Abstract
Background/Aim
Right lobe living donor (2/3rd partial hepatectomy) model is the best way to accurately study liver regeneration process in human beings. We aimed to study the kinetics of liver regeneration after 2/3rd partial hepatectomy in donors.
Methods
Retrospective analysis of prospectively maintained volumetric recovery data in donors was performed in 23 donors, who underwent 29 contrast-enhanced computed tomography within 3 months for various clinical indications.
Results
The absolute volumetric growth percentages were as follows: 37.60 ± 21.74 at 1st week, 92 ± 53.27 at 2nd week, 115.55 ± 59.65 at 4th week, and 110.79 ± 64.47 at 3 months. On sub-group analysis of our cohort, we found that 4.3%, 17%, 30.4%, and 39% donors attended ≥ 90% volumetric recovery at 1st, 2nd, 4th week, and 3 months, respectively. One patient at 4th week revealed 128% volumetric recovery. There was one more patient who exceeded original total liver volumes (TLV) (111% of TLV) at 2.5 months. The serum bilirubin and INR values peaked at postoperative day (POD) 3rd and then started showing a downward trend from POD 5th onwards.
Conclusion
Our study is the first to document complete volumetric recovery in donors as early as 3 weeks. Two of the donors overshot their original TLV during the early regenerative phase.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The fundamental principle in living donor liver transplant (LDLT) lies in the fact that it should provide enough liver volume (graft recipient weight ratio > 0.8) with a safe remnant (approximately 30% to 35% of total liver volume). The main pretext for safe donor hepatectomy is based primarily on the ability of the livers to regenerate. Right lobe living donor (2/3rd partial hepatectomy) model is the best way to accurately study regeneration process in human beings. Several studies addressing this issue have been done in the past with variable results. Some of the older studies done in the setting of LDLT demonstrated that liver regeneration occurs rapidly in the initial 4 weeks with the maximum occurring in the first week [1, 2]. A multi-center study from the West concluded that regeneration in donors is a brisk process and that substantial regeneration occurs by 3 months post-hepatectomy [3]. The majority of the studies were using a protocol-based imaging on donors at various time intervals to assess regeneration of remnant, posing an unwarranted risk of radiation to healthy donors [2, 3]. We have not used a protocol-based approach in our study in order to avoid unnecessary radiation and included only those donors in whom we had to undertake imaging studies for various clinical indications. In the present study, we have analyzed our prospectively collected imaging data from 23 donors for volumetric recovery in the early regenerative phase (≤ 3 months).
Methods
Between March 2013 and July 2017, we performed 194 liver transplantation (31 deceased donor and 163 LDLT). Our selection criteria for the living donors were as follows: age between 18 and 60 years, liver attenuation index (LAI) ≥ +5, minimum remnant liver volume of 30%, and no evidence of uncontrolled cardiopulmonary co-morbidities. The study was approved by an Institutional review board (Ethics committee). The study was based on prospectively maintained contrast-enhanced computed tomography (CECT) data, which was retrospectively analyzed for volumetric regeneration in 23 donors. A total of 29 CECT were included over a period of 3 months after donor hepatectomy. Clinical indications for imaging studies were as follows: unusual abdominal pain (n = 5), unexplained fever (n = 4), high total leukocyte count (n = 4), intra-abdominal bleed (n = 2), ascites (n = 2), ileus (n = 2), bile leak (n = 1), cholestasis (n = 1), dengue fever (n = 1), and obstructed incisional hernia (n = 1). Preoperative CECT with volumetric analysis using Myrian (R) XP intra-sense 1.18.0 software was used to calculate total liver volumes (TLV), right and left lobe or ramnant volumes, which acted as the baseline for the regeneration study. All the donors underwent right hepatectomy with subtotal/partial middle hepatic vein harvest, translating into more than 60% of the total liver volume (or 2/3rd partial hepatectomy model for human beings, range 56% to 69%). All the 23 donors underwent abdominal CECT for various clinical indications at different time points after hepatectomy (not protocol-based). We included only those CECT scans which were performed within 3 months after donor hepatectomy. We have arbitrarily defined early regenerative phase as 3 months for our study. The primary end-point of the study was to look for liver volumetric recovery at the end of 3 months. The secondary end-point was to look for a trend of functional (synthetic) recovery in donors (i.e. serum bilirubin and the international normalized ratio [INR], of prothrombin time values) at the end of 1 week as most of the donors get discharged by 9th-day after surgery. The following variables were included: donor’s age, sex, body mass index (BMI), LAI, duration between scans, TLV, preoperative remnant left liver volume (RLL), regenerated volumes at various time frames, absolute volumetric growth percentage (AVG%) and volumetric recovery percentage compared to TLV (VR%). Liver regeneration was studied by dividing time frame into 1 week, 2 weeks, 4 weeks, and 3 months periods. Liver volumes were calculated using Myrian (R) XP-intra-sense 1.18.0 software and compared with pre-transplant remnant volume in donors and graft volume in recipients. For Accuracy test of Myrian (R) XP-intra-sense 1.18.0 software, estimated and actual volumes of 60 consecutive donor cohort for graft (right lobe) had a Pearson’s coefficient of 0.83 (Sable. S, unpublished data). Liver volumes were calculated in cubic centimeters (cc) on imaging. The percentage growth remnant was calculated using the formula AVG% = difference between the regenerated volume on postoperative day (PODx) and pre-operative remnant volume/preoperative remnant volume multiplied by 100 and volumetric recovery (VR) % = difference between the regenerated volume on MODx multiplied by 100/TLV). All the donor liver biopsies (intraoperative) were histologically graded into three groups: (i) no steatosis: 10 donors, (ii) up to 5% steatosis: 10 donors, and (iii) 5% to 10% steatosis: 3 donors. None of the donors had more than 10% steatosis. Liver function was assessed using serum bilirubin and INR in the first postoperative week. Liver function test (LFT) including INR was done daily for first 3 days and later on alternate days till discharge. After discharge, LFTs were done only when clinically indicated. We did not include transaminases in our study as it represents only ischemia-reperfusion injury (or hepatocyte death) and does not represent the synthetic function.
Statistical analysis
Normality distribution of the data was tested using Shapiro-Wilk test. Statistical analysis was performed using SPSS software version 21.0.0. Results were expressed as median or mean and standard deviation or range for continuous variables. Univariate analysis was done using one-way analysis of variance as the data were normally distributed. Regression analysis was used to identify parameters associated with liver regeneration and determine the correlation between volumetric recovery percentage and various parameters included in the study (except for LFT). P-values of ≤ 0.05 were considered significant.
Results
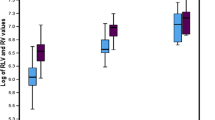
