Abstract
Angiostrongyliasis is a parasitic disease and a leading cause of human eosinophilic meningoencephalitis caused by rat lungworm Angiostrongylus cantonensis. This parasite infects a wide range of animal hosts, including snails and rats, which plays a significant role in zoonotic transmission. The study was conducted to determine the prevalence of A. cantonensis infection in freshwater snails and definitive rat hosts in the agricultural area in Ampayon, Butuan City, Philippines. A total of 54 rat samples and 719 snail individuals were collected in June and July 2020. An overall 2.36% prevalence rate of A. cantonensis snail infection was recorded, consisting of Pomacea canaliculata and Melanoides tuberculata, with a prevalence rate of 4.05% and 1.38%, respectively. Results revealed an overall prevalence of 38.9% in rat infection. Rattus tanezumi (48.48%) showed a higher infection than Rattus norvegicus (23.80%). Higher infection rates were found in rice field environments than residential houses, with 44.12% and 30% prevalence rates, respectively. Moreover, male rats showed higher infection rates (50%) than female rats (26.92%). Among age classes, adult rats had significantly higher infection rates (48.57%) than juvenile rats (21.05%). Correlation analysis showed a significant positive correlation between A. cantonensis infection intensity to the body length (r = 0.603; p = 0.001) and body weight (r = 0.715; p = 0.000) of rats. The study exemplifies the critical role of intermediate and definitive hosts for angiostrongyliasis. Infected freshwater snails and rats in rice fields make these agricultural areas a venue for A. cantonensis emergence. Integrated actions, health education campaigns, surveillance, hygiene, and good farming practices will help prevent the potential risk of the transmission of angiostrongyliasis in the area.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Angiostrongylus cantonensis, a parasitic nematode known as rat lungworm, is the leading cause of human eosinophilic meningoencephalitis and, if not managed correctly, can lead to serious life-threatening complications. The geographical distribution of prevalence spread globally from its presumed infection point in Southeast Asia (Jarvi and Prociv 2020; Jacob et al. 2021). The life cycle of this parasite involves rats as definitive hosts and mollusks as intermediate hosts. At the same time, other macro-invertebrate animals serve as paratenic hosts, and several other large wild and domestic animal vertebrates, including humans, are considered accidental or dead-end hosts (Hollingsworth et al. 2007). It has a very complex transmission that makes it challenging to assess; it involves parasitizing warm and cold-blooded animals that do not necessarily share the same geographical distribution and ecological niche (Lv et al. 2011). The majority of human infections generally occur after ingesting third stage (L3) larvae in infected gastropods, and water, or paratenic hosts such as amphibians and reptiles or known transport hosts such as crustaceans and arthropods (Steel et al. 2021; Modry et al. 2021). Rats play a major role in the maintenance of pathogen transmission cycles in multiple environments (Estaño et al. 2020).
Rats and snails are abundant in rural agricultural areas in the Philippines and represent a food source for some, especially if eating raw vegetables and snails, thus increasing the risk of angiostrongyliasis in humans (Tujan et al. 2016; Cawas et al. 2020). Rats can become infected after consuming an intermediate host containing L3 larvae. Infected mollusks represent a crucial component of disease epidemiology, which is closely associated with humans. The parasite larvae can utilize another intermediate host once they escape from the bodies of a dead mollusk, ensuring an uninterrupted life cycle posing a public health risk. Moreover, habitat fragmentation, and conversion of land use, such as agricultural practices, may also affect this parasite's life cycle and epidemiology (Castillo and Paller 2018). Thus, controlling mollusks and rats in agricultural areas is one way to prevent human infections (Tujan et al. 2016; Modrý et al. 2021). Vital information on the dynamics of A. cantonensis infection in a wild animal host is essential in venturing accurate surveillance and effective management strategies in disease control and elimination (Niebuhr et al. 2021). It is essential to assess the possible transmission route of the parasite due to its potential risks to both veterinary and public health to understand how to control and prevent its associated disease (Estaño et al. 2020). The study aims to determine A. cantonensis infection in rats and freshwater snails collected from agricultural areas in Ampayon, Butuan City, Southern Philippines.
