Abstract
Background
The successful application of ex vivo liver resection and autotransplantation (ERAT) has gained widespread attention for the treatment of end-stage hepatic alveolar echinococcosis, which is considered to be unresectable by conventional methods due to extensive invasion of the extra- and intrahepatic vasculature. However, data on remnant liver volume (RLV) are limited, and the safe volume limit of remnant liver is still unclear.
Methods
To determine the effect of liver volume in the technically developed era, we investigated the impact of the remnant liver-to-standard liver volume ratio (RLV/SLV) on the outcomes of ERAT.
Results
From February 2014 to May 2018, 56 ERAT procedures were performed. Eleven patients with an RLV/SLV < 40% (group S) were compared with 45 patients with an RLV/SLV ≥ 40% (group L). Serial changes in postoperative serum total bilirubin, alanine aminotransferase, aspartate aminotransferase, and international normalized ratio were comparable in both groups. The incidences of postoperative complications did not significantly differ between the two groups. Three patients died of intra-abdominal bleeding, acute cerebral hemorrhage, and severe liver dysfunction. In RLV estimation analysis, the actual RLV and RLV/SLV were significantly smaller than the expected RLV and RLV/SLV as determined by preoperative three-dimensional reconstruction software in patients with hepatic venous outflow obstruction.
Conclusion
Patients with a smaller RLV/SLV did not have outcomes inferior to those with a larger RLV/SLV. Further studies are warranted to clarify the factors that contribute to preoperative volumetric estimation and the safe lower limits for ERAT.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hepatic alveolar echinococcosis (HAE), caused by larval stage of Echinococcus multilocularis, is one of the most severe zoonotic parasitic diseases and is highly endemic in the central part of Western Europe, parts of the near East, Russia, the central Asian republics, China, Northern Japan, and Alaska.1,2 The expanding of HAE cases may be related to both the increased fox population and to the contribution of dog infection.1 HAE, known as “parasitic cancer,” manifests an insidious onset and slow progression, which often delay diagnosis.3 Radical resection combined with albendazole has been deemed the optimal treatment for HAE. When the disease progresses to the advanced stage with extensive invasion of the extra- and intrahepatic vasculature, conventional radical resection is extremely hazardous due to the potential risk of uncontrollable hemorrhage and a long ischemia time. Multiple unconventional methods including liver transplantation (LT)4 and ex vivo liver resection and autotransplantation (ERAT)5 have been used to address this complicated situation. We reported the first HAE patient treated with ERAT with replacement of the retrohepatic inferior vena cava (IVC) in 2014.6 Since then, the application of ERAT has been widely used in our center to treat end-stage HAE.
One of the complicated issues in ERAT is the threshold of remnant liver volume (RLV) required to meet functional demand. For improved surgical outcomes, we proposed that RLV be at least 40% of the estimated standard liver volume (SLV), which is in accordance with the experience in living donor liver transplantation (LDLT).7 Small-for-size graft, which is defined as a graft-to-recipient weight ratio < 0.8% or as a remnant liver-to-standard liver volume ratio (RLV/SLV) < 40%, has been associated with poor outcomes and increased mortality in LDLT recipients.8 It is widely accepted that a minimum threshold of 25 to 30% of the SLV for normal livers can meet the functional demand during major resection.9,10,11 However, an increased RLV has been associated with a decreased occurrence of posthepatectomy liver insufficiency.9 ERAT has a longer anhepatic time, more severe hemodynamic disturbances, longer liver cold ischemia time, and more complicated vascular reconstruction than LDLT or liver resection (LR).5 Moreover, a relatively long period between primary infection and surgery enables compensatory hypertrophy of the remnant liver, which leads to a remarkably varied RLV among end-stage HAE patients. Thus, volumetric estimation in ERAT may not always follow the same guidelines as LDLT. To the best of our knowledge, there are very few studies investigating the critical minimum RLV required to safely perform ERAT.
Volumetric estimation of the remnant liver can be performed preoperatively with a three-dimensional (3D) reconstruction system.12 The estimated liver volume has been demonstrated to have a good correlation with the actual RLV in many reports.13,14 However, the discrepancy between the estimated and actual liver volume often exists in patients with a high body mass index (BMI)15 or altered liver transection plane.16 Aside from these factors, preoperative hepatic venous outflow obstruction (HVOO) caused by a large HAE lesion has demonstrated major effects on the deviation between the estimated and actual RLV.
