Abstract
Dark fermentation process appears to be one of the most promising techniques to obtain clean energy, however, there are some limitations to this process. In the present study, stirred tank reactors under different modes [batch (BR) and continuous (CR)] were used to determine the evolution of hydrogen-producing microbial communities when nixtamalization (nejayote) and abattoir wastewater were co-digested. The inoculum consisted of granular sludge subjected to (a) thermal treatment (TT); and (b) UV irradiation. When the inoculum underwent TT (15 min at 100 °C) in BR (40 RPM, 35 ± 1 °C, pH 5.50 ± 0.05), the highest cumulative biohydrogen (bioH2) content was obtained (350 mL). In this case, the organic matter degradation reached 70%. In addition, the sequencing analysis showed Clostridium butyricum was the predominate strain (68–87%) between 48 and 72 h of the reaction. Conversely, when UV-pretreated inoculum was used, Clostridium butyricum only made up 1% after 60 h of reaction. The CR was operated for 20 cycles at a hydraulic retention time of 6 h. Volatile fatty acids confirmed butyrate acetate fermentation. Herein, the bioH2 yield was generated at a fivefold rate compared to previous studies. Specifically, 223 mL H2 g−1 total volatile solids and 8 mL H2 L−1 h−1 were produced. Moreover, Clostridium was present at 59–62% after 14 cycles, confirming a better efficiency in bioH2 production when the reactor works in continuous mode.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Statement of Novelty
-
The TT was the most effective treatment suppressing the methanogenic microorganisms
-
Clostridium was the main genera present in 59–62% after 14 cycles of fermentation
-
The maximum yield of bioH2 was 223 mL H2 g−1 TVS using a CRTT at HRT = 6 h
-
The bioH2 production was generated at a fivefold rate with CRTT in comparison to BRTT
Introduction
Population growth is closely related to increasing energy demands. Therefore, the consumption of fossil fuels, including coal and oil, and greenhouse gas emissions have increased substantially in recent years [1]. Hydrogen gas (H2) does not generate CO2 emissions and displays an energetic density of 120 kJ g−1 which is 2.5 times higher than that of gasoline [2]. It is considered an important candidate to replace fossil fuels. H2 is an odorless, colorless, and tasteless gas that is nonpolluting, as it only produces water after combustion [3]. When H2 is generated via a biological pathway, it is called biohydrogen (bioH2). These pathways include water biophotolysis, indirect biophotolysis, photofermentation, microbial electrolysis cells (MECs), and dark fermentation (DF) by green algae, cyanobacteria, photosynthetic bacteria, electrogenic bacteria, and anaerobic bacteria, respectively [2]. Dark fermentation is considered a simple process that has several advantages over the other biological technologies. In addition to not requiring light, DF has high conversion rates and can be performed with a variety of substrates, including municipal, industrial, and agricultural wastes [4,5,6,7,8,9].
Substrates for bioH2 production through DF must display a high carbohydrate content generated from sustainable and accessible resources, require minimal pretreatment, and, if possible, must be cost-effective [2]. According to previous studies, the efficiency of bioH2 production can be improved when two different substrates are used (co-digestion) because, under these conditions, a better buffer effect can be achieved [8, 9]. In addition, a better carbon: nitrogen (C/N) ratio can be obtained using two or more substrates, owing to their physicochemical characteristics. In fact, a C/N ratio of 20–40 is widely used as a standard parameter as it is associated with the enhancement of bioH2 production, owing to its buffering effect on the system [9, 10]. An optimal C/N ratio is required for the proper growth of microorganisms during this process. The optimization of the C/N ratio is one method used to reduce or avoid ammonia inhibition. When C is higher than the optimum N concentration in the C/N ratio, there is poor biogas production. However, when C is lower, this results in ammonia accumulation [11].
In addition to substrates, inoculum selection is extremely important in DF because it is the main component containing the microorganisms responsible for bioH2 production. An appropriate amount of inoculum is essential to achieve effective DF [12]. Enterobacter aerogenes, Clostridium butyricum, and Clostridium acetobutyricum were used as pure cultures for bioH2 production. However, mixed cultures of granular anaerobic sludge were also selected because they contained different microorganisms, including Clostridium spp., Prevotella spp., and Megasphaera spp. [9]. However, these mixed cultures require pretreatment before use in bioH2 production. One of the most frequently reported pretreatments is thermal treatment (TT). However, there are other options, including UV irradiation, alkaline and acid treatments, and microaeration, which require less energy and processing time. More in-depth studies of these processes are necessary to determine their impact on bioH2 production [12, 13].
Nejayote (NEJ) and abattoir wastewater (ABW) have high contents of biodegradable organic matter and are produced in large amounts in different countries. In Mexico, the annual generation of NEJ reaches 14,800 million liters [14], and the processing of cattle requires approximately 1,000 liters of water per animal. It is estimated that 95% of this water becomes wastewater [15]. Therefore, they can be used as substrates for DF [16]. Nixtamalization is a thermal-alkaline method applied to maize that allows the elimination of the pericarp from the kernel, generating two main products, nixtamal and NEJ. Nixtamal represents softened grains that serve as ingredients for the preparation of tortillas, flour, and other nixtamalized maize products, whereas NEJ is an alkaline wastewater resulting from cooking kernels [17, 18]. Previous studies have indicated that NEJ generation reaches 2.2–3.5 m3 t−1 of processed corn annually [17, 19]. Calcium hydroxide is added during the nixtamalization process, therefore displaying an alkaline pH (12–14) [14] in addition to a chemical oxygen demand (COD) of approximately 25–40 g L−1 and a TS concentration of approximately 9–25 g L−1 [20, 21]. In contrast, ABW has a COD of 20–24 g L−1, pH values between 6 and 7, and a TS concentration of 30–35 g L−1 [15].
García-Depraect et al., reported a bioH2 production of 6400 mL when tequila vinasses were co-digested with NEJ (ratio 20:80) and used in DF for 72 h at a 5.8 pH and a temperature of 35 °C, NEJ was used as the main source of carbon [14, 22]. This process was performed in a 3 L batch bioreactor in the presence of an inoculum containing Lactobacillus, Clostridium, Acetobacter, and Sporobacillus as the predominant species [14]. In addition, Maravilla et al., evaluated the effect of NEJ concentration on bioH2 yield. For this purpose, these researchers selected 250 mL anaerobic reactors with glucose as substrate and inoculum and maintained it at 37 °C. According to their results, the highest bioH2 composition (47.26 ppm) was obtained after 48 h, when NEJ was added at a rate of 40% [23]. Thus, NEJ has been used in mono-digestion and co-digestion processes to produce bioH2 via DF. However, 80% of NEJ components correspond to complex sugars that are hardly degradable by microorganisms. These polysaccharides include arabinose, xylose, glucose, and d-glucuronic acid, among others [23, 24]. Different treatments including sonochemistry can be used to improve the hydrolysis of these sugars. Sonochemical methods are efficient for the hydrolysis of lignocellulose and complex sugars [25, 26]. In contrast, ABW has been used in co-digestion with oil palm trunk hydrolysate (OPT) for bioH2 production as the main nitrogen source. Khamtib and Reungsang reported bioH2 production of 2604 ± 86 mL H2 L−1 substrate using ABW-OPT co-digestion in 120 mL batch reactors with an initial pH of 6.50 and at a temperature of 60 °C using Thermoanaerobacterium thermosaccharolyticm KKU19 as inoculum [27].
