Abstract
Methylphenidate (MPH) is the most commonly prescribed drug for the treatment of ADHD in males and females. However, a majority of previous studies investigated the effect of MPH in only males, and little is known regarding consequences of female exposure to MPH. This is unfortunate because the few studies that have been conducted indicate that females have a greater sensitivity to MPH. Previous research in male mice has shown that chronic exposure to MPH causes dopaminergic neurons within the nigrostriatal pathway to be more sensitive to the Parkinsonian toxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). However, estrogen has been shown to protect dopaminergic neurons from MPTP neurotoxicity. Therefore, in this study, we test the hypothesis that chronic MPH exposure in female mice will render dopaminergic neurons in the nigrostriatal pathway more sensitive to MPTP, and that estrogen may play a protective role. Interestingly, proestrus females exhibited greater sensitivity to MPTP, with significantly reduced dopaminergic neurons in the SN and significant increases in DA quinone production. Chronic MPH exposure contributed to GSH depletion, but surprisingly, it did not increase dopamine quinone levels or dopaminergic cell loss. There were no significant differences in anestrus animals, with the exception of a depletion in GSH seen when animals received chronic high-dose (10 mg/kg) MPH followed by MPTP. Thus, estrogen may actually sensitize neurons to MPTP in this model, and chronic MPH may contribute to GSH depletion within the striatum. This study provides insight into how chronic psychostimulant use may affect males and females differently.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Centers for Disease Control and Prevention (CDC) reports that as of 2016, 6.1 million children have been diagnosed with attention deficit hyperactivity disorder (ADHD) in the United States alone (Danielson et al. 2018). Methylphenidate (MPH) is the most commonly prescribed drug for the treatment of ADHD, and many children receive MPH from childhood to early adulthood; yet, most of the scientific literature focuses on understanding short-term consequences of MPH. As such, it is extremely important to examine the long-term consequences of MPH exposure. Additionally, the preponderance of previous studies investigated the effect of methylphenidate only in males, and little is known regarding consequences of female exposure to MPH. This is unfortunate as the fact that the few studies that have been conducted in females, suggest that they are more sensitivity to MPH (Brown et al. 2012). Additionally, given the same dosages, females have been shown to have higher brain concentrations of MPH than males (Bentley et al. 2015). Therapeutically, MPH functions by increasing the amount of dopamine (DA) and norepinephrine (NE) in the synaptic cleft as a result of its ability to block DA and NE transporters. Interestingly, there are sex differences reported in regard to dopaminergic tone (Cummins et al. 2014; Frolich et al. 2014). For example, in the striatum, females have a higher concentration of DA transporters and higher concentrations of DA release when compared with males (Walker et al. 2006). Furthermore, females have a greater DA turnover rate than males, a phenomenon that has been shown to be estrogen dependent based on the finding that an ovariectomy reduces the DA turnover rate and estrogen restores it (Dluzen et al. 1996). Moreover, natural fluctuations of estrogen in the estrous cycle are capable of altering the activity of DA in the striatum (Dluzen et al. 1996).
At a physiological pH, DA is capable of autoxidizing by dissociation of a hydroxyl group, creating a DA o-quinone. This unstable DA o-quinone then cyclizes to an aminochrome, which is reduced to leukoaminochrome o-semiquinone radicals, forming H2O2 and hydroxyl radicals. In addition to autoxidation, Fe + 2 and Cu + as well as enzymes with peroxidase activity are capable of catalyzing DA conversion to DA o-quinone, speeding up the reaction and increasing the levels of DA o-quinones (Klein et al. 2019). DA o-quinones are capable of disrupting normal cell function by forming adducts with cysteine residues and inducing oxidative stress throughout the cell (Monzani et al. 2018; Park et al. 2007). Interactions with cysteine residues can be particularly harmful to cell viability as cysteine residues are often found at the active site of enzymes. To prevent DA oxidation, excess DA is normally sequestered in vesicles with a lower pH (Eiden and Weihe 2011; Klein et al. 2019; Segura-Aguilar et al. 2014). However, since MPH is capable of increasing the concentration of DA in the synaptic cleft, it may allow for excess free DA to autoxidize and produce reactive oxygen species. Furthermore, estrogen is thought to play a protective role against oxidative stress, as an increase in oxidative stress was seen in ovariectomy mice, and estrogen was able to decrease the oxidative stress (Gaignard et al. 2015).
Fortunately, our brains are capable of quickly removing small amounts of DA o-quinone by using a natural antioxidant, glutathione (GSH) (Motaghinejad et al. 2016). In fact, GSH has been shown to prevent DA-induced cell death in a number of models (Park et al. 2007; Stokes et al. 2000; Zhou and Lim 2009). GSH forms conjugation reactions with quinones or aminochromes, eventually leading to the formation of 5-S-cysteinyl DA or 4-S-glutathionyl-5,6-dihydroxyindoline, respectively (Smeyne and Smeyne 2013). 5-S-cysteinyl DA and 4-S-glutathionyl-5,6-dihydroxyindoline may then be incorporated into neuromelanin (Zhou and Lim 2009), and some 5-S-cysteinyl DA is released into cerebral spinal fluid. However, GSH can become depleted when there is excess quinone production. If GSH is depleted, there are higher levels of free quinones, and therefore more free radicals, leading to oxidative stress and eventually neurotoxicity via apoptosis (Stokes et al. 2000). An estimated 80% of the DA in the brain is found within the nigrostriatal pathway (Golan et al. 2011; Stahl 2008). The primary responsibility of neurons within this pathway is purposeful movement, and damage to this pathway can result in tremors, spasms, tardive dyskinesia, and Parkinson’s disease (Dexter and Jenner 2013; Gibb and Lees 1988; Klein et al. 2019; Postuma et al. 2015). Therefore, drugs, such as MPH, that increase synaptic DA levels may have detrimental effects on the nigrostriatal pathway. In fact, MPH has been shown to induce oxidative stress by forming DA o-quinones and depleting GSH in the hippocampus and nigrostriatal pathway (Martins et al. 2006; Motaghinejad et al. 2016; Oakes et al. 2019).