Total 23 donors (14%) underwent 29 CECT (some of the donors required more than one scan). The mean age of the donors was 36.9 ± 11.2 (range: 20–58 y), out of them 13 (56%) were female. The mean BMI of the donors was 24.9 ± 4.0 (range 17.7–32.8 kg/m2) and their mean LAI was 8.7 ± 6.5. All the donors underwent right hepatectomy with subtotal MHV (harvesting middle hepatic vein keeping intact segment IVA drainage in the donors) [4] and average remnant volume was 36.5 ± 6.8% cc (left lobes). Tables 1 and 2 show the relevant liver volumetric data and biochemical functions of all the 23 donors. Fourteen donors did not reveal any significant findings on imaging. Two donors with intra-abdominal bleed were re-explored and later recovered uneventfully. One subject with bile and ascitic fluid leak from the main wound was managed successfully with percutaneous drainage. One donor with obstructed incisional hernia required emergency re-exploration and repair. There was no donor mortality. The average hospital stay was 9.7 ± 2.5 days. All the donors had normal histology except one, who had mild periportal fibrosis. None of the donors had macro-vesicular steatosis of more than 10% on histology. The liver volumetric recovery in the first week after donor hepatectomy was 58.11 ± 20% of the original TLV (one [4.3%] of the donors attained 90% of TLV) and absolute growth was 37.6 ± 21.7%. Within the 2nd postoperative week, the mean volumetric recovery was 71.9 ± 18.3% and absolute growth was 92 ± 53.3% of the remnant. In the 2nd week, volumetric recovery in 4/23 (17%) patients was ≥ 90% of TLV and absolute growth was ≥ 100% in 7/23 (30%) of the subjects. At the end of 3rd week, 10/23 (43%) subjects attained ≥ 80% and 5/23 (21%) ≥ 90% of volumetric recovery. There was one (4.3%) subject in this group who had 114% (original TLV) volumetric recovery within 3 weeks after donor hepatectomy (Fig. 2a, b). By the 4th week, 11/23 (47.8%) subjects had ≥ 80% volumetric recovery and 7/23 (30.4%) had ≥ 90% recovery (Fig. 1). In one subject in this group, the scan was repeated at the 4th week, which revealed further increase in volume to 128% of original TLV. There was one more patient who exceeded original TLV (111% of original) at 2.5 months (Fig. 2a, b). On sub-group analysis, we found that 2/23 (8.6%) donors achieved >100% volumetric recovery before 3 months. The absolute volumetric growth was 110 ± 53% and volumetric recovery as compared to TLV was 80.6 ± 19% at the end of 3 months. The mean volumetric growth percentages were as follows: 37.6 ± 21.74 at 1st week, 92 ± 53.27 at 2nd week, 115.55 ± 59.65 at 4th week, and 110.79 ± 64.47 at 3 months. The mean volumetric recovery percentages were as follows: 58.11 ± 20.5 at 1st week, 71.93 ± 18.38 at 2nd week, 76.68 ± 18.62 at 4th week, and 80.4 ± 19.38 at 3 months. On sub-group analysis, we found that 4.3%, 17%, 30.4%, and 39% donors attended ≥ 90% volumetric recovery (compared to TLV) at 1st, 2nd, 4th week, and 3 months, respectively. The mean serum bilirubin values were as follows: preoperative (baseline) 0.49 ± 0.22, POD 1: 1.46 ± 0.64, POD 3rd: 2.59 ± 1.60, POD 5th: 2.25 ± 1.61, and POD 7th: 1.57 ± 1.18. The INR values were preoperative (baseline): 1.02 ± 0.04, POD 1st: 1.25 ± 0.34, POD 3rd: 1.36 ± 0.22, POD 5th: 1.3 ± 0.16, and POD 7th: 1.17 ± 0.14 (Table 2 and Fig. 3). The serum bilirubin and INR values peaked at POD 3rd and then started showing downward trend from POD 5th onwards (Fig. 3). The serum bilirubin values did not reach the preoperative levels in the first postoperative week. INR values nearly attained the preoperative (baseline) levels at the end of 1 week. On regression analysis, age (p-value = 0.59), BMI (p-value = 0.11), LAI (p-value = 0.09), TLV (p-value = 0.51), and RLL (p-value = 0.77) had no significant bearing on volumetric recovery (Table 1).