Materials and methods
The locale of the study
Samples were collected June-July 2020 in agricultural areas consisting of residential houses and rice fields in Ampayon, Butuan City, Agusan del Norte, Caraga region, Mindanao island in Southern Philippines (Fig. 1). The area lies between the 8° 58′ 0′′ north latitude and 125° 36′ 15′′ east longitude with 19 m above sea level (masl). Based on topographic features, the area harbors a tropical rainforest climate. The existing land use consists of agricultural areas comprised of rice fields and residential houses, patches of secondary forest, and grassland utilized for animal pastures. The adjacent areas are also preserved to support and sustain necessary ecological functions, including the watershed in Taguibo, which serves as the area's main water source.
Snail collection and processing
The collection of snails was done in purposive sampling from 06:00 to 08:00 am by hand picking. Snail samples were individually placed in separate 50 mL glass containers filled with 25 mL water from the same source where the snail was collected and then exposed to direct sunlight between 8:00 am and 12:00 noon (Jumawan and Estaño 2021). The water in each container was examined for the presence of larvae. Snails that did not shed larvae were subjected to a crushing method. The snails were crushed, and each snail's hepatopancreas and other internal parts were isolated and squashed onto a glass slide. A drop of 0.95% physiological saline solution was added to the sample. The samples were viewed under a compound microscope to check for the presence of larvae (Paller et al. 2019).
Collection and processing of rat samples
A total of 54 invasive rat samples of the genus (Rattus spp.) were collected in agricultural areas in rice fields and residential houses. Ten sampling points from each site were randomly selected, proximate or same location where snail samples were collected, and three to five steel wire traps were baited with grilled coconut and dried fish. The trapped rat samples were then brought to the laboratory for processing and dissected for further examination. Morphometrics of rat samples were recorded, such as weight, total body length (TBL), fur color, sex, and place of the collection. Rat species were classified for age groups, sex and identified using the Synopsis of Philippine Mammals by Heaney et al. (2010) as a reference and with the aid of a rodent expert at the University. Age classes of rats were classified into adult and juvenile based on TBL, body weight, and reproductive status using mammae formula for females and the appearance of testis in males (Estaño et al. 2020).
Collection and processing of parasite in rats
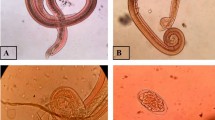
The collected rat samples were dissected, and the heart and lungs were isolated and examined for adult worms. Subsequently, the adult worms were collected and preserved with 70% ethanol. The collected adult worms were identified as A. cantonensis based on the appearance of a barber pole and copulatory bursa for female and male worms, respectively. Furthermore, the heart, lungs, and liver organs were artificially digested with a pepsin-HCL solution using a hot plate magnetic stirrer at 37 °C for one hour. The digested organs were filtered and placed in a Petri dish for microscopic examination of the larvae using a compound microscope (Estaño et al. 2020).
Data analysis
Pearson’s correlation analysis was used to correlate the intensity of A. cantonensis in the body length and body weight of rats. The Chi-square test of independence was used to compare the prevalence of A. cantonensis in rat species, sexes, ages, and habitats (Estaño et al. 2020). Mann Witney U test was used to compare the intensity between rat species, sexes, ages, and habitats. All data were tested for normality of distribution using SPSS version 20.0 software (IBM Corp. 2011), applying a 95% confidence level. The Shapiro–Wilk test was used to determine the normality of the data collected in the study. Statistical computations were performed using Quantitative Parasitology (QP) version 3.0. (Jumawan and Estaño, 2021).