Previous research in male mice has shown that chronic exposure to MPH causes dopaminergic neurons within the nigrostriatal pathway to be more sensitive to the Parkinsonian toxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) (Sadasivan et al. 2012). MPTP induces oxidative stress by blocking Complex I of the ETS, leading to mitochondrial dysfunction, loss of ATP production, and generation of free radicals including DA quinones. The loss of SNpc DA neurons subsequent to MPTP has allowed this chemical to be used to induce a model of the dopamine loss seen in Parkinson’s disease (Smeyne and Jackson-Lewis 2005). Furthermore, our lab has previously shown a dose-dependent increase in quinone formation in the striatum of male mice following chronic exposure to MPH (Oakes et al. 2019). Moreover, the increase in quinone formation in the striatum was accompanied by a depletion of GSH, and the depletion of GSH was enhanced when male mice were additionally exposed to MPTP (Oakes et al. 2019). Of note, estrogen has been found to be neuroprotective against MPTP, and it is thought that estrogen conveys neuroprotection by modulating the DA transporter (Dluzen et al. 1996). Interestingly, chronic use of psychostimulants appears to increase the risk of developing Parkinson’s disease (Curtin et al. 2015; Moratalla et al. 2017; Perfeito et al. 2013). Although estrogen has been shown to be protective against MPTP (Dluzen et al. 1996), human epidemiological studies have demonstrated that the use of stimulant drugs puts females at a greater risk of developing neurodegenerative disorders (Curtin et al. 2015). In contrast, estrogen is capable of working synergistically with psychostimulants, increasing the interactions between the psychostimulant and DA reward system (Curtin et al. 2015). Therefore, in this study, we test the hypothesis that chronic MPH exposure in female mice will render dopaminergic neurons in the nigrostriatal pathway more sensitive to MPTP, and that estrogen may play a protective role.
Materials and Methods
Mice and Drug Treatment
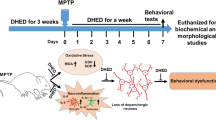
Experiments and procedures with the animals were performed following the regulations set forth by the NIH Guide for the Care and Use of Laboratory Animals. The protocols followed were approved by the University Committee on Animal Care (UCAC) at East Tennessee State University. Mice were allowed food and water ad libitum and were kept on a 12-h light and dark cycle. Adolescent female Swiss-Webster (MPTP-resistant (Hamre et al. 1999; Heikkila 1985) mice received intraperitoneal (i.p.) injections of saline, 1, or 10 mg/kg MPH (Sigma Aldrich, dissolved in 0.9% sterile saline) for 12 weeks. A dose of 1 mg/kg MPH was used because doses of less than 5 mg/kg MPH i.p. in rodents may represent the clinical treatment of ADHD (Gerasimov et al. 2000; Koda et al. 2010). In contrast, a dose of 10 mg/kg MPH i.p. was chosen as it may represent recreational misuse or use of MPH treatment in narcolepsy (Valvassori et al. 2007). Injections were administered using a school week (5 days/week) dosing schedule to prevent weight loss (Martins et al. 2004; Roche et al. 1979). Female mice were allowed to enter the estrus cycle as normal. The Whitten effect was used on the day of the last MPH injection by introducing male pheromones into half the cages prompting female mice to enter the estrus cycle at the same time (Gangrade and Dominic 1984; Whitten 1957). Half of the cages had no pheromones introduced keeping the female mice in anestrus via the Lee-Boot effect (Ma et al. 1998). Female mice were either in proestrus or in anestrus 7 days after the last MPH injection, which was confirmed by vaginal smear (McLean et al. 2012).
MPTP Dosing in Female Swiss-Webster Mice
Previous work has indicated that different strains of mice can have varied sensitivity to MPTP (Boyd et al. 2007; Hamre et al. 1999; Heikkila 1985; Hoskins and Davis 1989; Sedelis et al. 2000; Sonsalla and Heikkila 1988; Sundström et al. 1987; Vidyadhara et al. 2017). Swiss-Webster mice have been found to be MPTP-resistant, and thus, this strain is useful in examining changes in sensitivity to this Parkinsonian toxin. There is an established protocol for administration of MPTP (4 × 20 mg/kg, i.p.) in male Swiss-Webster mice (Hamre et al. 1999; Heikkila 1985). In addition, there appears to be differences in sensitivity to MPTP depending on gender (Alam et al. 2016; Disshon and Dluzen 2000; Dluzen et al. 1996; Sedelis et al. 2000). However, the use of the MPTP model for Parkinson’s has been limited in female models, and none of these studies used female Swiss-Webster mice as a model for MPTP. Therefore, the appropriate MPTP dose for female Swiss-Webster mice was determined by analyzing the survival rate at 4 different doses of MPTP. Anestrus female mice were challenged with injections of MPTP 4 times every 2 h. The MPTP doses given were 10 mg/kg, 12.5 mg/kg, 15 mg/kg, or 20 mg/kg (total dosage was 40 mg/kg, 50 mg/kg, 60 mg/kg, and 80 mg/kg, respectively). The probability of survival was calculated based on the number survived in a group divided by the total of the group. Each of the 4 groups had an n of 5. After this preliminary experiment, a dose of 12.5 mg/kg was chosen and utilized for the remaining work. Briefly, after the 12 weeks of MPH treatment, all animals were subjected to drug washout for 7 days, and then half of each group were treated with MPTP 4 times every 2 h (12.5 mg/kg, total of 50 mg/kg), while the other half was administered 4 injections of sterile 0.9% saline. Seven days after the MPTP injection, mice were sacrificed.