a (Donor 1) Post-contrast donor axial CT scan demonstrates right and left lobe highlighted in gray shade line of demarcation. Total liver volume (TLV) is 889 cm3. Left lobe has a volume of 286 cm3 (31% of TLV). 2b: Post-contrasts axial CT done in donor at 3 weeks demonstrates regeneration of the remnant left lobe (RLL, light green) with the volume of 1091 cm3 (114% of TLV). b (Donor 2) Post-contrast donor axial CT scan demonstrates right and left lobe highlighted in gray shade line of demarcation. Total liver volume (TLV) is 844 cm3. Left lobe has a volume of 308 cm3(36% of TLV). 2 Post-contrasts axial CT done in donor at 10 weeks demonstrates regeneration of the remnant left lobe (RLL, light green) with the volume of 943 cm3 (111% of TLV)
Discussion
In the current study, we found that complete liver volumetric recovery can occur as early as 3 to 4 weeks. This is probably the first study documenting volumetric recovery over-shooting the original TLV during early regenerative phase. One of the donors in our cohort had volumetric recovery of 128% of TLV within 3 weeks and the other had recovery 111% of TLV within 3 months. We did not perform serial imaging in our donors for the purpose of studying liver regeneration as it would pose unnecessary risk of radiation to them. Although our donors represent only those who had clinically some form of morbidity, these data reveal that regeneration is not affected by any of these morbidities. Early studies by Marcos et al. (2000) and Scatton et al. (2004) demonstrated liver regeneration occurs as early as 1 week, although they documented 100% regeneration it was only in terms of remnant growth. They did not take into consideration the original TLV of donor while reporting regeneration of remnant liver volume [1, 5]. One hundred percent regeneration of the remnant only means that RLL has doubled it volume but not reached the original TLV. Complete regeneration of liver within the first week (5–7 days) has only been document in rodent studies till date [6]. Gruttadauria et al. in 2012 demonstrated absolute volumetric growth by 94.7 ± 37.5% in 2 months, whereas our study revealed an absolute growth of ≥ 100% in one-third donors by the end of 2nd week [7]. This means the remnant volumes doubled in 30% of our donors at the end of 2nd week. In a study by Pomfret et al. RLL regenerated up to 70% when compared to TLV in 1 week and up to 80% (compared to TLV) in 1 month [8]. Another study by Pomfret et al. later demonstrated that liver regeneration is a long process and showed complete liver regeneration in only one donor at 12 months [9]. In general, this study showed the RLL reached 83.4 ± 9% compared to TLV in 12 months [9]. Our sub-group analysis revealed 30% donors attaining ≥ 90% volumetric recovery at 4 weeks and up to 40% donors attaining ≥ 90% volumetric recovery at the end of 3 months. In our study, we have noticed that not all the donors had uniform regenerative process; some of the donors showed incomplete/slow regeneration while others had surplus of original total liver volume in a very short period of time. We would also like to highlight that regeneration is variable in terms of duration and volume in different individuals and there are multiple factors which affect liver regeneration. In a study by Haga et al. RLL reached 68.9% of TLV in 1 month, 89.8% in 6 months, and about 80% in 1 year. None of the donors in his study reached preoperative TLV till 1 year [10]. Akoi et al. showed 51%, 57%, and 64% of TLV in 1, 2, 4 weeks, 74%, 77%, and 81% in 3, 6, 12 months, and 88% in 4 years [11]. In our study, we noticed average volumetric recovery of 71%, 76%, and 80% at 2 weeks, 4 weeks and 3 months suggesting a much rapid regeneration in our cohort. Reason for these differences remains unknown. Kim et al. (2013), showed 58.7 ± 7.4% and 81.5 ± 11.2% volumetric recovery (compared to TLV) within 1 week and 3 months after surgery which is comparable to volumetric data in our donor cohort [12]. It appears that liver regeneration is not affected by racial differences to a certain extent. Studies by Yokoi et al. (2005) and Chan et al. (2006) are the only studies documenting near complete volumetric recovery at 1 year and more than 2 years after donor hepatectomy [13, 14]. Although our study does not document the long-term volumetric recovery but two of our donors did show a complete regeneration within 3 months. Recent multicenter study by Olthoff et al. a in Adult-to-Adult Living Donor Liver Transplantation Cohort Study (A2ALL cohort, 2015) showed volumetric recovery in 6.3% (14/350) donors at the end of 3 months probably the only study reporting 100% volumetric recovery at 3 months [3]. Our results are comparable to A2ALL cohort 3-month volume model in donors in terms of complete volumetric recovery (i.e. 100% of TLV). Another recent study by Duclos et al. (2015) showed volumetric recovery of 64%, 71%, and 85% at the end of 7 days, 30 days and 1 year [15]. None of the donors achieved complete volumetric recovery in this study [15]. To the best of our knowledge, ours is the first human study documenting volumetric recovery in surplus of original total liver volume (> 100% of TLV) in two donors during early regenerative phase (≤ 3 months). Ours is also the first ever report from India to study regeneration kinetics in donors. The concept of “mitosis and apoptosis” or “heliostat control” in regeneration after partial hepatectomy model may be able to explain this over-shooting of liver volumes beyond original TLV [6, 16]. As hypothesized by Sakamoto et al. in rodent studies, a small wave of apoptosis of hepatocyte is noticed at the end of DNA synthesis that prevents over-shooting of regenerative response [16]. We believe that this apoptotic wave may be missing in some of the donors leading to surplus regeneration above TLV. Liver regeneration is a complex process associated with signalling cascades involving growth factors, cytokines, matrix remodelling and stimulation and inhibition of growth-related signals. It would be very difficult to pinpoint the exact cause leading to over-shooting of liver regeneration in donors.