In this study, we compared the surgical outcomes between ERAT patients with an actual RLV/SLV < 40% or ≥ 40% and analyzed the potential risk factors affecting postoperative complications. In addition, the deviation caused by HVOO in volumetric calculations and its impact on preoperative evaluations are also discussed.
Materials and Methods
The study was approved by the Ethics Committee of West China Hospital of Sichuan University (No. 2017-38) and was performed in accordance with the Declaration of Helsinki.
Study Populations
From February 2014 to May 2018, a total of 56 consecutive ERAT procedures were performed at our center. The patients, including 53 adults and 3 children, were divided into two groups according to the actual RLV/SLV: group L (n = 45), which consisted of patients with an actual ratio ≥ 40%; and group S (n = 11), which consisted of patients with an actual ratio < 40%.
Pretransplant Evaluation and Indications for ERAT
All patients underwent imaging studies including computed tomography (CT) (Fig. 1a) and magnetic resonance imaging to evaluate the characteristics of the lesion and vascular infiltration and to assess whether any extrahepatic metastasis existed. HVOO was defined as infiltration of the hepatic outflow of the remnant liver by HAE on CT. ERAT was considered when the following distinguishing features were present: (1) advanced HAE that was deemed “unresectable” with the use of traditional techniques because there was difficulty exposing or removing the lesion and a lack of reconstruction techniques and materials; (2) involvement of the hepatocaval region, three hepatic veins, and the retrohepatic vena cava or invasion of the tertiary branches of the portal veins and portal arteries requiring complex reconstruction with a prolonged ischemic time that the liver could not tolerate; and (3) good physiological state of the patient including normal liver and kidney function and extrahepatic echinococcosis lesions that could be surgically removed or controlled with albendazole.5
The key preoperative assessment and surgical procedures for ERAT for patients with advanced alveolar echinococcosis. a Preoperative CT revealed a large lesion in the right liver lobe, and the outflow of the remnant liver was invaded. b Preoperative 3D reconstruction of the HAE lesions, which provided information regarding the anatomy, estimated RLV/SLV, and surgical planning. c Venoplasty of the outflow tracts of the liver autograft during bench resection was performed to create a wider outflow orifice. d An extended incision was made in the anterior wall of the vena cava to increase the size of the outflow anastomosis
3D reconstruction software (IQQA-Liver; EDDA Technology, Inc., USA) (Fig. 1b) was used to calculate the RLV and to detect the main conduit anatomy and the spatial location of large lesions.12 The SLV was calculated based on the formula developed by Urata et al.17 The preliminary selection criteria for the remnant liver included an estimated RLV/SLV ≥ 40%. The actual RLV was obtained on the back table during surgery.
Surgical Procedure
The technical details of ERAT have been described previously.5 Surgery was performed through a reversed T-shaped incision. After mobilization of the liver, the phrenic veins were divided to expose the suprahepatic IVC, and the intrapericardial IVC was lowered. In five patients, it was necessary to open the pericardium from below to obtain sufficient length for the caval clamp. The portal structures were divided with sufficient length for reimplantation. The liver was removed and perfused with 0–4 °C histidine–tryptophan–ketoglutarate solution (Custodiol; Dr. Franz Kohler Chemie, Germany). An artificial vascular graft (InterGard; InterVascular SAS, Inc., La Ciotat, France) was used to reconstruct the IVC, and a portocaval shunt was then established to maintain stable hemodynamics.
During the bench resection, an extended right hepatectomy was performed in all patients using the Cavipulse Ultrasonic Surgical Aspirator (CUSA; Valleylab, Boulder, CO) device. Notably, the crucial conduit structures were carefully protected for subsequent reconstruction. After the lesion was totally removed, some conduit stumps required extra repair because of defects in the wall, an irregular shape of the orifice, a short length, or the presence of multiple stumps. The repair procedures including repair of vessel defects with patches, extension of stumps with autologous vascular grafts, and unification of multiple stumps were important for subsequent reconstruction procedures in the orthotopic position. Venoplasty was performed to create a wider outflow orifice (Fig. 1c).