Immunohistochemistry
Mice were sacrificed via transcardial perfusion with saline, followed by 4% paraformaldehyde. The brains were then removed, post-fixed overnight in fresh fixative, embedded in paraffin, and serially sectioned at 10 µm from the rostral hippocampus to the cerebellar-midbrain junction. Serial sections were mounted 5 sections per slide onto polyionic slides (Superfrost Plus, Fisher Scientific). Deparaffinized slides were incubated with an antibody against tyrosine hydroxylase to identify DA neurons (mouse monoclonal anti-tyrosine hydroxylase, Sigma-Aldrich; 1∶500 and biotinylated mouse IgG; 1:1000). A diaminobenzidine (DAB) reaction was used to yield a brown color to mark TH-expressing DA neurons. All tissue sections were counter stained with the Nissl stain Neutral Red for landmark identification and to allow visualization of SNpc neurons that were still alive but biochemically inactive. The total number of TH-positive neurons and TH-negative, Nissl-positive cells on both sides of the pars compacta region of the substantia nigra (SNpc) that had the characteristics of dopaminergic neurons were estimated using model-based stereological methods as described in Baquet et al. (Baquet et al. 2009). Experimental conditions were blinded during the analysis. An n of 3 to 5 mice was used for each group.
Quinone Assay
Mice were sacrificed by decapitation, and the striata were removed, flash-frozen in liquid nitrogen, and stored at −70 °C until analysis. An n of 4 to 5 mice was used for each group. The amount of free and protein bound ortho-quinones in the striatum was quantified using near-infrared fluorescence (nIRF) dot blots (Mazzulli et al. 2016). As reported previously, samples were homogenized in 300 µL of cold PBS by sonication, centrifuged for 5 min at 4 °C at 400 × g and the supernatant discarded. Free and protein-bound ortho-quinones were then extracted 3 times using the following procedure. Pellets were resuspended in cold lysis buffer then incubated for 30 min in an ice-water slurry. Samples were then frozen and thawed 3 times and then centrifuged for 30 min at 4 °C at 100,000 × g. The supernatant was saved as the Triton-soluble extraction and the Triton pellet was used for the second extraction. The Triton pellet was resuspended in SDS lysis buffer, boiled for 10 min, sonicated, and boiled for another 10 min and then centrifuged for 30 min at 22 °C at 100,000 × g. The supernatant was saved as the SDS-soluble extraction, and the SDS pellet was used for the third extraction. The SDS pellet was resuspended in 1 N sodium hydroxide (NaOH) and incubated overnight at 55 °C. The samples were then speed-vacuumed and resuspended in base buffer and saved as NaOH-soluble extraction. A standard curve was prepared using DOPAC oxidized with equimolar sodium periodate. Each sample extraction and standard were dot blotted onto a membrane and allowed to dry in a fume hood. Each extraction was normalized for protein content following a BCA protein assay. Dot blots were scanned at 700 nm on the Odyssey Infrared Imaging System (li-Cor) (Oakes et al. 2019).
Glutathione Assay
GSH content in the striatum was measured as previously stated (Oakes et al. 2019). A GSH assay kit (Cayman Chemical Co.) was utilized. Briefly, tissues were homogenized in 50 mM phosphate buffer (10 ml/g of tissue) and centrifuged (10,000 g for 15 min at 4 °C). The supernatant was removed and incubated for 5 min at room temperature with 10% metaphosphoric acid (v/v) and then centrifuged at 2000 g for 3 min. The supernatant was removed again and normalized for protein content following a Bradford protein assay. Samples were added to a 96-well plate and mixed with the reagent cocktail provided in the GSH assay kit and described in the protocol with no deviations, incubated for 25 min in the dark, and the absorbance was determined at 414 nm.
Statistical Analysis
Data are reported as means ± SEM, and the statistical analyses were performed using multiple variable analysis of variance (two-way ANOVA), followed by Tukey’s multiple-comparison test using GraphPad Prism software version 8. An n of 3 to 5 mice was used for each group. P values of < 0.05 were considered significant.
Results
MPTP Treatment in Female Swiss-Webster Mice
As mentioned previously, there is an established protocol for administration of MPTP (4 × 20 mg/kg, i.p.) in male Swiss-Webster mice (Hamre et al. 1999; Heikkila 1985). However, when we administered MPTP according to this protocol, 100% of the female Swiss-Webster mice died. Many died after the second and third injections, with the remaining mice dying shortly after the last injection. Previous work with other strains of mice (such as MPTP-sensitive C57BL/6 mice) has also shown complete lethality when female mice were given the same dose as males (4 × 20 mg/kg, i.p.) (Schwarting et al. 1999). While some peripheral toxicity is expected with MPTP, it is clear that female mice require MPTP dose adjustments. As such, we performed a preliminary experiment whereby we tested various doses of MPTP to determine the maximal dose we could use that would allow for at least 50% survival. As above, all doses were given 4 times, every 2 h and included individual injection of 20 mg/kg, 15 mg/kg, 12.5 mg/kg, or 10 mg/kg. The 20-mg/kg dose again produced a 100% death rate, and 15 mg/kg produced an 80% death rate. With a dose of 12.5 mg/kg, the death rate was 50%, and with 10 mg/kg, the death rate was 40%. Thus, for the remaining experiments, a dose of 12.5 mg/kg MPTP, every 2 h for a total of 4 injections, was utilized.