Our biochemical data suggest mild liver insufficiency is inevitable in first 72 h but by the end of 1-week serum bilirubin and INR values show a trend towards baseline values. Although serum bilirubin values showed a trend towards normalization it did not reach the baseline values. Serum INR values in our donor cohort reached normal range at the end of 1 week and as compared to preoperative (baseline) it was nearly the same. Contrary to the available literature [17,18,19], we believe the delayed return in serum bilirubin is most probably secondary to remnants without MHV rather than delay in synthetic function. We did not try to document liver functions (LFT) if donors were doing clinically well and did not follow any universal protocol for repeating LFTs after 1 week. Liver function tests were checked by protocol at 3 months and 1 year in all donors and had reached baseline values in all cases. We do not have fixed time point protocol LFTs between 1 week and 3 months. Two of our donors had ascites which suggest partial liver insufficiency although they did not meet post-hepatectomy liver failure (PHLF) criteria and recovered well, later both showed good liver volumetric recovery. There are multiple studies reporting complete normalization of liver function within 1 week [1, 9, 20, 21]. Some documenting functional recovery within 1 month [13, 22,23,24,25], while some reported that it may take up to 3 months to 1 year [12, 15, 26,27,28]. There was a lot of heterogeneity in these studies in terms of defining a normal liver function and the biochemical parameters used. Although most of these used serum bilirubin to define liver functions, but the definition of normal bilirubin levels remains unknown. Ideal definition of normalization of liver synthetic function would mean returning of values to their original baseline; however, while trying to document this would lead to unnecessary needle punctures in an otherwise healthy donor. Although we did have normalization of LFT in our donors at follow up ranging from 1 week to 3 months. We did not include this data in our study as it was not available for all 23 donors. We choose serum bilirubin and INR as a biochemical parameter to study synthetic function as they depict true function in the early post-hepatectomy phase. We did not use liver enzymes as it is not a marker for synthetic function and represent only damage/death of hepatocyte. Serum albumin has a very long half-life (3 weeks) and levels in the immediate postoperative periods may be falsely low due to hemodilution effect. Unlike other studies, we have included LFT data only for the first week to demonstrate the trend towards normalization of synthetic function. The serum bilirubin values and INR values peaked at POD 3rd and started showing down ward trend from POD 5th onwards. The average RLL (with steatosis less than 10%) in our donors was 37.5%, probably explaining the minimal derangement of liver function and the rapid trend towards recovery. The values did not reach the pre-op values in first postoperative week. We believe there is no clinical implication of repeating LFT’s once they show a trend towards normalization unless clinically required.
Recently, some of the studies have tried to analyze predictive factors associated with the regenerative process with variable results [3, 15]. Our study did not have any statistically significant association between age (p-value = 0.59), BMI (p-value = 0.11), LAI (p-value = 0.09), TLV (p-value = 0.51) and RLL (p-value = 0.77) and volumetric recovery. Our hypothesis to this is firstly the volume required to get a meaningful association is lacking in our study secondly there may be some inherent bias in donor selection which may have resulted in lack of significant association between these parameters. None of the donors had steatosis greater than 10% which may explain good regeneration in our cohort in terms of volumes and duration.