Before reimplantation of the autograft of the liver, the IVC was reconstructed with autologous vessels, allogenic vessels, or artificial grafts. In 18 patients, the temporary IVC, which was rebuilt with an artificial graft, was not removed because there was no suitable source to reconstruct the IVC. When the graft was ready, a total-clamping fashion was applied to control the IVC. An extended incision was made in the anterior wall of the vena cava to increase the size of the outflow anastomosis (Fig. 1d). All the crucial conduits were reconstructed in the following order: hepatic vein, portal vein, hepatic artery, and bile duct.
Postoperative Management
Color Doppler ultrasonography was used to measure the diameter of the portal vein and the portal vein peak velocity (PVV) on postoperative days (PODs) 1, 3, 5, and 7. The portal vein blood flow (PVF) was calculated based on the formula PVF (mL/min) = area × 0.57 × PVV × 60 (mL/min).18 PVF was also expressed as blood flow per minute per 100 g of liver graft tissue (PVF/100 g, mL/min/100 g). Once postoperative bleeding was excluded, a low molecular weight heparin sodium injection was administered from POD 2 until discharge. All patients were given albendazole (15 mg/kg/day) routinely for 2 years after ERAT.19 The patients returned for follow-up visits every 3–6 months after discharge.
Postoperative complications were graded based on the Clavien–Dindo classification.20 Postoperative mortality was defined as death within 30 days of surgery or during the postoperative hospital stay. Small-for-size syndrome (SFSS) was defined as a small partial liver graft with the presence of two of the following on three consecutive days: bilirubin above 100 μmol/L, international normalized ratio (INR) above 2, or grade 3/4 encephalopathy after excluding other causes of graft dysfunction.21
Statistical Analysis
Categorical variables are expressed as numbers and were analyzed using the χ2 test or Fisher’s exact test. Normally distributed continuous variables are expressed as the means ± SD and analyzed using t test. Non-normally distributed continuous variables are expressed as medians and interquartile ranges (IQRs) and analyzed using the Mann–Whitney U test. Linear regression was used to compare the relationship between continuous variables. All statistical analyses were two-tailed, and P values < 0.05 were regarded as statistically significant. The statistical software package SPSS 22.0 (IBM Corp., Armonk, NY, USA) was used to analyze relevant data.
Results
Demographic and Surgical Characteristics
The demographic and surgical procedural characteristics for all 56 patients are summarized in Table 1. The mean age of the patients was 35.5 ± 10.0 years, and 24 of them were males. The mean BMI was 22.1 ± 3.3 kg/m2. The mean actual RLV/SLV was significantly lower in group S than in group L (0.35 ± 0.03 vs. 0.69 ± 0.18, P < 0.001). Four patients had an RLV/SLV < 35%, and the minimum actual RLV/SLV was 31.5%. There were no significant differences between group L and group S in terms of age, sex, BMI, lesion size, lesion number, preoperative serum levels of total bilirubin (TB), alanine aminotransferase (ALT), aspartate aminotransferase (AST), and INR, preoperative percutaneous transhepatic cholangial drainage (PTCD), and previous history of hepatectomy. Thirteen patients in group L and one patient in group S underwent preoperative PTCD to reduce TB levels to comply with standards. One patient in group L refused PTCD, although she had a TB level of 174.7 μmol/L. She ultimately underwent ERAT because the duration of the cholestatic jaundice was less than 1 week and the estimated RLV/SLV was 82.9%. One patient in group S and four patients in group L had a history of previous LR.
The median intraoperative blood loss was 2150 mL (IQR, 1500–3000 mL). The mean operative time was 761.5 ± 137.8 min (range, 540–1170 min). The mean anhepatic time was 317.8 ± 71.1 min (range, 122–488 min). The median transfusion volume of packed red blood cells was 6 U (IQR, 4–10 U), and only three (5.4%) patients required no red blood cells. The median transfusion volume of fresh-frozen plasma was 600 mL (IQR, 75–1595 mL), and 12 (25%) patients required no fresh-frozen plasma. Regarding the intraoperative data, no significant differences were identified between the two groups.