The following formulas were used to compute prevalence and mean intensity:
Results
Prevalence of Angiostrongylus infection in snails
Of the 719 collected snails in agricultural areas in Ampayon Butuan City, an overall 2.36% (95% CI 1.21–5.87) prevalence rate of A. cantonensis infection recorded from irrigation and rice field was observed. Among the collected snail species, only Pomacea canaliculata and Melanoides tuberculata harbor infection with a prevalence rate of 4.05% (95% CI 2.43–6.69) and 1.38% (95% CI 0.45–3.97), respectively. The examined snails were collected in three identified habitat types, where 143 snail individuals were collected in creeks, 114 in irrigation, and 462 snail individuals were collected in rice fields. No infection was observed in creeks, while snails in irrigation harbors had a 1.75% (95% CI 0.48–6.17) prevalence rate, and rice field habitat recorded the highest with 3.24% (95% CI 1.98–5.28) prevalence rate (Table 1).
Prevalence of Angiostrongylus infection in rats
A summary of the results of the 54 rat samples collected and examined for A. cantonensis infection is provided in Table 2. Results revealed an overall prevalence of 38.9% (95% CI 21.3–53.78) with a mean intensity of 5 ± 3.06 worms per rat. Of the rat species, Rattus tanezumi showed a higher infection with 48.48% (95% CI 32.5–64.78) than Rattus norvegicus with a 23.80% (95% CI 10.63–45.09) prevalence rate. Also, the mean intensity was observed to be higher in R. tanezumi (7 ± 2.37) than in R. norvegicus (3 ± 1.06). Statistical analysis revealed a significant difference between rat species for both prevalence rate (p = 0.008) and mean intensities (p = 0.021). In terms of collection sites, rice field areas showed higher infection than residential areas, with 44.12% (95% CI 28.88–60.55) and 30% (95% CI 14.55–51.9) prevalence rates, respectively. Likewise, the rice field areas (6 ± 1.44) showed higher than residential areas (5 ± 1.83) for mean intensity. However, no significant difference in A. cantonensis infection between sites. Moreover, male rats showed higher infection with a 50% (95% CI 32.63–67.37) prevalence rate and mean intensity of 8 ± 1.90 worms/rat, while female rats showed 26.92(95% CI 13.71–46.08) prevalence rate and mean intensity of 3 ± 0.79 worms/rat, significant at p = 0.040 and p = 0.023 respectively. Among age classes, the prevalence in adult rats was significantly higher, 48.57% (95% CI 33.02–64.43), than the juvenile rats, 21.05% (95% CI 8.508–43.33) with a mean intensity of 8 ± 1.61 and 1 ± 0.41 worms/rat, respectively (p = 0.010).
Association of rat’s total body length and weight with parasite intensity
The results reveal that A. cantonensis parasite infection intensity was associated with body length and weight. Pearson's correlation analysis showed a significant positive correlation between A. cantonensis parasite infection intensity with the total body length (r = 0.603; p = 0.001) and body weight (r = 0.715; p = 0.000) of rats, that is, as rat's body length and weight increases the parasite intensity also increases, albeit moderately (Fig. 2).
Discussion
Snails play a significant role in the transmission of A. cantonensis and agricultural areas favored the zoonosis of this parasite. These areas serve as an interface of various animals consisting of intermediate, paratenic, and definitive hosts. Most of the recorded infections in the Philippines were recovered in rice field habitat (Castillo and Paller 2018; Cawas et al. 2020). This study collected several samples in rice field since freshwater snails are naturally abundant in this area which maintains their ecological and physiological requirements. Most of the infected snails harbor in rice field areas, and a few samples in irrigation were also positive for A. cantonensis. No infection was observed in the creek habitat since it has fast-moving water. Rice fields, as the favored habitat of parasitic transmission, may facilitate the intermediate and definitive host ecological relationship. Only two species harbor infection among the three freshwater snail species collected, the Pomacea canaliculata and Melanoides tuberculata. It is worth noting that these freshwater snails are consumed by locals in the area and other provinces in the Philippines (Tujan et al. 2016). Males occasionally consume this snail with alcoholic drinks, and locals prefer to eat snails (raw or cooked) and undercooked vegetables (Cawas et al. 2020). Although total infection reported a low prevalence of A. cantonensis in freshwater snails, this may result from several factors, such as the seasonal timing of infection in the definitive host. The ecological characteristics of P. canaliculata and M. tuberculata, such as their benthic life cycle, may also be accounted for the low prevalence of A. cantonensis infection. Previous studies suggest that A. cantonensis infection in terrestrial snails and slugs are higher than in freshwater snails (Deng et al. 2008). Notably, only a few species of freshwater snails naturally transmit A. cantonensis because rat feces containing its L1 are diluted in freshwater bodies (Lv et al. 2011). Of the two infected snails, P. canaliculata harbors the highest infection, and this snail has a larger size than M. tuberculata. A study by Niebuhr et al. (2021) observed higher infection in larger size snails than in snails with smaller sizes. It also increases the potential risk of infection to humans as locals widely consume more larger snails than small snails.