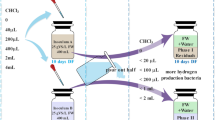
Accordingly, this study aimed to determine the effect of the type of pretreatment of granular sludge (GS) inoculum: TT (100 °C/30 min) and UV irradiation (254 nm/15 min), as well as the operational mode of reactors (batch (BR) and continuous (CR)) using co-digestion of NEJ-ABW previously treated with sonochemistry in 5 L reactors for bioH2 production. In addition, the evolution of microbial communities during DF was studied.
Materials and Methods
Inoculum
In this study, GS inoculum obtained from an anaerobic reactor operating at the wastewater treatment plant of a brewery located in Monterrey, Mexico was used. Samples were collected according to NOM-004-SEMARNAT-2002 using random sampling [8]. For each sample, the temperature and pH were measured in situ before placing them in 1 L plastic bottles after rinsing 3 times with the same sludge and stored at 4 °C until required. In this study, two different pretreatments (TT and UV irradiation) were used to suppress the growth of methanogenic microorganisms and promote bioH2 production [28]. In the thermal pretreatment, the inoculum was heated for 30 min at 100 °C using a hot plate (Cimarec, Thermo Scientific). For UV irradiation, a UV lamp (Coospider CUV-7/11, 7 W) was used at a wavelength of 254 nm. During the procedure, the UV lamp was placed inside a quartz-cooling jacket that was immersed in a 1 L beaker containing the inoculum. The solution was stirred continuously to ensure homogenization. The complete system was placed in a dark wooden box. The inoculum was then irradiated for 15 min.
Co-digestion Procedure and Characterization
In this study, DF was performed through the co-digestion of NEJ and ABW, which have been characterized before its use. The substrate mixture was adjusted to a (C/N) ratio of 30. Before DF, hydrolysis of the polysaccharides present in the NEJ-ABW co-digestion was performed through a sonochemical procedure at 20 kHz for 30 min using an ultrasonic processor (750 watts Cole-Parmer) [25]. Subsequently, pH was adjusted to 5.50 ± 0.05 to provide the optimal environment for bioH2 production [28]. To adjust the pH, NaOH 1N and H2SO4 5N solutions were used.
Set Up of Bioreactors
In these experiments, 5 L bioreactors (Sartorius Stedim Biostat A) with a working volume of 3 L were used. The bioreactors were set up in batch and continuous modes. Two separate reactors were used in the BR experiments to determine the effect of inoculum pretreatment on bioH2 production and one in the CR experiment to determine the impact of operational conditions. As previously indicated, the inoculum was subjected to (a) TT and (b) UV. In addition, the continuous reactor with thermal treatment (CRTT) displayed the same characteristics as the batch reactor thermal treatment (BRTT). The three bioreactors were continuously stirred at 40 RPM and maintained at a temperature of 35 °C. The CRTT was fed using an internal Biostat A peristaltic pump and an external peristaltic bomb (Diagger Thermo Scientific) was used to extract the bioreactor effluent to maintain a hydraulic retention time (HRT) of 6 h. The dissolved oxygen, pH, and temperature were automatically regulated with the bioreactor software. To maintain the proper pH, NaOH 1N and H2SO4 1N were added as required. The biogas produced during the DF was quantified using the water displacement method [30]. In the experiments, the inoculum and the reinforced clostridial medium (RCM) (10 g L−1 meat extract, 10 g L−1 peptone, 3 g L−1 yeast extract, 5 g L−1 d-glucose, 1 g L−1 starch, 5 g L−1 NaCl, 3 g L−1 C2H3NaO2, 0.5 g L−1 l-cysteine chloride, and 0.5 g L−1 agar-agar) were added to the reactor at a 10% v/v ratio with respect to the working volume. The selected working volume was 3 L, and NEJ-ABW co-digestion was performed at a C/N ratio of 30 and a pH of 5.5. The BRTT and BRUV were operated for 72 h and 60 h with an initial total volatile solid (TVS) concentration of 11,326 and 14,307 mg L−1, respectively. Whereas the CRTT was operated for 20 cycles at an HRT of 6 h with an organic loading rate (OLR) of 27 ± 8 g L−1 d along the process.
Analytical Methods
For substrate characterization, the following parameters were determined: (a) pH, using a pH meter (Thermo Scientific); (b) total carbohydrates (CH) using the Dubois method [29]; (c) chemical oxygen demand (COD), according to NMX-AA-030-SCFI-2001 using the methodology reported by Lirio María Reyna-Gómez et al., [9]; and (d) total nitrogen (TN) was quantified using the standardized Hach method (Hach kit 10–150 mg/L). A UV-Vis Perkin Elmer Lambda 365 spectrometer was used for the CH, COD, and TN measurements.
The composition of biogas and volatile fatty acids (VFAs) were determined by gas chromatography (7820A, Agilent Technologies), as previously reported [30]. The biogas composition was determined using a thermal conductivity detector, a silica capillary column (30 m × 0.53 mm, Supelco Carboxen 1006 plot), and argon as the carrier gas. In addition, VFAs were analyzed using a flame ionization detector (FID) with an HP-Inowax column (50 m × 0.20 mm) and argon as the carrier gas.
Samples from the BRTT and BRUV were analyzed every 12 h, whereas the CRTT samples were analyzed at the end of each cycle. Physicochemical analyses were performed as previously mentioned. The measured parameters included COD, pH, and CH. Total solids (TS) and TVS were determined using the methodologies reported in the “Standard Methods for the Examination of Water and Wastewater” [31].
The bioH2 yield was calculated using equation 1 for BR and equation 2 for CR:
\(Where {V}_{{BioH}_{2}}\) is the volume of bioH2 produced and TVScons is the concentration of TVS consumed during the DF process.
where: \({Y}_{Bio{H}_{2}}\) is expressed as a function of the OLR for TVS in the influent (mL bioH2 g−1 TVSinfluent), BH2PR is the bioH2 production rate (mLH2 h−1), TVSinfluent is the TVS concentration in the influent gTVS mL−1, Finfluent is the daily volume of the influent (mL h−1).