Remnant Liver Volume Calculation
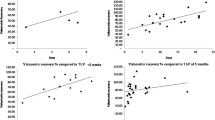
According to the hepatic venous outflow obstruction, 26 patients were assigned to the HVOO group and 30 patients were assigned to the no-HVOO group. In the HVOO group, the actual RLV and RLV/SLV were significantly smaller than the expected RLV (708 ± 226 mL vs. 868 ± 259 mL, P < 0.001) and expected RLV/SLV (0.65 ± 0.22 vs. 0.79 ± 0.23, P < 0.001), with a deviation ratio of 0.14 ± 0.09. However, the actual RLV and RLV/SLV were comparable in the no-HVOO group. The relationship between the estimated and actual RLV/SLV was linear in the both the HVOO group and the no-HVOO group (Fig. 2): in the HVOO group, the actual RLV/SLV was calculated as 0.866 × estimated RLV/SLV ratio − 0.036 (R2 = 0.835; P < 0.001), and in the no-HVOO group, the actual RLV/SLV was calculated as 0.983 × estimated RLV/SLV ratio − 0.034 (R2 = 0.930; P < 0.001).
Postoperative Complications and Survival Rates
Postoperative liver function returned to normal soon after surgery. Serial changes in postoperative serum TB, ALT, AST, and INR in the two groups are shown in Fig. 3, and no significant differences were identified between group L and group S.
Postoperative data are listed in Table 2. The median duration of the postoperative hospital stay in group S was significantly longer than that in group L (P = 0.007). The median duration of postoperative intensive care unit stay in group S was longer than that in group L, albeit not significantly (P = 0.241).
As shown in Table 2, the incidence of postoperative bleeding, pleural effusion, infection, biliary complications, and vascular complications did not significantly differ between group L and group S. There were no significant differences between the two groups regarding the Clavien–Dindo grades of postoperative complications. Three of the four patients who had an RLV/SLV < 35% (31.5, 32.0, 32.2, and 32.9%) developed postoperative complications. The patient with a minimal RLV/SLV experienced coagulation disorders that presented as continuous drainage of bloody liquid. He recovered with a transfusion of fresh-frozen plasma and coagulation drugs. The most frequent cause of complications was biliary leakage, with an incidence of 16.1% (nine patients). Three patients underwent endoscopic nasobiliary drainage, and the remaining six patients retained the drainage tube for continued drainage. One patient developing biliary stenosis and underwent reoperation 16 months after ERAT. One patient developed a venous outflow obstruction during the postoperative hospital stay, while two other patients developed late-onset venous outflow obstruction. The causes of the three deaths that occurred in group L were intra-abdominal bleeding originating from the intercostal arteries, acute cerebral hemorrhage, and severe liver dysfunction. No patients in group S died. Only one patient died due to liver dysfunction. Her actual RLV/SLV ratio was 0.8, and she had no underlying liver disease. Preoperative examination of the patient revealed that the right renal vein was invaded; thus, resection and reconstruction of right renal vein were necessary.
During an average of 16 months (range, 2–54 months) of follow-up, no recurrence occurred, and one patient was lost to follow-up. The overall survival rate of the whole cohort was 94.5%.
Changes in Portal Hemodynamics
The changes in portal hemodynamics after the surgery are shown in Fig. 4. PVF in group S increased significantly and peaked on POD 1, while in group L, PVF peaked on POD 3. An obvious decreasing trend was identified during the continuous observation course (1263 mL/min at POD 1 vs. 848 mL/min at POD 7, P < 0.05, group S; 1161 mL/min at POD 1 vs. 1005 mL/min at POD 7, P < 0.05, group L). As shown in Fig. 4, PVF/100 in group S was significantly higher than that in group L throughout the 7-day observation period (238 mL/min/100 g vs. 143 mL/min/100 g at POD 1, 235 mL/min/100 g vs. 165 mL/min/100 g at POD 3, 194 mL/min/100 g vs. 148 mL/min/100 g at POD 4, 164 mL/min/100 g vs. 136 mL/min/100 g at POD 7, P < 0.05).