Dopamine Cell Count in the Substantia Nigra
We conducted a stereological analysis of TH + cells within the SNpc in order to determine how chronic MPH followed by saline/MPTP affected numbers of dopaminergic neurons within that brain region. Females were further subdivided based on the stage of the estrus cycle at the time of MPTP exposure, as proestrus animals have high estrogen levels and anestrus animals have low estrogen levels. Interestingly, in proestrus females exposed to chronic saline treatment, MPTP caused a significant decrease in the number of dopaminergic neurons within the SNpc (Fig. 1m). This is surprising given that the dose is lower than doses used in males, and these mice are typically MPTP-resistant (Hamre et al. 1999). Although this trend was observed in animals exposed to chronic MPH, there were no significant differences. Additionally, there were no significant differences observed in anestrus animals across any of the treatments (Fig. 1n).
Representative images of the substantia nigra par compacta (SNpc) of proestrus female mice treated with saline a–d, 1 mg/kg e–h, or 10 mg/kg i–l MPH for 12 weeks followed by MPTP. Stereological estimates of dopaminergic neuron numbers in the SNpc of proestrus m or anestrus n female mice. Female Swiss-Webster mice received intraperitoneal injections of saline, 1, or 10 mg/kg MPH for 12 weeks (labeled at the bottom of the bar graph) followed by either saline (black bars) or MPTP (gray bars). Female mice were confirmed to be in proestrus or anestrus at the time of the injection, and tissue was collected 7 days later. Data are expressed as means ± SEM (n = 3 to 5). Two-way ANOVA followed by Tukey’s post hoc analyses
Dopamine O-Quinone Concentration in the Striatum
Dopamine may contribute to neurotoxicity when it oxidizes to a DA o-quinone. As such, we quantified levels of quinones within the terminal region of the nigrostriatal pathway, the striatum. The production of DA o-quinones was significantly increased in proestrus mice that were exposed to chronic saline followed by MPTP compared with those exposed only to saline (Fig. 2a). Interestingly, again, no significant differences were observed in proestrus mice exposed to chronic MPH followed by MPTP or saline. Moreover, no significant differences were observed among anestrus subgroups (Fig. 2b). However, anestrus mice exposed to chronic saline followed by MPTP displayed significantly lower dopamine quinone production compared their proestrus counterparts (Fig. 2a and b).
The concentration of free and protein-bound ortho-quinones in the striata of proestrus a or anestrus b female mice. Female Swiss-Webster mice received intraperitoneal injections of saline, 1, or 10 mg/kg MPH for 12 weeks followed by either saline or MPTP. Female mice were confirmed to be in proestrus or anestrus at the time of the injection. Data are expressed as means ± SEM (n = 4 to 5). Two-way ANOVA followed by Tukey’s post-hoc test. *p < 0.05 vs. proestrus saline + MPTP
Glutathione Concentration in the Striatum
GSH is an important antioxidant within the CNS that can conjugate to DA ortho-quinones in an effort to prevent formation of free radicals. Thus, subsequently, we quantified striatal GSH levels in proestrus and anestrus subgroups following chronic saline or MPH exposure, followed by saline or MPTP. Interestingly, in proestrus females, a significant decrease in GSH levels was observed following chronic exposure to both low (1 mg/kg) and high (10 mg/kg) doses of MPH (Fig. 3a). Furthermore, mice exposed to chronic MPH followed by MPTP displayed even greater depletion in GSH levels, with mice receiving 10 mg/kg MPH followed by MPTP having the lowest levels. In anestrus females, chronic MPH did not deplete GSH, but when chronic MPH animals also received MPTP, there was a significant depletion in GSH levels within the striatum (Fig. 3b).
Glutathione (GSH) concentration in the striata of proestrus a or anestrus b female mice. Female Swiss-Webster mice received intraperitoneal injections of saline, 1, or 10 mg/kg MPH for 12 weeks followed by either saline or MPTP. Female mice were confirmed to be in proestrus or anestrus at the time of the injection. Data are expressed as means ± SEM (n = 3 to 5). Two-way ANOVA followed by Tukey’s post-hoc test. *p < 0.05 vs. proestrus saline + saline, **p < 0.05 vs. proestrus saline + MPTP
Discussion
Previously, we found that chronic MPH increased quinone formation and depleted GSH in the striatum of male Swiss-Webster mice, and MPTP caused an even greater depletion in GSH (Oakes et al. 2019). In this study, we examined the effect of chronic MPH exposure in female Swiss-Webster mice on dopaminergic neurons in the nigrostriatal pathway and whether or not those dopaminergic neurons became sensitive to the neurotoxin, MPTP. We also investigated the role estrogen may play by utilizing the estrus cycle. Firstly, we elucidated the appropriate MPTP dose for female Swiss-Webster mice. Swiss-Webster mice are known to be MPTP-resistant (Heikkila 1985), and estrogen has been shown to be neuroprotective (Dluzen and Horstink 2003; McArthur and Gillies 2011). Unfortunately, when female Swiss-Webster mice are administered the typical acute MPTP regimen (20 mg/kg i.p. × 4 injections), it results in complete lethality, consistent with other mouse strains (Schwarting et al. 1999). This is likely due to peripheral toxicity, as female mice are particularly vulnerable to cardiovascular side effects in response to MPTP (Jackson-Lewis and Przedborski 2007). However, death due to MPTP-induced cardiovascular issues is unrelated to the loss of dopaminergic neurons in the nigrostriatal pathway; thus, female mice treated with MPTP may succumb and die due to cardiovascular events, before dopaminergic neuron loss in the SNpc may be observed. (Jackson-Lewis and Przedborski 2007). We found that a dose of 12.5 mg/kg MPTP given 4 times i.p. allowed for 50% or greater survival in female Swiss-Webster mice, and interestingly, it did produce some dopaminergic neuron loss within the SNpc.