DNA Extraction and Analysis
The microbial compositions of the BR and CR were determined. For this purpose, the following samples were analyzed: (a) BRTT at 24 and 72 h, (b) BRUV at 60 h, and (c) cycles 7 and 14. DNA extraction was done in a 0.25 g sample using the QIAGEN DNeasy PowerSoil kit [8]. In this case, cell lysis was promoted through mechanical and chemical methods that caused bacterial cell walls to rupture [32], allowing for the release of genetic material. Nucleic acids were adsorbed using different salts, and genomic DNA was captured on a silica membrane. After centrifugation (30 s at 10,000×g), DNA was rinsed and eluted [33]. After DNA extraction, sequencing was performed to identify the microbial species present in the samples, as previously described. The DNA samples were sent to the RTL Genomics Laboratory in Texas, USA, where amplicon sequencing was performed by PCR of the 16s rRNA gene with the aid of a MiSeq Illumina platform.
Results and Discussion
Substrate Characterization
Substrate properties have been reported to substantially affect the bioH2 yield and stability of co-digestion systems [34]. Thus, the physicochemical characterization of substrates is required to properly design bioreactors and processes (Table 1). The results indicated that in the case of NEJ, the substrate presented an alkaline pH (10.35 ± 1.97), TN of 179 ± 80 mg L−1, high CH concentration (17,747 ± 1910 mg L−1), and a COD of 35,212.0 ± 2,054.0 mg L−1 [14, 17, 19]. In addition, ABW displayed a pH of 5.69 ± 0.06, low CH concentration (1217 ± 358 mg L−1), COD of 15,969.0 ± 4,936.0, and TN levels of 2588 ± 793 mg L−1 [16, 20, 21]. Which are similar to those reported by García-Depraect et al., as they used NEJ as a substrate in DF to produce bioH2. These researchers reported that NEJ presented a pH of 12 ± 0.2, TN levels of 440 ± 21.2 mg L−1, and a CH concentration of 16,015.8 ± 1649.7 mg L−1 [14]. The pH of NEJ varies because different corn grinders use different amounts of calcium hydroxide during nixtamalization. With respect to ABW, Khamtib, and Reungsang [27] reported pH values of 7.12 ± 0.14, which are different from those obtained herein (5.69 ± 0.06). It was also found that the system contained CH concentrations of 440 ± 80 mg L−1, almost a third of the values (1217 ± 358 mg L−1) observed in this study. The addition of ABW to the feedstock offers a valuable opportunity to enhance process stability and improve the carbon and nitrogen balance. The contribution of N by ABW can bolster the buffer capacity [35]. Furthermore, it resulted in a significant decrease in feedstock pH compared to that of the NEJ. According to these data, both substrates were successfully used in the co-digestion when the C/N ratio was adjusted to 30. This C/N ratio is extremely important because it directly affects bioH2 production during DF by providing a balance of micro-and macronutrients and promoting the buffering effect [8, 9, 36].
However, given the alkaline pH of NEJ, the 5 N H2SO4 solution was added until a pH of 5.5 ± 0.05 was reached. According to the literature, this pH is within the reported range and can be used even when the inoculum is pretreated to suppress methanogens [37, 38].
Effect of Inoculum Pretreatment on NEJ-ABW Co-digestion Using Batch Bioreactors
Organic Matter Removal
Figure 1 shows the results of CH consumption in the BRTT and BRUV bioreactors. In the first experiment, NEJ-ABW co-digestion showed CH consumption between 10 and 35% from the beginning of the process and up to 72 h. In contrast, BRUV presented a CH consumption of 37% during the first 12 h, reaching 70% after 60 h. These differences may be due to the higher microbial diversity of BRUV. This probably occurred because, in batch reactors, UV treatment was less effective than TT in ensuring the abatement of methanogenic activity. The TT allowed the selection of species participating in bioH2 generation. To the best of our knowledge, CH removal using NEJ-ABW co-digestion has not been previously reported. Previous studies have used thermally pretreated GS as an inoculum in co-digestion processes using BR [8, 9]. In other experiments, CH was removed in 30–80% and 25–70% after 30 h of co-digestion with cheese whey-brewery and -bakery wastewater, respectively. According to the authors, the balance between macro-and micronutrients (including CH) and a C/N ratio of 30 favor the metabolic pathways involved in bioH2 generation. In addition, in mono-digestion processes, CH removal was up to 50% when the C/N ratio varied between 15 and 30 [8]. Moreover, Del Angel-Acosta et al., performed a co-digestion of NEJ brewery wastewater. Their data was similar to that of this study as they indicated a CH removal of 68% after 122 h [16].
Table 2 displays COD, TS, and TVS removal, as well as the percent of bioH2 present in the biogas after NEJ-ABW co-digestion in batch reactors. In BRTT, the highest COD reduction was observed at 12 h. After this point, COD mostly showed a percent removal of 37 ± 10% and decreased to 19% at the end of the reaction. This behavior may be caused by the growth of bioH2-producing microorganisms within the first few hours. The BRUV showed greater stability as compared to BRTT, as a COD removal of 30.0 ± 4% was achieved during the 60 h of reaction. Even when BRUV contained a higher microbial diversity (Fig. 7) metabolic pathways may present a limiting factor [37]. Thus, in our experiments, slightly higher COD removal was reached as compared to that reported by Garcia-Peña et al., who indicated a value of 25% when fruits and vegetable residues were used as substrates [39].
The data also indicated a final TS reduction of 16 ± 2 and 17 ± 2% for BRTT and BRUV, respectively. The reactor with the highest microbial diversity exhibited the greatest organic matter reduction. The same behavior was observed when TVS removal was quantified. In this case, 39% of TVS was reduced in BRUV after 60 h, whereas 27% was removed in BRTT after 72 h.
BioH2 Production and Biogas Composition
Figure 2 and Table 2 show the bioH2 production and COD, TS, and TVS removal in the BRTT and BRUV bioreactors, respectively. After 72 and 60 h, the accumulation of bioH2 reached 359 and 191 mL, respectively. In addition, after DF in BRTT, 66 ± 27% of produced biogas corresponded to bioH2 and the remainder to CO2. Nevertheless, between 36 and 48 h, a reduction in bioH2 generation occurred only in 13 ± 2% (Table 2). The absence of CH4 in the biogas resulted from the efficiency of the inoculum pretreatment method. However, BRTT reactivation after 60 h probably occurred because of changes in the microbial consortium [40]. With respect to BRUV, the composition of the biogas corresponded to bioH2 (15 ± 4%), methane (19 ± 6%), and the rest was CO2 at 60 h. Thus, bioH2 production was affected after 60 h of DF (Table 2) [28, 37].