Infection of A. cantonensis in freshwater snails Pomacea canaliculata and Melanoides tuberculata in the Philippines was first reported in the rice field of Nueva Ecija, the province known as the rice granary of the northern Philippines (Tujan et al. 2016). This study recovered infection in freshwater in agricultural areas in Ampayon Butuan City, Mindanao Island, in Southern Philippines. Other records regarding A. cantonensis infection in intermediate host Acatina fulica and Laevicaulis alte, were also reported from Metro Manila (Constantino-Santos et al. 2014). The recorded infection in this study illustrates the links of transmission of this parasite which intermediate host like snails plays an essential role in the completion of life cycles. Inside the intermediate host, it develops to third-stage infective larvae. It will result in further transmission in a wide-ranging host, which includes planarians, prawns, crabs, frogs, and lizards may serve as paratenic hosts in which infective larvae reside and undergo no further development (Wang et al. 2008). Agricultural areas have a high potential venue for human infections, which may occur through ingesting intermediate or paratenic hosts. Also, the latter is often eaten raw, or their juices used in the preparation of local dishes, infective stage larvae may also leave mollusks and contaminate vegetables such as lettuce and other green leafy vegetables that are widely planted adjacent in the rice field areas.
Invasive rats are abundant in agricultural areas and exploit the crops resulting in declining agricultural production, making them one of the major agricultural pests. Food availability also influences rat habitat preferences (Quilla and Paller 2020). They also served as a reservoir host of various parasitic diseases. The occurrence of con-generic and syntopic rat species plays an important role also in angiostrongyliasis infection. In this study, Rattus tanezumi was ubiquitously found in both habitats in rice field and residential houses, recorded with the highest infection. It may suggest that R. tanezumi can act as a bridge rat hosts species carrying pathogens across different habitats. At the same time, the Norway rat Rattus norvegicus was found only in residential houses associated with poor sanitation. This rat species is an opportunist and highly adaptive to a broad spectrum of conditions, enabling them to successfully inhabit and survive in human habitations (Estaño et al. 2020). This rat species harbor infection with A. cantonensis, and angiostrongyliasis has spread far beyond its original boundaries in R. norvegicus.
Regarding habitat comparison, the abundance of rat diet in agricultural areas includes rice, coconut, maize, gastropods, and insects. Angiostrongylus cantonensis requires gastropods as intermediate hosts, which abound in rice field areas. These factors would have affected rat species' distribution of favored food, behavior, and ability to use different areas or habitats (Harper and Bunbury 2015). In this study, residential houses and rice fields in the agricultural areas show no difference in A. cantonensis infection. Since R. tanezumi species may venture into other areas, including residential houses, they will shift to other food items available in agricultural areas if rice grains are unavailable during rice planting and growing season. In these factors, R. tanezumi in the rice field and residential houses in agricultural areas both carries the potential risk for angiostrongyliasis transmission. Notably, some R. tanezumi occupied residential houses for searching of food and considered their temporary refugia when the rice field was cleared for planting preparations. Interestingly, locals who consume rats prefer to utilize rats captured in rice field as locals think that rodents in rice fields are cleaner and safer to eat than those captured in residential houses. These rats can quickly adjust to environmental changes and inhabit almost all habitats, causing them to be sentinels for the rapid transmission of various infectious diseases, especially parasites, to humans.