Our data indicate that MPTP was capable of decreasing the number of dopaminergic neurons within the SNpc of proestrus females. In addition, increased quinone formation is seen in the striatum of proestrus female mice treated with MPTP. In contrast, anestrus females had no significant differences in the number of dopaminergic neurons in the SNpc. Following this trend, anestrus females showed no significant differences in quinone formation in the striatum. Taken together, these results suggest that high physiological levels of estrogen in proestrus females may actually sensitize female Swiss-Webster mice to MPTP. These data conflict with previous studies that have shown that physiological levels of estrogen can be neuroprotective against neurotoxins such as MPTP, and proestrus females have less dopaminergic neuron loss when compared with diestrus females (Dluzen and Horstink 2003; Dluzen et al. 1996; Gomez-Mancilla and Bédard 1992; McArthur and Gillies 2011). While this may be true, these studies used different animal models and dosing regimens. For example, Gomez-Mancilla and Bédard utilized a female monkey model and administered MPTP immediately prior to administration of estrogen (Gomez-Mancilla and Bédard 1992). In studies that utilized mouse models, different strains were used, and the female mice were ovariectomized and then given a bolus of estrogen at the time of the MPTP injection (Dluzen and Horstink 2003; Dluzen et al. 1996; McArthur and Gillies 2011). However, it is also of note that levels of estrogen above physiological levels have been shown to worsen the dopaminergic neuron loss within the SNpc in response to MPTP (Bourque et al. 2009; McArthur and Gillies 2011). In general, studies have shown that female mice exhibit more variability in MPTP-induced neuronal damage to dopaminergic neurons when compared with males (Przedborski et al. 2001). Given the effect of MPTP on dopaminergic neuron number in the SNpc and quinone production in the striatum, it was surprising that GSH was not significantly depleted in proestrus females exposed to chronic saline followed by MPTP. However, females are known to have high concentrations of GSH, which may explain why significant depletions in GSH were not seen (Gaignard et al. 2015; Smeyne et al. 2007).
Another unexpected finding was that chronic MPH did not seem to sensitize female mice to SNpc dopaminergic cell loss in response to MPTP, as it does in males (Sadasivan et al. 2012). Although there was a trend towards a decreased neuron number within the SNpc in the proestrus groups exposed to MPH, there were no significant differences, unlike the mice treated with chronic saline. Additionally, although there was a trend towards an increase in DA quinone levels within the striatum; this was also not significantly changed in the chronic MPH mice. Again, this is in contrast to results observed in males, although notably, males are able to tolerate and receive a higher dose of MPTP than could be utilized in females (Oakes et al. 2019). Furthermore, MPTP induces a retrograde cell death, where synaptic terminals die prior to cell bodies and may experience toxicity at lower doses of MPTP (Al Sweidi et al. 2012). Therefore, the MPTP dose of 12.5 mg/kg may have been insufficient to result in cell body toxicity but was sufficient in destroying the axon terminals. This could potentially lead to less DA release in the striatum, and therefore, decreased quinone production in the striatum as well.
Of note, chronic MPH did appear to induce a significant depletion in striatal GSH in proestrus females, and GSH was further reduced by MPTP. This suggests that the GSH available was capable of handling the extra quinone production that was induced by chronic MPH and MPTP. Of note, females are known to have increased concentration of GSH in mitochondria when compared with males (Gaignard et al. 2015; Turrens 2003). Therefore, it is possible that the increased concentrations of GSH were capable of dispatching any quinones that formed, preventing excess quinones and loss of dopaminergic cell bodies within the SNpc.
In conclusion, female Swiss-Webster mice show dopaminergic neuron loss within the SNpc at a MPTP dose of 12.5 mg/kg i.p. × 4 injections, when MPTP is administered in the proestrus period. Additionally, an increase in levels of quinones within the striatum was also observed when proestrus females were administered chronic saline followed by MPTP. Again, this is surprising given the Swiss-Webster mouse is traditionally more resistant to MPTP and the dose of MPTP utilized in females was less than that typically used in males. Taken together, these results demonstrate that estrogen may sensitize dopaminergic neurons within the SNpc to the Parkinsonian toxin, MPTP. Finally, although long-term MPH did not appear to increase SNpc neuron loss or quinone formation, it did produce a significant depletion in GSH levels within the striatum, that was further reduced with MPTP. Thus, these data may provide insight into possible consequences of long-term MPH exposure in a female model.