The results showed that the BRTT and BRUV yielded 112 and 38 mL H2 g−1 TVS; and 1.96 and 0.94 mL H2 L−1 h−1, respectively. Accordingly, the performance of the bioreactors was completely different. Specifically, BRTT presented an efficiency and productivity of 81 and 57% higher, compared to BRUV.
Table 3 presents bioH2 production when different substrates and GS were used in the DF process, as well as the results obtained in the present study. These data indicate that the yields obtained in our experiments were 8 times higher than those obtained in previous studies [39, 41, 42]. In contrast, bioH2 production in the present study was slightly lower than that shown in Table 3. This behavior was attributed to the use of more complex substrates. In addition, microorganisms require more time to adapt to the carbon sources (NEJ) [17].
Volatile Fatty Acids
Figure 3a and b show the production of VFAs during DF in the co-digestion of NEJ-ABW in the BRTT (12, 24, 48, and 72 h) and BRUV (12 and 60 h). In BRTT (Fig. 3a), the predominant compound was acetic acid (24–18 mmol), followed by formic acid (5–2 mmol), propionic acid (~1 mmol), and butyric acid (<1 mmol). The highest VFA concentration (38 mmol) was observed after 12 h. At this point, 27 mmol acetic acid was generated. The maximum bioH2 production occurred at this time, reaching 127 mL of bioH2. This value represents 66% of bioH2 present in the biogas. These metabolites indicate that butyrate-acetate fermentation occurred [43]. These data agree with those reported by Khamtib and Reungsang, who co-digested palm tree hydrolysates and ABW. They also found that the highest bioH2 production during the DF process was reached when acetic acid and butyric acid presented the maximum concentrations [27].
The VFA concentration was slightly higher in BRUV than that of BRTT (Fig. 3b), as 43 mmol of these compounds was quantified after 13 h of DF. In addition, 13 mmol corresponded to formic acid. In addition, VFA concentrations decreased after 60 h. This was attributed to changes in the metabolic pathways associated with CH4 production. The concentration of formic acid was maintained at a constant. According to Chong et al., VFA accumulation hinders the DF by increasing the ionic strength of the medium. However, this process did not completely inhibit the methanogenic hydrogen-consuming bacteria present in the inoculum [44].
Effect of Continuous Operation on NEJ-ABW Co-digestion
Organic Matter Removal
Figure 4 shows CH consumption when NEJ-ABW was digested for 20 cycles in CRTT operated at an HRT of 6 h. The highest variation in CH degradation was observed in the first cycle (60%). In the second cycle, degradation decreased to 40% and increased until reaching 70%, a potential cause of this variation can be the addition of RCM. It then decreased during cycles 11 and 12 before increasing again. This behavior could be explained by the adaptation of the microbial species involved in the co-digestion process.
According to the results, 85% of CH was removed in CRTT in cycle 13. In contrast, the BRTT showed a reduction of 37% CH. Thus, when DF was carried out in continuous mode, the microbial communities were able to adapt better because of the constant presence of nutrients in the substrate [39, 45]. These results are superior to those reported in previous studies in which NEJ was used as the main source of CH [16].
Table 4 shows the data for organic matter removal (COD, TS, and TVS) during NEJ-ABW co-digestion in CRTT with an OLR of 27 ± 8 g L−1 d. Results correspond to cycles 3, 7, 10, and 17 when the reactor was operated at an HRT of 6 h. The data indicated that 22 and 42% of COD removal was achieved and there was no significant difference. These numbers were higher than those obtained with the BRTT. In the experiments, COD was maintained between 10 and 15 g L−1 and removal was 3–28%, which is in correspondence to CH reduction. Thus, CRTT showed higher consumption than BRTT because, in the former, more nutrients were available due to 6 h of HRT, which facilitated the adaptation of bioH2 producing microorganisms [37]. The TVS were degraded at a rate between 20 and 49%. These data were similar to those published in [8]. These researchers reported a TS and TVS removal of 20 and 40%, respectively, when cheese whey and brewery wastewater were co-digested in the presence of GS using an up-flow anaerobic sludge blanket (UASB) reactor operated at an HRT of 9 h.
BioH2 Production and Yield
Figure 5 shows cumulative bioH2 production when NEJ-ABW was used as the substrate in the DF of the CRTT operated at an HRT of 6 h. After 20 cycles, the bioH2 volume was 1805 mL, which is 5 times higher than that obtained using BRTT [46]. However, bioH2 showed a concentration of 10–20% over the 20 cycles. It is likely that the operating conditions affected these results as bioH2 producing microorganisms were probably washed out [8, 47] and microorganisms indigenous to the substrate probably proliferated.
Given that during the 20 cycles a high amount of bioH2 was accumulated, CRTT showed a higher yield (223 mL H2 g−1 TVS) and productivity (8 mL H2 L−1 h−1) than BRTT (Table 3). Garcia-Peña et al. used fruit and vegetable residues and reported a yield of 60 mL H2 g−1 TVS and a productivity of 1.58 mL H2 L−1 h−1 [40]. In contrast, Reyna-Gómez obtained a yield of 25 mL H2 g−1 TVS and a productivity of 2.6 mL H2 L−1 h−1 when they performed the co-digestion of fruit peels and sewage sludge [30]. The values obtained in the present study were slightly higher than those previously reported. However, when NEJ and tequila vinasses were used as substrates, a yield of 91 mL H2 g−1 COD, and productivity of 102 mL H2 L−1 h−1 were achieved [34].
Volatile Fatty Acids
Figure 6 shows the VFAs produced during cycles 7, 10, and 14. The highest concentration in the three cycles corresponded to acetic acid, with values between 13 and 17.5 mmol. These data agree with the results obtained for bioH2 (9–20% in the biogas). In addition, in the three cycles, CH removal was approximately 70%. Our results showed that other metabolic pathways were present in cycle 10, after which the concentration of propionic acid was increased to 8 mmol. The highest VFA concentration was observed during this cycle. It is likely that this process negatively affected bioH2 production. Propionic acid inhibits bioH2 generation during DF. To produce propionic acid, molecular H2 must be consumed [43, 48]. This process has been previously reported by Cruz-López et al. They observed high acetic acid concentrations when a continuous bioreactor was operated at an HRT of 9 h during the co-digestion of cheese whey and brewery wastewater [8]. Similarly, in cycle 10, the concentration of propionic acid was increased to 550 mg L−1 [8] and acetic acid was the predominant acid. For this reason, bioH2 production remained constant.