This study is not without limitations, most important of which is its retrospective nature. The relatively small number of patients included in our study limited the number of variables that could be simultaneously studied on regression analysis. This is a single-center study performed in a tertiary care highly specialized hospital and so our results cannot be generalized to larger heterogeneous populations. Our liver volumetry data represent a capture of regeneration within 3 months and as most of the donors had only single CECT so it would be difficult to estimate the rate of liver regeneration and how it may differ inter- individually. It would be interesting to follow up the two patients who had over-shooting of liver volumes within 3 weeks and 3 months to know whether the mitosis effect sustains or apoptosis takes over and brings down the volumes to original TLV. However, there is no clinically justifiable reason to subject them to another CECT.
In conclusion, our study is the first to document complete volumetric recovery in donors as early as 3 weeks, unlike previous studies where the donor had incomplete restoration of volumes even at the end of 1 year. Interestingly, two of the donors overshot their original TLV during the early regenerative phase. Serum bilirubin and INR values peak at 3rd day and show downward trend by the end of 1 week. Age, BMI, LAI, TLV, and RLL did not have a significant effect on volumetric recovery.
References
Marcos A, Fisher RA, Ham JM, et al. Liver regeneration and function in donor and recipient after right lobe adult to adult living donor liver transplantation. Transplantation. 2000;69:1375–9.
Humar A, Kosari K, Sielaff TD, et al. Liver regeneration after adult living donor and deceased donor split-liver transplants. Liver Transpl. 2004;10:374–8.
Olthoff KM, Emond JC, Shearon TH, et al. Liver regeneration after living donor transplantation: adult-to-adult living donor liver transplantation cohort study. Liver Transpl. 2015;21:79–88.
Soin AS, Mohanka R, Singla P, et al. Segment IV preserving middle hepatic vein retrieval in right lobe living donor liver transplantation. J Am Coll Surg. 2011;213:e5–16.
Scatton O, Belghiti J, Dondero F, et al. Harvesting the middle hepatic vein with a right hepatectomy does not increase the risk for the donor. Liver Transpl. 2004;10:71–6.
Michalopoulos GK. Liver regeneration. J Cell Physiol. 2007;213:286–300.
Gruttadauria S, Parikh V, Pagano D, et al. Early liver regeneration of remnant liver volume after right hepatectomy for living donation: a multiple regression analysis. Liver Transpl. 2012;18:907–13.
Pomfret EA, Pomposelli JJ, Lewis WD, et al. Live donor adult liver transplantation using right lobe grafts: donor evaluation and surgical outcome. Arch Surg. 2001;136:425–33.
Pomfret EA, Pomposelli JJ, Gordon FD, et al. Liver regeneration and surgical outcome in donors of right-lobe liver grafts. Transplantation. 2003;76:5–10.
Haga J, Shimazu M, Wakabayashi G, et al. Liver regeneration in donors and adult recipients after living donor liver transplantation. Liver Transpl. 2008;14:1718–24.
Aoki T, Imamura H, Matsuyama Y, et al. Convergence process of volumetric liver regeneration after living-donor hepatectomy. J Gastrointest Surg. 2011;15:1594–601.
Kim SJ, Na GH, Choi HJ, You Y, Kim DG. Effect of donor right hepatectomy on splenic volume and platelet count for living donor liver transplantation. J Gastrointest Surg. 2013;17:1576–83.
Yokoi H, Isaji S, Yamagiwa K, et al. Donor outcome and liver regeneration after right-lobe graft donation. Transpl Int. 2005;18:915–22.
Chan SC, Lo CM, Wong Y, Liu CL, Fan ST. Long-term biological consequences of donor right hepatectomy including the middle hepatic vein in adult-to-adult live donor liver transplantation. Liver Transpl. 2006;12:259–63.
