unavoidable necessity.
References
Bird G, Boewer PA, Macklin MG, Baltenan P, Driga B, Serban M, Zaharia S (2003) The solid state partitioning of contaminant metals and As in river channel sediments of the mining affected drainage basin, northwestern Romania and eastern Hungary. Appl Geochem 18:1583–1595. doi:10.1016/S0883-2927(03)00078-7
Dean JR (2007) Bioavailability, bioaccessibility and mobility of environmental contaminants, vol 92. Wiley, England, pp 106–292
Dolenec T, Serafimovski T, Tasev G, Dobnikar M, Dolenec M, Rogan N (2007) Major and trace elements in paddy soil contaminated by Pb–Zn mining: a case study of Kočani Field, Macedonia. Environ Geochem Health 29:21–32. doi:10.1007/s10653-006-9057-x
Filgueiras AV, Lavilla I, Bendicho C (2002) Chemical sequential extraction for metal partitioning in environmental solid samples. J Environ Monit 4:823–857. doi:10.1039/b207574c
Fuentes A, Llorens M, Saez J, Soler M, Ortuno J, Meseguer V (2004) Simple and sequential extraction of heavy metals from different sewage sludges. Chemosphere 54:1039–1047. doi:10.1016/j.chemosphere.2003.10.029
German Federal Ministry of the Environment (1992) Novelle zur Verordnung über das Aufringen von Klärschlamm (Bundesgesetzblatt)
Jung MC (2001) Heavy metal contamination of soils and waters in and around the Imcheon Au-Ag mine, Korea. Appl Geochem 16:1369–1375. doi:10.1016/S0883-2927(01)00040-3
Kabata-Pendias A (1993) Behaviour properties of trace metals in soils. Appl Geochem 2:3–9. doi:10.1016/S0883-2927(09)80002-4
Kabata-Pendias A, Pendias H (1984) Trace elements in soils and plants. CRC Press, Boca Raton
Kazi T, Jamali G, Kazi G, Arain M, Afridi H, Siddiqui A (2002) Evaluating the mobility of toxic metals in untreated industrial wastewater sludge using a BCR sequential procedure and a leaching test. Anal Bioanal Chem 374:255–261. doi:10.1007/s00216-002-1482-9
Korre A, Durucan S, Koutroumani A (2002) Quantitative-spatial assessment of the risks associated with high Pb loads in soils around Lavrio, Greece. Appl Geochem 17:1029–1045. doi:10.1016/S0883-2927(02)00058-6
Lee CG, Chon HT, Jung MC (2001) Heavy metal contamination in the vicinity of the Daduk Au-Ag-Pb-Zn mine in Korea. Appl Geochem 16:1377–1386. doi:10.1016/S0883-2927(01)00038-5
Li XD, Coles BJ, Ramsey MH, Thornton I (1995) Sequential extraction of soils for multielement analysis by ICP-AES. Chem Geol 124:109–123. doi:10.1016/0009-2541(95)00029-L
Liu H, Probst A, Liao B (2005) Metal contamination of soils and crops affected by the Chenzhou lead/zinc mine spill (Hunan, China). Sci Total Environ 339:153–166. doi:10.1016/j.scitotenv.2004.07.030
Lu A, Zhang X (2005) Environmental geochemistry study of arsenic in Western Hunan mining area P.R. China. Environ Geochem Health 27:313–320. doi:10.1007/s10653-004-5735-8
Müller G (1969) Index of geoaccumulation in sediments of the Rhine River. Geojournal 2:108–118
Müller G (1979) Schwermetalle in den sedimentaen des Rheins-Veranderungen seit 1971. Umsch Wiss Tech 79:778–783
Serafimovski T, Aleksandrov M (1995) Lead and zinc deposits and occurrences in the Republic of Macedonia. Special edition of RGF, No. 4, 387 pgs., with extended summary in English, Stip
Serafimovski T, Alderton DHM, Mullen B, Fairall K (2004) Pollution associated with metal mining in Macedonia. 32nd International Geological Congress, FL
Singh M, Ansari AA, Müller G, Singh IB (1997) Heavy metals in freshly deposit sediments of the Gomati River (a tributary of the Ganga River): effects of human activities. Env Geol 29:246–252. doi:10.1007/s002540050123
Tack FMG, Verloo MG (1995) Chemical speciation and fractionation in soil and sediment heavy-metal analysis—a review. Int J Environ Anal Chem 59:225–238
Taylor SR, Mclennan SM (1995) The geochemical evolution of the continental crust. Rev Geeophys 33:611–627. doi:10.1029/95RG00262
Tessier A, Campbell PGC, Bisson M (1979) Sequential extraction procedure for the speciation of particulate trace metals. Anal Chem 51:844–851. doi:10.1021/ac50043a017
Uradni List RS (1996) Uredba o mejnih opozorilnih in kritičnih emisijskih vrednostih nevarnih snovi v tleh. Uradni list 68:5773–5774
Ure AM (1996) Single extraction schemens for soil analysis and related applications. Sci Tot Environ 17:178–183
Witte KM, Wanty RB, Ridley WI (2004) Engelman Spruce (Picea engelmannii) as a biological monitor of changes in soil metal loading related to past mining activity. Appl Geochem 19:1367–1376. doi:10.1016/j.apgeochem.2004.01.022
Wong SC, Li XD, Zhang G, Qi SH, Min YS (2002) Heavy metals in agricultural soils of the Pearl River Delta, South China. Environ Pollut 119:33–44. doi:10.1016/S0269-7491(01)00325-6
Acknowledgments
The research was financially supported by the Slovenian Research Agency (ARRS), contract number 1000-05-310229.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rogan, N., Dolenec, T., Serafimovski, T. et al. Distribution and mobility of heavy metals in paddy soils of the Kočani Field in Macedonia. Environ Earth Sci 61, 899–907 (2010). https://doi.org/10.1007/s12665-009-0405-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-009-0405-x