Microbial Composition in Bioreactors
To characterize the microbiome, samples were collected from the reactor at different reaction times. DNA extraction was performed, and nucleic acids were identified by amplicon sequencing using the MiSeq Illumina platform. Figure 7 shows the microbial diversity at the species level for BRTT and BRUV. In the BRTT, Clostridium was the dominant species with 87 and 68% after 24 and 72 h, respectively. As shown in this Figure, after 24 h, the predominant species was Clostridium butyricum (87%), followed by Clostridium beijerinckii with only 1% of total Clostridium. Moreover, after 72 h, higher Clostridium diversity was observed. In this case, Clostridium lundense was present in 25%, Clostridium sp. in 13%, C. beijerinckii in 12%, C. butyricum in 9%, Clostridium sporogenes in 5%, and Clostridium oryzae in only 1%.
The presence of C. butyricum was confirmed 24 h after the BRTT. This species is known as a bioH2 producer when mesophilic conditions prevail [40]. It is also known for its high bioH2 conversion rate regardless of substrate complexity. A maximum bioH2 yield of 3.6 mol H2 mol−1 hexose was reported when C. butyricum was used to digest organic residues. This value is similar to the maximum theoretical bioH2 yield of 4 mol H2 mol−1 hexose [49]. Other authors have used C. butyricum as an inoculum in addition to pure glucose and hydrolyzed starch as substrates where yields of 1.98 and 1.5 mol H2 mol−1 glucose were produced, respectively [50, 51]. The C. butyricum genome contains the hydA gene, which encodes hydrogenases. These enzymes are involved in monosaccharide metabolism and bioH2 production [52].
In the BRTT, C. beijerinckii was the predominant species. Both C. butyricum and C. Beijerinckii are strict anaerobic bacteria that have been identified as highly efficient bioH2 producers. This is due to their theoretical yields of approximately 4 mol bioH2 mol−1 glucose. However, the maximum experimental value appeared to be 2 mol bioH2 mol−1 glucose [47, 53]. C. beijerinckii is predominantly found in microbial consortia used in bioH2 production under mesophilic conditions. García-Depraect and León-Becerril used a continuous stirred-tank reactor (CSTR) loaded with tequila vinasses at a temperature of 35 °C. They reported a yield of 124 mL H2 g−1 TVS and identified C. beijerinckii as the predominant species [54]. Rambabu et al., performed the fermentation of rice industry wastewater in a BR with the same bacteria under mesophilic conditions (37 °C) and reported a yield of 215 mL H2 L−1 [55]. Thus, if the proper conditions are met, the presence of these two species guarantees efficient bioH2 production.
In our experiments, C. lundense was present in higher proportions in the BRTT after 72 h. Even when this species does not produce bioH2, it is usually studied because it can hydrolyze lipids. Thus, it may participate in the hydrolysis of DF [56]. These microorganisms are spore-forming bacteria that can grow under mesophilic conditions and at pHs between 5.5 and 7 [56, 57]. In the present study, it was also determined that Lactobacillus shenzhenensi represented 20% of the BRTT microbiota after 72 h. Even when Lactobacillus is not able to form spores [58], their presence in the final stages of DF may be due to the protection provided by GS [42]. It was also observed that the percentage of Lactobacillus shenzhenensi increased from 1 to 20% during the last 48 h of bioreactor operation. The positive effects of lactic acid bacteria on DF have been extensively discussed [37]. In the present study, our data indicated that Lactobacillus inhibited bioH2 production because of competition with bacteria of the genus Clostridium [40].
Similar to BRTT, 73% of BRUV microbiota corresponded to Clostridium. After 60 h of BRUV operation, among the predominant species, Clostridium frigoriphilum (46%), Clostridium sp. (15%), Clostridium perfringens (4%), Clostridium estertheticum (3%), and C. oryzae (1%) have all shown potential as bioH2 producers; however, as shown in Fig. 7, only 1% was featured by C. butyricum, which is a highly efficient bioH2 producer [59]. According to these results, the low bioH2 production observed in the BRUV was caused by the low rate of BioH2-producing Clostridium. The predominant species in this reactor, C. frigoriphilum, and C. estertheticum, have been reported to be tolerant to low temperatures and are responsible for the decomposition of vacuum-packaged frozen meat [60]. The presence of these species was probably due to the nature of the ABW used during substrate co-digestion. In these experiments, meat was used to prepare a synthetic solution to simulate the abattoir wastewater [61].
In addition, it is likely that the presence of CH4 in BRUV was responsible for the low bioH2 production. No methanogens were identified during this stage of the study. However, after 60 h of DF, 21% of the microbiota were not characterized. Some methanogenic species may be present in this portion of the microbiota. These microorganisms also participated in organic matter degradation.
The CRTT microbiota was analyzed in cycles 7 and 14. The highest CH consumption (75%) was observed in cycle 7. In addition, the maximum bioH2 production (17% bioH2 in the biogas, 131 mL total bioH2) was observed in cycle 14.
Figure 8 presents CRTT microbial diversity at the species level during cycles 7 and 14, where Clostridium represented the most predominant genera at concentrations of 59 and 62%, respectively. During cycle seven, the distribution was Clostridium sp., (47%), C. frigoriphilum (5%), C. oryzae (4%), C. butyricum (1%), C. beijerinckii (1%), and Clostridium carboxidivorans (1%). To the best of our knowledge, this is the first time Clostridium carboxidivorans have been identified in these reactor types. These bacteria are classified as acetogenic, and produce acetate, ethanol, butyrate, and butanol [62]. Therefore, it is considered to be a bioH2-producing bacteria. However, C. butyricum, C. acetobutyricum, and C. beijerinckii [63] displayed higher efficiencies than the former. During cycle 14, lower Clostridium diversity was observed. Specifically, Clostridium spp. and C. oryzae were found in proportions of 97 and 3%, respectively. However, a high proportion of these bacteria might be responsible for the high bioH2 production. Also, in cycle 7, a total of 4% Ethanoligenens sp., were quantified. These bacteria produced up to 2.6 mol bioH2/mol substrate [40, 55]. They also showed high hydrolytic capacity. Therefore, they show high performance during CH removal, and consequently, elevated CH consumption was observed in this cycle [40]. Other bacteria that were identified included Leuconostoc sp. (6%) and Lactobacillus oligofermenans (2%), which hinder bioH2 production as they compete for the substrate with bioH2-producing species [45]. Notably, Lactobacillales produce different CH-degrading enzymes including fructokinase, lactate dehydrogenase, and glucose 6 phosphate dehydrogenase [64]. In contrast to cycle 7, reduced microbial diversity was observed in cycle 14 because only Clostridiaceae and a small percentage of Ethanoligenens spp. were identified. The absence of Lactobacillus resulted in low bioH2. In addition, a high proportion of Clostridium species was observed during this cycle.