This study revealed that male rat was more vulnerable to infections. Male rats have higher hyperactivity movement in adulthood than females, the unpremeditated choice before puberty, and increased inefficient impulse control regardless of hormonal status (Bayless et al. 2012, 2013). The result of this present survey is consistent with the previous study of Estaño et al. (2020), conducted in the Northern Philippines, where the mobility of A. cantonensis to new areas is primarily mediated by male rats, most of whom have a greater dispersal possibility than females. Factors such as sex hormones, body size, immune competence, home range, dispersal rates, and mobility differ between sexes. Breeding females forage for food in rice fields to meet the nutritional needs for lactation while raising offspring. At the same time, males were more active and traveled significantly longer than females occupying a more expansive area, making them more likely to acquire the infective stage of the parasites (Tew and Macdonald 1994; Heaney et al. 1999; Soliman et al. 2001). These factors affect why infection is higher in male rats than in females for both prevalence rate and mean intensity. Nonetheless, infections persist in females, and both sexes are still vulnerable to harboring infections due to these behaviors.
Several factors that affect A. cantonensis infections, including climate, food availability, and environmental conditions, could influence the morphometric measurements of rats (Singleton et al. 2010). This study shows a significant difference in infections between age comparisons. These results show consistent findings to the previous survey by Estaño et al. (2020) of non-native rats collected in Mount Makiling Forest Reserve and its adjacent areas in Luzon Island, Northern Philippines. The high infection in adult rats has more prolonged exposure to acquired parasite infection compared to juveniles living for a short time than adults residing for an extended period. The results of age influence the adult showed a higher prevalence and mean intensity compared to juvenile. Adult rats have a more extensive niche availability in most parasites due to their behavior and ability to travel, seeking food accessibility (Tujan et al. 2016). With that factors, parasite fitness can correlate strongly with host fitness and size (Tseng and Myers 2014). Moreover, the result showed a strong positive correlation between A. cantonensis infection intensity and rats' body weight and body length. The intensity of parasite infection will increase as the body length and weight of the rat hosts increases. These findings agreed with the study of Castillo and Paller (2018) and the previous study of Estaño et al. (2020) that large-bodied rat hosts provide more niches and nutrients for parasites. The heavier hosts are more prone to interact in breeding functions, trade-offs against the immune defense, and increased exposure to prolonged infections (Perrin et al. 1996; Schwanz 2008).
The infection of A. cantonensis is challenging to assess, and the human-animal environmental interface in agricultural areas favors the transmission of angiostrongyliasis. Since the parasite may infect a wide range of animal hosts, it will significantly impact agricultural areas and public health. Changes in land use practices and environmental modifications may also result in changes in the epidemiology of this parasite. Diagnostics and clinical observations demonstrate that eosinophilic meningitis is caused by several helminths and non-helminth parasites (Graeff-Teixeira et al. 2009), and A. cantonensis is the most common cause of this disease (Wang et al. 2008). The Department of Health in the Philippines has reported cases of meningitis for the past several years, and it was recorded as one of the leading causes of child mortality (Ma et al. 2018). Though it was not solely based on A. cantonensis infection, the prevalence of the parasite is of concern, and even more diagnosis of this parasite in humans is neglected most of the time (Tujan et al. 2016). So far, there are only a few sporadic reported cases in the country (Alto 2001; Hochberg et al. 2007), but the cases could be higher. The problem in the Philippines is the lack of disease diagnosis (Estaño et al. 2020). With the new records of infection, both intermediate and definitive animal hosts in the country may indicate the infection is becoming increasingly important as the topographic features, local living conditions, and practices allow it to spreads to more locations. Understanding the transmission dynamics of A. cantonensis involving rats, its intermediate host snails, and other reservoirs hosts concerning human behavior, climate, and environmental changes are significant in the control and prevention. Thus, it is vital to assess the possible transmission route of the parasite due to its risks to both veterinary and public health.