Discussion
HAE is a severe helminthic zoonosis in the northern hemisphere, especially in western China.22 Radical resection is regarded as the first-line treatment. However, many patients lose the opportunity to receive radical resection during the early stage of disease because of delayed diagnosis caused by a long asymptomatic onset. LT has been recommended to treat those patients with “unresectable” but not metastatic HAE lesions.4 However, the utilization of LT has been limited by the shortage of donors and high incidence of post-transplantation relapse related to the mandatory use of immunosuppressive agents. Since Wen et al.23 reported a patient treated with ERAT for end-stage HAE in 2011, some other centers and our own have started to use this technique.5,6,24,25 The experience in previous studies has implied that patient selection, especially regarding volumetric calculations, is of utmost importance for achieving satisfactory outcomes after ERAT. This is currently the first series to investigate the impact of RLV on the outcomes of patients treated with ERAT. According to the present study, we found no inferior results in patients with smaller remnant liver compared to those with larger remnant livers, as long as the RLV was within the accepted clinical limits. When the RLV/SLV was below 40%, a lower liver volume did not have significant influence on the outcomes of ERAT.
Experiences gained from conventional LR have indicated that after an extended hepatectomy involving 70 to 75% of the liver, the liver can still function well in non-cirrhosis patients,9,11 while the graft-to-standard liver volume ratio must be at least 40% at most LDLT transplant centers.7 These experiences were also applied in the practice of ERAT, as our preliminary results suggested that the RLV/SLV should be at least 40% to avoid SFSS,5 which is similar to the results of other related reports.24 However, we were reluctant to fully adhere to the doctrines of LR or LDLT because of the unique features of this surgery and the recipients. First, the long cold ischemia time needed during ex vivo resection is a major feature of ERAT that distinguishes this procedure from LDLT; furthermore, it is the main cause of ischemia/reperfusion injury and is associated with poor-quality grafts and a high incidence of postoperative biliary and arterial complications in LT recipients.26 In the present study, the cold ischemia time is approximately equal to the anhepatic time (317.8 ± 71.1 min), which is obviously longer than that in LDLT. Second, ERAT is a more complicated procedure than LDLT and conventional hepatectomy, especially in terms of conduit reconstruction, which leads to a longer operative time (761.5 ± 137.8 min) and more intraoperative blood loss (mean, 2150 mL; IQR, 1500–3000 mL). The operative time and intraoperative blood loss have been regarded as poor prognostic indicators for graft loss and severe hepatic dysfunction for LT recipients27 and hepatectomy patients.11 In addition, we noticed that in some cases, the liver graft significantly shrank after it was retrieved from the abdominal cavity. These special circumstances naturally remind us that the safe limits of ERAT may not totally comply with those used in other procedures.
To verify the validity of existing safety threshold, we compared the outcomes from a group of patients with an RLV/SLV < 40% and a group of patients with an RLV/SLV > 40%; both groups exhibited acceptable results without significant differences. We attribute these comparable outcomes to several reasons. First, most patients had no underlying liver disease or major comorbidities. Seven patients were infected with hepatitis B virus, but none had developed cirrhosis. Fifty patients had Child–Pugh A liver function, while the remaining six had Child–Pugh B preoperatively. Second, during the slow progression of HAE, the lesions gradually compress one of the portal branches leading to increased blood inflow and hypertrophy of the residual liver. This process could be considered as a natural portal vein embolization. The actual RLV/SLV is reported to be 0.62 ± 0.21 (range, 0.31–1.17), which can quite adequately meet the functional demands in most cases. Third, the smooth intraoperative course was guaranteed by delicate surgical technique based on our accumulated experiences with LDLT.28 In addition to achieving the proper RLV/SLV ratio, we also took effective measures to ensure that every graft had good outflow drainage.5 If necessary, the outflow tract was lengthened and broadened with an autologous patch before reimplantation. Therefore, the RLV/SLV can be relaxed to 35% when a patient has normal liver function and no underlying liver disease.
The visible shrinkage of liver graft observed intraoperatively was also confirmed by quantitative analysis; this report is also the first on this often overlooked deviation. We initially suspected that this deviation was related to patency of the residual hepatic outflow. Further analysis revealed that in patients with preoperative HVOO, the deviation between the estimated RLV/SLV and the actual value was 0.14 ± 0.09, despite the findings of good volumetric consistency between the estimated and the actual values had been reported in a previous study on the application of 3D reconstruction in ERAT.12 Different from harvesting a liver graft from a healthy donor, ERAT is performed in patients whose intrahepatic vasculature is extensively infiltrated by large end-stage HAE lesions. Residual venous drainage with severe infiltration or compression may chronically lead to severe congestion of the liver. The final liver volume can certainly be reduced if the congested blood is drained out after the liver is harvested. Moreover, the discrepancy among patients with HVOO also varied significantly, which may simultaneously be determined by portal inflow, the level of outflow obstruction, and compensatory hypertrophy of the liver. Further study is warranted to clarify the factors contributing to this volumetric estimation deviation.