Conclusions
In this study, the effect of inoculum pretreatment on bioH2 production was determined. Our data indicated that the best pretreatment corresponded to TT because it resulted in the most effective microbial diversity for bioH2 production compared to UV treatment. In this case, the predominant bacterial species was Clostridium, which produces bioH2. In addition, the highest bioH2 productivity (8 mL H2 L−1 h−1) and maximum yield (223 mL H2 g−1 TVS) were obtained when the reactor was operated in continuous mode at an HRT of 6 h. The results presented herein prove that continuous bioH2 production can be achieved through co-digestion of DF with NEJ-ABW. Therefore, we present an alternative method for treating wastewater generated by these industries.
Data Availability
Data are available from the corresponding author on request.
References
Abas, N., Kalair, A., Khan, N.: Review of fossil fuels and future energy technologies. Futures 69, 31–49 (2015). https://doi.org/10.1016/j.futures.2015.03.003
Zhang, L., Zhang, B., Zhu, X., Chang, H., Wang, S.O.H.: Role of bioreactors in microbial biomass and energy conversion. In: Liao, Q., Chang, J., Herrmann, C., Xia, A. (eds.) Bioreactors for microbial biomass and energy conversion, pp. 39–80. Springer, Singapore (2018)
Johnston, B., Mayo, M.C., Khare, A.: Hydrogen: the energy source for the 21st century. Technovation 25(6), 569–585 (2005). https://doi.org/10.1016/j.technovation.2003.11.005
Hallenbeck, P.C., Ghosh, D.: Advances in fermentative biohydrogen production: the way forward? Trends Biotechnol. 27(5), 287–297 (2009). https://doi.org/10.1016/j.tibtech.2009.02.004
Akinbomi, J., Taherzadeh, M.J.: Evaluation of fermentative hydrogen production from single and mixed fruit wastes. Energies 8(5), 4253–4272 (2015). https://doi.org/10.3390/en8054253
Alexandropoulou, M., Antonopoulou, G., Lyberatos, G.: Food industry waste’s exploitation via anaerobic digestion and fermentative hydrogen production in an up-flow column reactor. Waste Biomass Valor. 7(4), 711–723 (2016). https://doi.org/10.1007/s12649-016-9544-y
Valta, K., Damala, P., Angeli, E., Antonopoulou, G., Malamis, D., Haralambous, K.J.: Current treatment technologies of cheese whey and wastewater by Greek cheese manufacturing units and potential valorisation opportunities. Waste Biomass Valor. 8, 1649–1663 (2017). https://doi.org/10.1007/s12649-017-9862-8
Cruz-López, A., Cruz-Méndez, A., Suárez-Vázquez, S.I., Reyna-Gómez, L.M., Pecina-Chacón, D.E., de León Gómez, H.: Effect of hydraulic retention time on continuous biohydrogen production by the codigestion of brewery wastewater and cheese whey. Bioenergy Res. (2022). https://doi.org/10.1007/s12155-022-10399-0
Reyna-Gómez, L.M., Molina-Guerrero, C.E., Alfaro, J.M., Vázquez, S.I.S., Robledo-Olivo, A., Cruz-López, A.: Effect of carbon/nitrogen ratio, temperature, and inoculum source on hydrogen production from dark codigestion of fruit peels and sewage sludge. Sustainable 11(7), 1–13 (2019). https://doi.org/10.3390/SU11072139
Preethi, T.M.M., Usman, J.R., Banu, M.G., Kumar, G.: Biohydrogen production from industrial wastewater: an overview. Bioresour. Technol. Rep. 7, 100287 (2019). https://doi.org/10.1016/j.biteb.2019.100287
Odejobi, O.J., Ajala, O.O., Osuolale, F.N.: Anaerobic co-digestion of kitchen waste and animal manure: a review of operating parameters, inhibiting factors, and pretreatment with their impact on process performance. Biomass Convers. Biorefin. 13(7), 5515–5531 (2023). https://doi.org/10.1007/s13399-021-01626-3
Ghimire, A., et al.: Effects of operational parameters on dark fermentative hydrogen production from biodegradable complex waste biomass. Waste Manag. 50, 55–64 (2016). https://doi.org/10.1016/j.wasman.2016.01.044
Argun, H., Dao, S.: Bio-hydrogen production from waste peach pulp by dark fermentation: effect of inoculum addition Hidayet. Int. J. Hydrogen Energy 42(4), 2569–2574 (2017). https://doi.org/10.1016/j.ijhydene.2016.06.225
García-Depraect, O., Gómez-Romero, J., León-Becerril, E., López-López, A.: A novel biohydrogen production process: co-digestion of vinasse and Nejayote as complex raw substrates using a robust inoculum. Int. J. Hydrogen Energy 42(9), 5820–5831 (2017). https://doi.org/10.1016/j.ijhydene.2016.11.204
Hernández, S.C., Jiménez, L.D., García, J.A.B.: Potential of energy production from slaughterhouse wastewater. Interciencia 43(8), 558–565 (2018)
Del Angel-Acosta, Y.A., Alvarez, L.H., Garcia-Reyes, R.B., Carrillo-Reyes, J., Garcia-Gonzalez, A., Meza-Escalante, E.R.: Co-digestion of corn (nejayote) and brewery wastewater at different ratios and pH conditions for biohydrogen production. Int. J. Hydrogen Energy 46(54), 27422–27430 (2021). https://doi.org/10.1016/j.ijhydene.2021.05.208
España-Gamboa, E., Domínguez-Maldonado, J.A., Tapia-Tussell, R., Chale-Canul, J.S., Alzate-Gaviria, L.: Corn industrial wastewater (nejayote): a promising substrate in Mexico for methane production in a coupled system (APCR-UASB). Environ. Sci. Pollut. Res. 25(1), 712–722 (2018). https://doi.org/10.1007/s11356-017-0479-z
Díaz-Montes, E., Castro-Muñoz, R.: Analyzing the phenolic enriched fractions from Nixtamalization wastewater (Nejayote) fractionated in a three-step membrane process. Curr. Res. Food Sci. 5, 1–10 (2022). https://doi.org/10.1016/j.crfs.2021.11.012
Salmerón-Alcocer, A., et al.: Aerobic treatment of maize-processing wastewater (nejayote) in a single-stream multi-stage bioreactor. J. Environ. Eng. Sci. 2(5), 401–406 (2003). https://doi.org/10.1139/s03-046
Castro-Muñoz, R., Fíla, V., Durán-Páramo, E.: A review of the primary by-product (nejayote) of the nixtamalization during maize processing: potential reuses. Waste Biomass Valor. 10, 13–22 (2019). https://doi.org/10.1007/s12649-017-0029-4