Duclos J, Bhangui P, Salloum C, et al. Ad integrum functional and volumetric recovery in right lobe living donors: is it really complete 1 year after donor hepatectomy? Am J Transplant. 2016;16:143–56.
Sakamoto T, Liu Z, Murase N, Ezure T, et al. Mitosis and apoptosis in the liver of interleukin-6–deficient mice after partial hepatectomy. Hepatology. 1999;29:403–11.
Cattral MS, Molinari M, Vollmer CM Jr, et al. Living-donor right hepatectomy with or without inclusion of middle hepatic vein: comparison of morbidity and outcome in 56 patients. Am J Transplant. 2004;4:751–7.
Yang HR, Jeng LB, Li PC, et al. Living donor right hepatectomy with inclusion of the middle hepatic vein: outcome in 200 donors. Transplant Proc. 2012;44:460–2.
de Villa VH, Chen CL, Chen YS, et al. Right lobe living donor liver transplantation—addressing the middle hepatic vein controversy. Ann Surg. 2003;238:275–82.
Fan ST, Lo CM, Liu CL, Yong BH, Chan JK, Ng IO. Safety of donors in live donor liver transplantation using right lobe grafts. Arch Surg. 2000;135:336–40.
Inomata Y, Uemoto S, Asonuma K, Egawa H. Right lobe graft in living donor liver transplantation. Transplantation. 2000;69:258–64.
Fujita S, Kim ID, Uryuhara K, et al. Hepatic grafts from live donors: donor morbidity for 470 cases of live donation. Transpl Int. 2000;13:333–9.
Sakamoto S, Uemoto S, Uryuhara K, et al. Graft size assessment and analysis of donors for living donor liver transplantation using right lobe. Transplantation. 2001;71:1407–13.
Ito T, Kiuchi T, Egawa H, et al. Surgery-related morbidity in living donors of right-lobe liver graft: lessons from the first 200 cases. Transplantation. 2003;76:158–63.
Cho JY, Suh KS, Kwon CH, et al. Outcome of donors with a remnant liver volume of less than 35% after right hepatectomy. Liver Transpl. 2006;12:201–6.
Nadalin S, Testa G, Malagó M, et al. Volumetric and functional recovery of the liver after right hepatectomy for living donation. Liver Transpl. 2004;10:1024–9.
Rudow DL, Brown RS, Emond JC, Marratta D, Bellemare S, Kinkhabwala M. One-year morbidity after donor right hepatectomy. Liver Transpl. 2004;10:1428–31.
Ibrahim S, Chen CL, Wang CC, et al. Liver regeneration and splenic enlargement in donors after living-donor liver transplantation. World J Surg. 2005;29:1658–66.
Acknowledgements
We would like to express our gratitude and thanks to Dr. Agrima Gera, Dr. Nilesh Gumardhar, and Mr. Rahul Jadhav for their support till completion of this article.
Author information
Authors and Affiliations
Contributions
Study concept and protocol design, and initial draft for manuscript: SS and VK, data collection, and volumetric recovery data analysis; SS, SM, SS and KY. Critical revision of manuscript and intellectual content: SS, AC, VV, SK, and VK.
Corresponding author
Ethics declarations
Conflict of interest
SS, SM, SS, KY, AC, SK, VV, and VK declare that they have no conflict of interest.
Ethics statement
The authors declare that the study was performed in a manner conforming to the Helsinki declaration of 1975, as revised in 2000 and 2008 concerning human and animal rights, and the authors followed the policy concerning informed consent as shown on Springer.com.
Rights and permissions
About this article
Cite this article
Sable, S.A., Maheshwari, S., Sharma, S. et al. Kinetics of liver regeneration in donors after living donor liver transplantation: A retrospective analysis of “2/3rd partial hepatectomy” model at 3 months. Indian J Gastroenterol 37, 133–140 (2018). https://doi.org/10.1007/s12664-018-0838-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12664-018-0838-9