According to the experiences with LDLT, the leading factor associated with SFSS is reported to be portal hyperperfusion in addition to other possible mechanisms including outflow problems, donor age, graft size, and quality.29,30 In addition, smaller grafts are associated with elevated sinusoidal pressure caused by an excessive portal flow/graft volume ratio and over-regeneration of the graft.31 To prevent SFSS, several reports have recommended that the PVF/100 g value (representing the capacity of the hepatic sinusoids to accommodate portal inflow) should not exceed 250 mL/min/100 g.32 Troisi et al.33 reported on five patients who received smaller grafts with PVF/100 g values above 250 mL/min/100 g, three of whom developed SFSS without inflow modulation. We identified two patients with POD 1 PVF/100 g values ≥ 250 mL/min/100 g in group S, but all of them experienced a smooth postoperative recovery. Eventually, the PVF/100 g values of all the patients decreased to an acceptable value within the first postoperative week. Several patients underwent concurrent splenectomy due to severe hypersplenism rather than inflow modulation. Since the study population was small, we cannot draw any conclusions about splenectomy for preventing SFSS in ERAT.
This single-center study is limited by its retrospective nature and the relatively small number of included patients. Considering that the present study represents the first and largest cohort dedicated to validating the appropriate safety limits in ERAT, some of the presented results may be useful for guiding surgeons in clinical practice and for shedding light on further exploration of patient selection for ERAT.
Conclusion
The prognosis of the patients with a smaller RLV/SLV is comparable to that of those with a larger RLV/SLV. An RLV/SLV ≥ 40% is not always necessary to achieve good outcomes. For patients with normal liver function, it is rational to relax the threshold of the RLV/SLV. Shrinkage of the remnant liver in patients with HVOO should also be carefully considered during volumetric evaluation. Increased experience and further study are warranted to determine a widely applicable safety limit for ERAT.
References
Conraths FJ, Probst C, Possenti A, Boufana B, Saulle R, La Torre G, Busani L, Casulli A. Potential risk factors associated with human alveolar echinococcosis: systematic review and meta-analysis. PLoS neglected tropical diseases. 2017;11(7):e0005801.
Vuitton DA, Zhou H, Bresson-Hadni S, Wang Q, Piarroux M, Raoul F, Giraudoux P. Epidemiology of alveolar echinococcosis with particular reference to China and Europe. Parasitology. 2003;127 Suppl:S87–107.
Brunetti E, Kern P, Vuitton DA. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta tropica. 2010;114(1):1–16.
Aydinli B, Ozturk G, Arslan S, Kantarci M, Tan O, Ahiskalioglu A, Ozden K, Colak A. Liver transplantation for alveolar echinococcosis in an endemic region. Liver Transpl. 2015;21(8):1096–1102.
Yang X, Qiu Y, Huang B, Wang W, Shen S, Feng X, Wei Y, Lei J, Zhao J, Li B, Wen T, Yan L. Novel techniques and preliminary results of ex vivo liver resection and autotransplantation for end-stage hepatic alveolar echinococcosis: a study of 31 cases. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2018;18(7):1668–1679.
Jianyong L, Jingcheng H, Wentao W, Lunan Y, Jichun Z, Bing H, Ding Y. Ex vivo liver resection followed by autotransplantation to a patient with advanced alveolar echinococcosis with a replacement of the retrohepatic inferior vena cava using autogenous vein grafting: a case report and literature review. Medicine (Baltimore). 2015;94(7):e514.
Miller CM, Quintini C, Dhawan A, Durand F, Heimbach JK, Kim-Schluger HL, Kyrana E, Lee SG, Lerut J, Lo CM, Pomfret EA. The International Liver Transplantation Society Living Donor Liver Transplant Recipient Guideline. Transplantation. 2017;101(5):938–944.
Sugawara Y, Makuuchi M, Takayama T, Imamura H, Dowaki S, Mizuta K, Kawarasaki H, Hashizume K. Small-for-size grafts in living-related liver transplantation. J Am Coll Surg. 2001;192(4):510–513.