Campechano Carrera, E.M., de Dios Figueroa, J., Cárdenas, G.A., Villa, H.E., Martínez Flores, S.J., Sandoval, J., Luna Bárcenas, J.G.: New ecological nixtamalisation process for tortilla production and its impact on the chemical properties of whole corn flour and wastewater effluents. Int. J. Food Sci. Technol. 47(3), 564–571 (2012). https://doi.org/10.1111/j.1365-2621.2011.02878.x
García-Depraect, O., Rene, E.R., Gómez-Romero, J., López-López, A., León-Becerril, E.: Enhanced biohydrogen production from the dark co-fermentation of tequila vinasse and nixtamalization wastewater: novel insights into ecological regulation by pH. Fuel 253, 159–166 (2019). https://doi.org/10.1016/j.fuel.2019.04.147
Maravilla, A.R., Marion, S.T., Samaniego, J.G., Romero, F.T.: Producción de hidrógeno por digestion anaerobia del nejayote. Fuel 253, 159 (2016)
Zúñiga-Sánchez J.O.: Evaluación del uso del nejayote para la producción de goma xantano por Xanthomonas campestris y su escalamiento a nivel semi-piloto. p. 61, (2020)
Ong, V.Z., Wu, T.Y.: An application of ultrasonication in lignocellulosic biomass valorisation into bio-energy and bio-based products. Renew. Sustain. Energy Rev. 132, 109924 (2020). https://doi.org/10.1016/j.rser.2020.109924
Stefanovic, B., Rosenau, T., Potthast, A.: Effect of sonochemical treatments on the integrity and oxidation state of cellulose. Carbohydr. Polym. 92(1), 921–927 (2013). https://doi.org/10.1016/j.carbpol.2012.09.039
Khamtib, S., Reungsang, A.: Co-digestion of oil palm trunk hydrolysate with slaughterhouse wastewater for thermophilic bio-hydrogen production by Thermoanaerobacterium thermosaccharolyticm KKU19. Int. J. Hydrogen Energy 39(13), 6872–6880 (2014). https://doi.org/10.1016/j.ijhydene.2014.02.073
Yun, J., Kim, T.G., Cho, K.S.: Suppression of methanogenesis in hydrogen fermentation by intermittent feeding. J. Environ. Sci. Heal. Part A 50(14), 1458–1467 (2015). https://doi.org/10.1080/10934529.2015.1074480
Dubois, M., Gilles, K.A., Hamilton, J.K., Rebers, P.A., Smith, F.: Colorimetric method for determination of sugars and related substances. Anal. Chem. (1956). https://doi.org/10.1021/ac60111a017
Reyna-Gómez, L.M., Cruz-López, A., Alfaro, J.M., Suárez-Vázquez, S.I.: Evaluation of the production of biohydrogen during the co-digestion of organic wastes in an upflow hybrid anaerobic reactor. Chem. Eng. J. 425, 129235 (2021). https://doi.org/10.1016/j.cej.2021.129235
APHA: Standard methods for the examination of water and wastewater. Stand. Methods, (2005). ISBN 9780875532356
Madigan M.T., Martinko J.M., Bender K.S., Buckley D.H., Stahl D.A.: Brock—biologia de los microorganismos. In: Metabolismo microbiano, (2015)
Casanova-Mina, A.A., Suárez-Vázquez, S.I., Acuña-Askar, K., Alfaro-Barbosa, J.M., Cruz-López, A.: Continuous dark fermentation by industrial food wastewater: the effect of hydraulic retention time on hydrogen production and microbial variation. Biomass Convers. Biorefin. (2023). https://doi.org/10.1007/s13399-023-04596-w
Wong, Y.M., Wu, T.Y., Juan, J.C.: A review of sustainable hydrogen production using seed sludge via dark fermentation. Renew. Sustain. Energy Rev. 34, 471–482 (2014). https://doi.org/10.1016/j.rser.2014.03.008
Basak, B., Fatima, A., Jeon, B.-H., Ganguly, A., Chatterjee, P.K., Dey, A.: Process kinetic studies of biohydrogen production by co-fermentation of fruit-vegetable wastes and cottage cheese whey. Energy Sustain. Dev. 47, 39–52 (2018). https://doi.org/10.1016/j.esd.2018.08.004
Vijayaraghavan, K., Ahmad, D., Soning, C.: Bio-hydrogen generation from mixed fruit peel waste using anaerobic contact filter. Int. J. Hydrogen Energy (2007). https://doi.org/10.1016/j.ijhydene.2007.07.001
Bernardo Ruggeri, S.S., Tommasi, T.: BioH2 & BioCH4 through anaerobic digestion: from research to full-scale applications. Springer, London (2015)
Cisneros-Pérez, C., Carrillo-Reyes, J., Celis, L.B., Alatriste-Mondragón, F., Etchebehere, C., Razo-Flores, E.: Inoculum pretreatment promotes differences in hydrogen production performance in EGSB reactors. Int. J. Hydrogen Energy 40(19), 6329–6339 (2015). https://doi.org/10.1016/j.ijhydene.2015.03.048
Garcia-Peña, E.I., Canul-Chan, M., Chairez, I., Salgado-Manjarez, E., Aranda-Barradas, J.: Biohydrogen production based on the evaluation of kinetic parameters of a mixed microbial culture using glucose and fruit-vegetable waste as feedstocks. Appl. Biochem. Biotechnol. 171, 279–293 (2013). https://doi.org/10.1007/s12010-013-0341-9
Dzulkarnain, E.L.N., Audu, J.O., Wan Dagang, W.R.Z., Abdul-Wahab, M.F.: Microbiomes of biohydrogen production from dark fermentation of industrial wastes: current trends, advanced tools and future outlook. Bioresour. Bioprocess. 9(16), 1–25 (2022). https://doi.org/10.1186/s40643-022-00504-8
Abubackar, H.N., et al.: Effects of size and autoclavation of fruit and vegetable wastes on biohydrogen production by dark dry anaerobic fermentation under mesophilic condition. Int. J. Hydrogen Energy 44(33), 17767–17780 (2019). https://doi.org/10.1016/j.ijhydene.2019.05.106
Reyna-Gómez, L.M.: Producción de H2 a partir de la codigestión de cáscaras de frutas y lodos de una planta tratadora de agua residual a escala laboratorio. Universidad Autónoma de Nuevo León, San Nicolás de los Garza (2021)
Wang, J., Yin, Y.: Progress in microbiology for fermentative hydrogen production from organic wastes. Crit. Rev. Environ. Sci. Technol. 41(1), 825–865 (2019). https://doi.org/10.1080/10643389.2018.1487226
Chong, M.-L., Sabaratnam, V., Shirai, Y., Hassan, M.A.: Biohydrogen production from biomass and industrial wastes by dark fermentation. Int. J. Hydrogen Energy 34(8), 3277–3287 (2009). https://doi.org/10.1016/j.ijhydene.2009.02.010
Tapia-Venegas, E., et al.: Adaptation of acidogenic sludge to increasing glycerol concentrations for biohydrogen production. Appl. Microbiol. Biotechnol. 99, 8295–8308 (2015). https://doi.org/10.1007/s00253-015-6832-6