Conclusion and recommendation
The study reports on the infection of A. cantonensis in intermediate freshwater snails and definitive rat hosts collected in agricultural areas in Ampayon, Butuan City, Southern Philippines. Human cases of angiostrongyliasis have yet to be recorded in Mindanao island, Southern Philippines, but this could be due to misdiagnoses and a lack of readily available diagnostic tools. The higher infections in the adult stage and males show a higher vulnerability to transmitting pathogens. Furthermore, the highest infection in R. tanezumi conveys that this species acts as a bridge that carries pathogens across different habitats. Humans can be infected by ingesting infective stage larvae in contaminated water, raw vegetables, and other raw foods. The result calls for a prompt health education campaign and supports interventions, including operational components such as pest control and sanitation, environmental management, proper diagnosis, human case studies, and surveillance. Moreover, good agricultural practices and hygienic awareness will help prevent the potential risk of the transmission of angiostrongyliasis in the area.
References
Alto W (2001) Human infections with Angiostrongylus cantonensis. Pac Health Dialog 8(1):176–182 (PMID: 12017820)
Bayless DW, Darling JS, Stout WJ, Daniel JM (2012) Sex differences in attentional processes in adult rats as measured by performance on the 5-choice serial reaction time task. Behav Brain Res 235(1):48–54. https://doi.org/10.1016/j.bbr.2012.07.028
Bayless DW, Darling JS, Daniel JM (2013) Mechanisms by which neonatal testosterone exposure mediates sex differences in impulsivity in prepubertal rats. Hor Behav 64(5):764–769. https://doi.org/10.1016/j.yhbeh.2013.10.003
Castillo DSC, Paller VGV (2018) Occurrence of Angiostrongylus cantonensis in rodents from the rice granary of the Philippines and associated risk factors for zoonotic transmission. J Paras Dis 42(3):350–356. https://doi.org/10.1007/s12639-018-1005-z
Cawas JR, Quisao CJT, Castillo DSC, Pornobi KO (2020) Prevalence of Angiostrongylus cantonensis among different species of snails in the village of Bagong Sikat Munoz, Nueva Ecija, Philippines and its associated risk factors for zoonotic transmission. J Paras Dis 44(2):388–394. https://doi.org/10.1007/s12639-020-01200-0
Constantino-Santos DMA, Basiao ZU, Wade CM, Santos BS, Fontanilla IKC (2014) Identification of Angiostrongylus cantonensis and other nematodes using the SSU rDNA in Achatina fulica populations of Metro Manila. Trop Biomed 31(2):327–335 (PMID: 25134902)
Deng ZH, Zhang QM, Lin RX, Huang S, Zhang Y, Lv S, Liu H, Hu L, Pei F, Wang J, Ruan C (2008) Survey on the natural focus of angiostrongyliasis in Guandong province. S Chi J Prev Med 34:42–45 (PMID: 20411741)
Estaño LA, Bordado AMD, Paller VGV (2020) Angiostrongylus cantonensis infection of non-native rats in Mount Makiling Forest reserve, the Philippines. Parasitology 148(2):143–148. https://doi.org/10.1017/S0031182020001511
Graeff-Teixeira C, da Silva ACA, Yoshimura K (2009) Update on eosinophilic meningoencephalitis and its clinical relevance. Clin Micro Rev 22(2):322–348
Harper GA, Bunbury N (2015) Invasive rats on tropical islands: their population biology and impacts on native species. Glob Eco Conserv 3:607–627. https://doi.org/10.1016/j.gecco.2015.02.010
Heaney LR, Balete DS, Rickart EA, Utzurrum RCB, Gonzales PC (1999) Mammalian diversity on Mount Isarog, a threatened center of endemism on Southern Island, Philippines. Field Zoo New Ser 95:1–58
Heaney LRM, Dolar ML, Balete DS, Esselstyn JA, Rickart EA, Sedlock JL (2010) Field Museum website, http://www.fieldmuseum.org/philippine_mammals/.