Pak LM, Chakraborty J, Gonen M, Chapman WC, Do RKG, Groot Koerkamp B, Verhoef K, Lee SY, Massani M, van der Stok EP, Simpson AL, Memorial Sloan Kettering Cancer Center Hepatopancreatobiliary S. Quantitative imaging features and postoperative hepatic insufficiency: a multi-institutional expanded cohort. J Am Coll Surg. 2018;226(5):835–843.
Kim HJ, Kim CY, Park EK, Hur YH, Koh YS, Kim HJ, Cho CK. Volumetric analysis and indocyanine green retention rate at 15 min as predictors of post-hepatectomy liver failure. HPB: the official journal of the International Hepato Pancreato Biliary Association. 2015;17(2):159–167.
Schindl MJ, Redhead DN, Fearon KC, Garden OJ, Wigmore SJ, Edinburgh Liver S, Transplantation Experimental Research G. The value of residual liver volume as a predictor of hepatic dysfunction and infection after major liver resection. Gut. 2005;54(2):289–296.
He YB, Bai L, Jiang Y, Ji XW, Tai QW, Zhao JM, Zhang JH, Liu WY, Wen H. Application of a three-dimensional reconstruction technique in liver autotransplantation for end-stage hepatic alveolar echinococcosis. J Gastrointest Surg. 2015;19(8):1457–1465.
He YB, Bai L, Aji T, Jiang Y, Zhao JM, Zhang JH, Shao YM, Liu WY, Wen H. Application of 3D reconstruction for surgical treatment of hepatic alveolar echinococcosis. World Journal of Gastroenterology. 2015;21(35):10200–10207.
Radtke A, Sotiropoulos GC, Nadalin S, Molmenti EP, Schroeder T, Lang H, Saner F, Valentin-Gamazo C, Frilling A, Schenk A, Broelsch CE, Malago M. Preoperative volume prediction in adult living donor liver transplantation: how much can we rely on it? American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2007;7(3):672–679.
Baskiran A, Kahraman AS, Cicek IB, Sahin T, Isik B, Yilmaz S. Preoperative evaluation of liver volume in living donor liver transplantation. Northern clinics of Istanbul. 2018;5(1):1–5.
Hiroshige S, Shimada M, Harada N, Shiotani S, Ninomiya M, Minagawa R, Soejima Y, Suehiro T, Honda H, Hashizume M, Sugimachi K. Accurate preoperative estimation of liver-graft volumetry using three-dimensional computed tomography. Transplantation. 2003;75(9):1561–1564.
Urata K, Kawasaki S, Matsunami H, Hashikura Y, Ikegami T, Ishizone S, Momose Y, Komiyama A, Makuuchi M. Calculation of child and adult standard liver volume for liver transplantation. Hepatology (Baltimore, Md). 1995;21(5):1317–1321.
Moriyasu F, Ban N, Nishida O, Nakamura T, Miyake T, Uchino H, Kanematsu Y, Koizumi S. Clinical application of an ultrasonic duplex system in the quantitative measurement of portal blood flow. Journal of clinical ultrasound: JCU. 1986;14(8):579–588.
Guidelines for treatment of cystic and alveolar echinococcosis in humans. WHO Informal Working Group on Echinococcosis. Bulletin of the World Health Organization. 1996;74(3):231–242.
Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibanes E, Pekolj J, Slankamenac K, Bassi C, Graf R, Vonlanthen R, Padbury R, Cameron JL, Makuuchi M. The Clavien–Dindo classification of surgical complications: five-year experience. Annals of surgery. 2009;250(2):187–196.
Dahm F, Georgiev P, Clavien PA. Small-for-size syndrome after partial liver transplantation: definition, mechanisms of disease and clinical implications. Am J Transplant. 2005;5(11):2605–2610.
Deplazes P, Rinaldi L, Alvarez Rojas CA, Torgerson PR, Harandi MF, Romig T, Antolova D, Schurer JM, Lahmar S, Cringoli G, Magambo J, Thompson RC, Jenkins EJ. Global distribution of alveolar and cystic echinococcosis. Advances in parasitology. 2017;95:315–493.
Wen H, Dong JH, Zhang JH, Zhao JM, Shao YM, Duan WD, Liang YR, Ji XW, Tai QW, Aji T, Li T. Ex vivo liver resection followed by autotransplantation for end-stage hepatic alveolar echinococcosis. Chinese medical journal. 2011;124(18):2813–2817.