References
Al Sweidi S, Sánchez MG, Bourque M, Morissette M, Dluzen D, Di Paolo T (2012) Oestrogen receptors and signalling pathways: implications for neuroprotective effects of sex steroids in Parkinson’s disease. J Neuroendocrinol 24:48–61. https://doi.org/10.1111/j.1365-2826.2011.02193.x
Alam G, Miller DB, O’Callaghan JP, Lu L, Williams RW, Jones BC (2016) MPTP neurotoxicity is highly concordant between the sexes among BXD recombinant inbred mouse strains. Neurotoxicology 55:40–47. https://doi.org/10.1016/j.neuro.2016.04.008
Baquet ZC, Williams D, Brody J, Smeyne RJ (2009) A comparison of model-based (2D) and design-based (3D) stereological methods for estimating cell number in the substantianigra pars compacta (SNpc) of the C57BL/6J mouse. Neuroscience 161:1082–1090. https://doi.org/10.1016/j.neuroscience.2009.04.031
Bentley J, Snyder F, Brown SD, Brown RW, Pond BB (2015) Sex differences in the kinetic profiles of d- and l- methylphenidate in the brains of adult rats. Eur Rev Med Pharmacol Sci 19:2514–2519
Bourque M, Dluzen DE, Di Paolo T (2009) Neuroprotective actions of sex steroids in Parkinson’s disease. Front Neuroendocrinol 30:142–157. https://doi.org/10.1016/j.yfrne.2009.04.014
Boyd JD, Jang H, Shepherd KR, Faherty C, Slack S, Jiao Y, Smeyne RJ (2007) Response to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) differs in mouse strains and reveals a divergence in JNK signaling and COX-2 induction prior to loss of neurons in the substantianigra pars compacta. Brain Res 1175:107–116. https://doi.org/10.1016/j.brainres.2007.07.067
Brown RW, Hughes BA, Hughes AB, Sheppard AB, Perna MK, Ragsdale WL, Roeding RL, Pond BB (2012) Sex and dose-related differences in methylphenidate adolescent locomotor sensitization and effects on brain-derived neurotrophic factor. J Psychopharmacol (Oxford, England) 26:1480–1488. https://doi.org/10.1177/0269881112454227
Cummins ED, Griffin SB, Duty CM, Peterson DJ, Burgess KC, Brown RW (2014) The role of dopamine D1 and D2 receptors in adolescent methylphenidate conditioned place preference: sex differences and brain-derived neurotrophic factor. Dev Neurosci 36:277–286. https://doi.org/10.1159/000360636
Curtin K, Fleckenstein AE, Robison RJ, Crookston MJ, Smith KR, Hanson GR (2015) Methamphetamine/amphetamine abuse and risk of Parkinson’s disease in Utah: a population-based assessment. Drug Alcohol Depend 146:30–38. https://doi.org/10.1016/j.drugalcdep.2014.10.027
Danielson ML, Bitsko RH, Ghandour RM, Holbrook JR, Kogan MD, Blumberg SJ (2018) Prevalence of parent-reported ADHD diagnosis and associated treatment among US children and adolescents, 2016. J Clin Child Adolesc Psychol 47:199–212. https://doi.org/10.1080/15374416.2017.1417860
Dexter DT, Jenner P (2013) Parkinson disease: from pathology to molecular disease mechanisms. Free Radic Biol Med 62:132–144. https://doi.org/10.1016/j.freeradbiomed.2013.01.018
Disshon KA, Dluzen DE (2000) Estrogen reduces acute striatal dopamine responses in vivo to the neurotoxin MPP+ in female, but not male rats. Brain Res 868:95–104. https://doi.org/10.1016/s0006-8993(00)02329-5
Dluzen D, Horstink M (2003) Estrogen as neuroprotectant of nigrostriatal dopaminergic system: laboratory and clinical studies. Endocrine 21:67–75. https://doi.org/10.1385/endo:21:1:67
Dluzen DE, McDermott JL, Liu B (1996) Estrogen alters MPTP-induced neurotoxicity in female mice: effects on striatal dopamine concentrations and release. J Neurochem 66:658–666
Eiden LE, Weihe E (2011) VMAT2: a dynamic regulator of brain monoaminergic neuronal function interacting with drugs of abuse. Ann N Y Acad Sci 1216:86–98. https://doi.org/10.1111/j.1749-6632.2010.05906.x
Frolich J, Banaschewski T, Dopfner M, Gortz-Dorten A (2014) An evaluation of the pharmacokinetics of methylphenidate for the treatment of attention-deficit/ hyperactivity disorder. Expert Opin Drug Metab Toxicol 10:1169–1183. https://doi.org/10.1517/17425255.2014.922542
Gaignard P, Savouroux S, Liere P, Pianos A, Thérond P, Schumacher M, Slama A, Guennoun R (2015) Effect of sex differences on brain mitochondrial function and its suppression by ovariectomy and in aged mice. Endocrinology 156:2893–2904. https://doi.org/10.1210/en.2014-1913
Gangrade BK, Dominic CJ (1984) Studies of the male-originating pheromones involved in the Whitten effect and Bruce effect in mice. Biol Reprod 31:89–96. https://doi.org/10.1095/biolreprod31.1.89
Gerasimov MR, Franceschi M, Volkow ND, Gifford A, Gatley SJ, Marsteller D, Molina PE, Dewey SL (2000) Comparison between intraperitoneal and oral methylphenidate administration: a microdialysis and locomotor activity study. J Pharmacol Exp Ther 295:51–57
Gibb WR, Lees AJ (1988) A comparison of clinical and pathological features of young- and old-onset Parkinson’s disease. Neurology 38:1402–1406. https://doi.org/10.1212/wnl.38.9.1402
Golan DE, Tashjian AH, Armstrong EJ (2011) Principles of pharmacology: the pathophysiologic basis of drug therapy. Principles of Pharmacology: The Pathophysiologic Basis of Drug Therapy, 3 edn. LWW
Gomez-Mancilla B, Bédard PJ (1992) Effect of estrogen and progesterone on L-dopa induced dyskinesia in MPTP-treated monkeys. Neurosci Lett 135:129–132. https://doi.org/10.1016/0304-3940(92)90152-w