Kovalev, A.A., et al.: Biochemical hydrogen potential assay for predicting the patterns of the kinetics of semi-continuous dark fermentation. Bioresour. Technol. 376, 128919 (2023). https://doi.org/10.1016/j.biortech.2023.128919
Sivagurunathan, P., Anburajan, P., Kumar, G., Kim, S.H.: Effect of hydraulic retention time (HRT) on biohydrogen production from galactose in an up-flow anaerobic sludge blanket reactor. Int. J. Hydrogen Energy (2016). https://doi.org/10.1016/j.ijhydene.2016.06.047
Liang, Z., et al.: Removal of volatile organic compounds (VOCs) emitted from a textile dyeing wastewater treatment plant and the attenuation of respiratory health risks using a pilot-scale biofilter. J. Clean. Prod. (2020). https://doi.org/10.1016/j.jclepro.2020.120019
Kivistö, A., Santala, V., Karp, M.: Non-sterile process for biohydrogen and 1,3-propanediol production from raw glycerol. Int. J. Hydrogen Energy 38(27), 11749–11755 (2013). https://doi.org/10.1016/j.ijhydene.2013.06.119
Argun, H., Kargi, F., Kapdan, I.K.: Microbial culture selection for bio-hydrogen production from waste ground wheat by dark fermentation. Int. J. Hydrogen Energy 34(5), 2195–2200 (2009). https://doi.org/10.1016/j.ijhydene.2008.12.066
Baeyens, J., et al.: Reviewing the potential of bio-hydrogen production by fermentation. Renew. Sustain. Energy Rev. 131, 11–23 (2020). https://doi.org/10.1016/j.rser.2020.110023
Wang, M.Y., Olson, B.H., Chang, J.S.: Relationship among growth parameters for Clostridium butyricum, hydA gene expression, and biohydrogen production in a sucrose-supplemented batch reactor. Appl. Microbiol. Biotechnol. 78, 525–532 (2008). https://doi.org/10.1007/s00253-007-1317-x
Behera, B.K., Varma, A.: Microbial resources for sustainable energy. Springer, Switzerland (2016)
García-Depraect, O., León-Becerril, E.: Fermentative biohydrogen production from tequila vinasse via the lactate-acetate pathway: operational performance, kinetic analysis and microbial ecology. Fuel 234, 151–160 (2018). https://doi.org/10.1016/j.fuel.2018.06.126
Rambabu, K., Bharath, G., Thanigaivelan, A., Das, D.B., Show, P.L., Banat, F.: Augmented biohydrogen production from rice mill wastewater through nano-metal oxides assisted dark fermentation. Bioresour. Technol. 319, 124243 (2021). https://doi.org/10.1016/j.biortech.2020.124243
Cirne, D.G., Delgado, O.D., Marichamy, S., Mattiasson, B.: Clostridium lundense sp. Nov., a novel anaerobic lipolytic bacterium isolated from bovine rumen. Int. J. Syst. Evol. Microbiol. 56(3), 625–628 (2006). https://doi.org/10.1099/ijs.0.63730-0
Wikoff, W.R., et al.: Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc. Natl. Acad. Sci. USA 106(10), 3698–3703 (2009). https://doi.org/10.1073/pnas.0812874106
Zou, Y., et al.: Lactobacillus shenzhenensis sp. Nov., isolated from a fermented dairy beverage. Int. J. Syst. Evol. Microbiol. 63(5), 1817–1823 (2013). https://doi.org/10.1099/ijs.0.041111-0
Bando, Y., Fujimoto, N., Suzuki, M., Ohnishi, A.: A microbiological study of biohydrogen production from beer lees. Int. J. Hydrogen Energy 38(6), 2709–2718 (2013). https://doi.org/10.1016/j.ijhydene.2012.11.142
Mang, S., Schwaiger, K., Lindner, R., Gareis, M., Dorn-In, S.: High incidence of cold-tolerant Clostridium frigoriphilum and C. algidicarnis in vacuum-packed beef on retail sale in Germany. Int. J. Food Microbiol. 340, 109053 (2021). https://doi.org/10.1016/j.ijfoodmicro.2021.109053
Barba, F.J., Ahrné, L., Xanthakis, E., Landerslev, M.G., Orlien, V.: Innovative technologies for food preservation. In: Barba, F.J., Orlien, V., Sant’Ana, A.S., Kouba, M. (eds.) Innovative technologies for food preservation: inactivation of spoilage and pathogenic microorganisms, pp. 25–51. Elsevier, Amsterdam (2018)
Liou, J.S.C., Balkwill, D.L., Drake, G.R., Tanner, R.S.: Clostridium carboxidivorans sp. Nov., a solvent-producing clostridium isolated from an agricultural settling lagoon, and reclassification of the acetogen Clostridium scatologenes strain SL1 as Clostridium drakei sp. Nov. Int. J. Syst. Evol. Microbiol. 55(5), 2085–2091 (2005). https://doi.org/10.1099/ijs.0.63482-0
Murray M.-Y: Comprehensive biotechnology, Pergamon, ISBN: 9780080885049. Second Ed., (2011)
Mazareli, R.C.D.S., et al.: Metagenomic analysis of autochthonous microbial biomass from banana waste: screening design of factors that affect hydrogen production. Biomass Bioenergy 138, 105573 (2020). https://doi.org/10.1016/j.biombioe.2020.105573
Acknowledgements
The authors acknowledge SENER-CONACyT-Sustentabilidad Energética for providing funding (CONACYT Research Project Code No. 249908), UANL for providing financial support through the PAICYT Research Program (485-IT2022), and the Environmental Engineering Research Laboratory of the UANL Institute of Civil Engineering. RCCF thanks CONACYT for the graduate scholarship No. 1080269.
Funding
This work was supported by SENER-CONACyT-Sustentabilidad Energética and Universidad Autónoma de Nuevo León, Mexico. RCCF has received research support from CONACyT, Mexico.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by RCCF and LMRG. The first draft of the manuscript was written by RCCF and ACL. All authors commented on previous versions of the manuscript and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Ethical Approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Campos-Flores, R.C., Reyna-Gómez, L.M., Suárez-Vázquez, S.I. et al. Effect of Inoculum Pretreatment and Operational Mode of Reactor on BioH2 Production from Nixtamalization (Nejayote) and Abattoir Wastewater. Waste Biomass Valor 15, 2145–2158 (2024). https://doi.org/10.1007/s12649-023-02279-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-023-02279-1