Wen H, Dong JH, Zhang JH, Duan WD, Zhao JM, Liang YR, Shao YM, Ji XW, Tai QW, Li T, Gu H, Tuxun T, He YB, Huang JF. Ex vivo liver resection and autotransplantation for end-stage alveolar echinococcosis: a case series. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2016;16(2):615–624.
Wang H, Liu Q, Wang Z, Zhang F, Li X, Wang X. Clinical outcomes of ex vivo liver resection and liver autotransplantation for hepatic alveolar echinococcosis. Journal of Huazhong University of Science and Technology Medical sciences = Hua zhong ke ji da xue xue bao Yi xue Ying De wen ban = Huazhong keji daxue xuebao Yixue Yingdewen ban. 2012;32(4):598–600.
Rammohan A, Govil S, Vargese J, Kota V, Reddy MS, Rela M. Changing pattern of biliary complications in an evolving liver transplant unit. Liver Transpl. 2017;23(4):478–486.
Ikegami T, Yoshizumi T, Sakata K, Uchiyama H, Harimoto N, Harada N, Itoh S, Nagatsu A, Soejima Y, Maehara Y. Left lobe living donor liver transplantation in adults: what is the safety limit? Liver Transpl. 2016;22(12):1666–1675.
Zhang W, Tan Y, Shen S, Jiang L, Yan L, Yang J, Li B, Wen T, Zeng Y, Wang W, Xu M. Adult to adult right lobe living donor liver transplantation: does biological relationship matter? Medicine (Baltimore). 2017;96(4):e4139.
Yao S, Kaido T, Uozumi R, Yagi S, Miyachi Y, Fukumitsu K, Anazawa T, Kamo N, Taura K, Okajima H, Uemoto S. Is portal venous pressure modulation still indicated for all recipients in living-donor liver transplantation? Liver Transpl. 2018.
Uemura T, Wada S, Kaido T, Mori A, Ogura Y, Yagi S, Fujimoto Y, Ogawa K, Hata K, Yoshizawa A, Okajima H, Uemoto S. How far can we lower graft-to-recipient weight ratio for living donor liver transplantation under modulation of portal venous pressure? Surgery. 2016;159(6):1623–1630.
Kiuchi T, Tanaka K, Ito T, Oike F, Ogura Y, Fujimoto Y, Ogawa K. Small-for-size graft in living donor liver transplantation: how far should we go? Liver Transpl. 2003;9(9):S29–35.
Chang CD, Cheng YF, Chen TY, Tsang LL, Ou HY, Yu CY, Hsu HW, Chen CL, Concejero AM, Huang TL. Portal venous pressure in adult living donor liver transplantation. Transplantation proceedings. 2014;46(3):696–698.
Troisi R, Cammu G, Militerno G, De Baerdemaeker L, Decruyenaere J, Hoste E, Smeets P, Colle I, Van Vlierberghe H, Petrovic M, Voet D, Mortier E, Hesse UJ, de Hemptinne B. Modulation of portal graft inflow: a necessity in adult living-donor liver transplantation? Ann Surg. 2003;237(3):429–436.
Acknowledgements
The authors thank American Journal Experts for language editing.
Author information
Authors and Affiliations
Contributions
Study conception and design: W. T.W.
Acquisition of data: S.S., Y.W.Q., X.W.Y.
Analysis and interpretation of data: S.S., Y.W.Q.
Drafting of manuscript: S.S., Y.W.Q.
Critical revision: W.T.W.
Corresponding author
Ethics declarations
Disclosure
Supported by grants of the National Natural Science Foundation of China (no. 81770566), the New Medical Technology Foundation of West China Hospital of Sichuan University (no. 2016-036), and the Department of Science and Technology of Sichuan Province (no. 2016FZ0076).
Disclosure Information
Nothing to disclose.
Rights and permissions
About this article
Cite this article
Shen, S., Qiu, Y., Yang, X. et al. Remnant Liver-to-Standard Liver Volume Ratio Below 40% is Safe in Ex Vivo Liver Resection and Autotransplantation. J Gastrointest Surg 23, 1964–1972 (2019). https://doi.org/10.1007/s11605-018-4022-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-018-4022-4