Hamre K, Tharp R, Poon K, Xiong X, Smeyne RJ (1999) Differential strain susceptibility following 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) administration acts in an autosomal dominant fashion: quantitative analysis in seven strains of Musmusculus. Brain Res 828:91–103
Heikkila RE (1985) Differential neurotoxicity of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in Swiss-Webster mice from different sources. Eur J Pharmacol 117:131–133. https://doi.org/10.1016/0014-2999(85)90482-0
Hoskins JA, Davis LJ (1989) The acute effect on levels of catecholamines and metabolites in brain, of a single dose of MPTP in 8 strains of mice. Neuropharmacology 28:1389–1397. https://doi.org/10.1016/0028-3908(89)90015-4
Jackson-Lewis V, Przedborski S (2007) Protocol for the MPTP mouse model of Parkinson’s disease. Nat Protoc 2:141–151. https://doi.org/10.1038/nprot.2006.342
Klein MO, Battagello DS, Cardoso AR, Hauser DN, Bittencourt JC, Correa RG (2019) Dopamine: functions, signaling, and association with neurological diseases. Cell Mol Neurobiol 39:31–59. https://doi.org/10.1007/s10571-018-0632-3
Koda K, Ago Y, Cong Y, Kita Y, Takuma K, Matsuda T (2010) Effects of acute and chronic administration of atomoxetine and methylphenidate on extracellular levels of noradrenaline, dopamine and serotonin in the prefrontal cortex and striatum of mice. J Neurochem 114:259–270. https://doi.org/10.1111/j.1471-4159.2010.06750.x
Ma W, Miao Z, Novotny MV (1998) Role of the adrenal gland and adrenal-mediated chemosignals in suppression of estrus in the house mouse: the lee-boot effect revisited. Biol Reprod 59:1317–1320. https://doi.org/10.1095/biolreprod59.6.1317
Martins MR, Reinke A, Petronilho FC, Gomes KM, Dal-Pizzol F, Quevedo J (2006) Methylphenidate treatment induces oxidative stress in young rat brain. Brain Res 1078:189–197. https://doi.org/10.1016/j.brainres.2006.01.004
Martins S, Tramontina S, Polanczyk G, Eizirik M, Swanson JM, Rohde LA (2004) Weekend holidays during methylphenidate use in ADHD children: a randomized clinical trial. J Child Adolesc Psychopharmacol 14:195–206. https://doi.org/10.1089/1044546041649066
Mazzulli JR, Burbulla LF, Krainc D, Ischiropoulos H (2016) Detection of free and protein-bound ortho-quinones by near-infrared fluorescence. Anal Chem 88:2399–2405. https://doi.org/10.1021/acs.analchem.5b04420
McArthur S, Gillies GE (2011) Peripheral vs. Central sex steroid hormones in experimental Parkinson’s disease Front Endocrinol 2:82. https://doi.org/10.3389/fendo.2011.00082
McLean AC, Valenzuela N, Fai S, Bennett SA (2012) Performing vaginal lavage, crystal violet staining, and vaginal cytological evaluation for mouse estrous cycle staging identification. J Vis Exp JoVE:e4389 https://doi.org/10.3791/4389
Monzani E, Nicolis S, Dell’Acqua S, Capucciati A, Bacchella C, Zucca FA, Mosharov EV, Sulzer D, Zecca L, Casella L (2018) Dopamine, oxidative stress and protein-quinone modifications in Parkinson’s and other neurodegenerative diseases. Angew Chem Int Ed Engl. https://doi.org/10.1002/anie.201811122
Moratalla R, Khairnar A, Simola N, Granado N, García-Montes JR, Porceddu PF, Tizabi Y, Costa G, Morelli M (2017) Amphetamine-related drugs neurotoxicity in humans and in experimental animals: main mechanisms. Prog Neurobiol 155:149–170. https://doi.org/10.1016/j.pneurobio.2015.09.011
Motaghinejad M, Motevalian M, Shabab B (2016) Effects of chronic treatment with methylphenidate on oxidative stress and inflammation in hippocampus of adult rats. Neurosci Lett 619:106–113. https://doi.org/10.1016/j.neulet.2015.12.015
Oakes HV, Ketchem S, Hall AN, Ensley T, Archibald KM, Pond BB (2019) Chronic methylphenidate induces increased quinone production and subsequent depletion of the antioxidant glutathione in the striatum. Pharmacol Rep 71:1289–1292. https://doi.org/10.1016/j.pharep.2019.08.003
Park ES, Kim SY, Na JI, Ryu HS, Youn SW, Kim DS, Yun HY, Park KC (2007) Glutathione prevented dopamine-induced apoptosis of melanocytes and its signaling. J Dermatol Sci 47:141–149. https://doi.org/10.1016/j.jdermsci.2007.03.009
Perfeito R, Cunha-Oliveira T, Rego AC (2013) Reprint of: revisiting oxidative stress and mitochondrial dysfunction in the pathogenesis of Parkinson disease-resemblance to the effect of amphetamine drugs of abuse. Free Radical Biol Med 62:186–201. https://doi.org/10.1016/j.freeradbiomed.2013.05.042
Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, Obeso J, Marek K, Litvan I, Lang AE, Halliday G, Goetz CG, Gasser T, Dubois B, Chan P, Bloem BR, Adler CH, Deuschl G (2015) MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord 30:1591–1601. https://doi.org/10.1002/mds.26424
Przedborski S, Jackson-Lewis V, Naini AB, Jakowec M, Petzinger G, Miller R, Akram M (2001) The parkinsonian toxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP): a technical review of its utility and safety. J Neurochem 76:1265–1274. https://doi.org/10.1046/j.1471-4159.2001.00183.x
Roche AF, Lipman RS, Overall JE, Hung W (1979) The effects of stimulant medication on the growth of hyperkinetic children. Pediatrics 63:847–850
Sadasivan S, Pond BB, Pani AK, Qu C, Jiao Y, Smeyne RJ (2012) Methylphenidate exposure induces dopamine neuron loss and activation of microglia in the basal ganglia of mice. PLoS ONE 7:e33693. https://doi.org/10.1371/journal.pone.0033693
Schwarting RK, Sedelis M, Hofele K, Auburger GW, Huston JP (1999) Strain-dependent recovery of open-field behavior and striatal dopamine deficiency in the mouse MPTP model of Parkinson’s disease. Neurotox Res 1:41–56. https://doi.org/10.1007/bf03033338
Sedelis M, Hofele K, Auburger GW, Morgan S, Huston JP, Schwarting RK (2000) MPTP susceptibility in the mouse: behavioral, neurochemical, and histological analysis of gender and strain differences. Behav Genet 30:171–182
Segura-Aguilar J, Paris I, Munoz P, Ferrari E, Zecca L, Zucca FA (2014) Protective and toxic roles of dopamine in Parkinson’s disease. J Neurochem 129:898–915. https://doi.org/10.1111/jnc.12686
Smeyne M, Boyd J, Shepherd KR, Jiao Y, Pond BB, Hatler M, Wolf R, Henderson C, Smeyne RJ (2007) GSTpi expression mediates dopaminergic neuron sensitivity in experimental parkinsonism. Proc Natl Acad Sci USA 104:1977–1982. https://doi.org/10.1073/pnas.0610978104
Smeyne M, Smeyne RJ (2013) Glutathione metabolism and Parkinson’s disease. Free Radical Biol Med 62:13–25. https://doi.org/10.1016/j.freeradbiomed.2013.05.001
Smeyne RJ, Jackson-Lewis V (2005) The MPTP model of Parkinson’s disease. Brain Res Mol Brain Res 134:57–66. https://doi.org/10.1016/j.molbrainres.2004.09.017
Sonsalla PK, Heikkila RE (1988) Neurotoxic effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and methamphetamine in several strains of mice. Prog Neuropsychopharmacol Biol Psychiatry 12:345–354. https://doi.org/10.1016/0278-5846(88)90054-1
Stahl S (2008) Stahl’s essential psychopharmacology: neuroscientific basis & practical applications. Cambridge Medicine, 3 edn. Cambridge University Press
Stokes AH, Lewis DY, Lash LH, Jerome WG 3rd, Grant KW, Aschner M, Vrana KE (2000) Dopamine toxicity in neuroblastoma cells: role of glutathione depletion by L-BSO and apoptosis. Brain Res 858:1–8
Sundström E, Strömberg I, Tsutsumi T, Olson L, Jonsson G (1987) Studies on the effect of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) on central catecholamine neurons in C57BL/6 mice. Comparison with three other strains of mice. Brain Res 405:26–38. https://doi.org/10.1016/0006-8993(87)90986-3
Turrens JF (2003) Mitochondrial formation of reactive oxygen species. J Physiol 552:335–344. https://doi.org/10.1113/jphysiol.2003.049478
Valvassori SS, Frey BN, Martins MR, Réus GZ, Schimidtz F, Inácio CG, Kapczinski F, Quevedo J (2007) Sensitization and cross-sensitization after chronic treatment with methylphenidate in adolescent Wistar rats. Behav Pharmacol 18:205–212. https://doi.org/10.1097/FBP.0b013e328153daf5
Vidyadhara DJ, Yarreiphang H, Raju TR, Alladi PA (2017) Admixing of MPTP-resistant and susceptible mice strains augments nigrostriatal neuronal correlates to resist MPTP-induced neurodegeneration. Mol Neurobiol 54:6148–6162. https://doi.org/10.1007/s12035-016-0158-y
Walker QD, Ray R, Kuhn CM (2006) Sex differences in neurochemical effects of dopaminergic drugs in rat striatum Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 31:1193–1202. https://doi.org/10.1038/sj.npp.1300915
Whitten MK (1957) Effect of exteroceptive factors on the oestrous cycle of mice. Nature 180:1436. https://doi.org/10.1038/1801436a0
Zhou ZD, Lim TM (2009) Roles of glutathione (GSH) in dopamine (DA) oxidation studied by improved tandem HPLC plus ESI-MS. Neurochem Res 34:316–326. https://doi.org/10.1007/s11064-008-9778-6
Acknowledgements
The authors would like to thank the ETSU Division of Laboratory Animal Research (DLAR) staff, particularly Dr. Greg Hanley, Jennie Hoard, and Robin King who provided guidance and assistance with the mouse model. We would also like to acknowledge Angela Hanley for her assistance with animal collections.
Funding
This work was supported by the American Foundation of Pharmaceutical Education and the Gatton College of Pharmacy Department of Pharmaceutical Sciences.
Author information
Authors and Affiliations
Contributions
Conceptualization: Hannah V. Oakes, Brooks B. Pond. Methodology: Hannah V. Oakes, Brooks B. Pond, Richard J. Smeyne. Formal analysis and investigation: Hannah V. Oakes, David McWethy, Shannon Ketchem, Lily Tran, Kaitlyn Phillips, Laura Oakley, Richard J. Smeyne, Brooks B. Pond. Writing - original draft preparation: Hannah V. Oakes. Writing - review and editing: Hannah V. Oakes, Richard J. Smeyne, Brooks B. Pond. Funding acquisition: Hannah V. Oakes, Brooks B. Pond; Supervision: Richard J. Smeyne, Brooks B. Pond.
Corresponding author
Ethics declarations
Ethics Approval
All protocols followed were approved by the University Committee on Animal Care (UCAC) at East Tennessee State University. Experiments and procedures with the animals were performed following the regulations set forth by the NIH Guide for the Care and Use of Laboratory Animals.
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Oakes, H.V., McWethy, D., Ketchem, S. et al. Effect of Chronic Methylphenidate Treatment in a Female Experimental Model of Parkinsonism. Neurotox Res 39, 667–676 (2021). https://doi.org/10.1007/s12640-021-00347-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-021-00347-9