Hochberg NS, Park SY, Blackburn BG, Sejvar JJ, Gaynor K, Chung H, Effler PV (2007) Distribution of eosinophilic meningitis cases attributable to Angiostrongylus cantonensis. Hawaii Emerg Infect Dis 13:1675–1680. https://doi.org/10.3201/eid1311.070367
Hollingsworth RG, Kaneta R, Sullivan JJ, Bishop HS, Qvarnstrom Y, Da Silva AJ, Robinson DG (2007) Distribution of Parmarion cf martensi (Pulmonata: Helicarionidae), a New Semi-Slug Pest on Hawai ‘i Island, and Its Potential as a Vector for Human Angiostrongyliasis1. Pac Sci 61(4):457–467. https://doi.org/10.2984/1534-6188(2007)61[457:DOPCMP]2.0.CO;2
Jacob J, Tan G, Lange I, Saeed H, Date A, Jarvi S (2021) In vitro efficacy of anthelmintics on Angiostrongylus cantonensis L3 larvae. Parasitology 148(2):240–250. https://doi.org/10.1017/S0031182020001146
Jarvi S, Prociv P (2020) Angiostrongylus cantonensis and neuroangiostrongyliasis (rat lungworm disease). Parasitology 148(2):129–132. https://doi.org/10.1017/S003118202000236X
Jumawan JC, Estaño LA (2021) Prevalence of Schistosoma japonicum in bovines and Oncomelania hupensis quadrasi from ricefifields surrounding Lake Mainit, Philippines. J Paras Dis. https://doi.org/10.1007/s12639-021-01372-3
Lv S, Zhang QM, Steinmann P, Deng ZH, Lin RX, Huang S, Zhang Y, Liu H, Hu L, Pei F, Wang J, Ruan C (2011) The emergence of angiostrongyliasis in the People’s Republic of China: the interplay between invasive snails, climate change and transmission dynamics. Freshwat Bio 56(4):717–734. https://doi.org/10.1111/j.1365-2427.2011.02579.x
Ma M, Zhang M, Qiu Z (2018) Eosinophilic meningitis caused by Angiostrongylus cantonensis in an infant: a case report. Medicine. https://doi.org/10.1097/MD.0000000000010975
Modrý D, Fecková B, Putnová B, Manalo S, Otranto D (2021) Alternative pathways in Angiostrongylus cantonensis (Metastrongyloidea: Angiostrongylidae) transmission. Parasitology 148(2):167–173. https://doi.org/10.1017/S0031182020001857
Niebuhr CN, Siers SR, Leinbach IL, Kaluna LM, Jarvi SI (2021) Variation in Angiostrongylus cantonensis infection in definitive and intermediate hosts in Hawaiʻi, a global hotspot of rat lungworm disease. Parasitology 148(2):133–142. https://doi.org/10.1017/S003118202000164X
Paller VG, Macaraig JRM, Verona RT, Estaño LA (2019) Cercarial fauna of freshwater snails in selected agricultural areas in Laguna, Philippines. Helminthologia 56(1):81–86. https://doi.org/10.2478/helm-2018-0040
Perrin N, Christe P, Richner H (1996) On host life-history response to parasitism. Oikos 75:317–320. https://doi.org/10.1016/s1286-4579(00)00389-0
Quilla MHD, Paller VGV (2020) Histopathological features and prevalence of Capillaria hepatica infection in Rattus spp. in Philippine Mount Makiling forest reserve and its adjacent areas. J Paras Dis 44(2):338–348. https://doi.org/10.1007/s12639-019-01189-1
Schwanz LE (2008) Chronic parasitic infection alters reproductive output in deer mice. Behav Ecol Sociobiol 62(8):1351–1358. https://doi.org/10.1007/s00265-008-0563-y
Singleton GR, Belmain S, Brown PR, Aplin K, Htwe NM (2010) Impacts of rodent outbreaks on food security in Asia. Wildl Res 37(5):355–359. https://doi.org/10.1071/WR10084
Soliman S, Marzouk AS, Main AJ, Montasser AA (2001) Effect of sex, size, and age of commensal rat hosts on the infestation parameters of their ectoparasites in a rural area of Egypt. J Parasitol 87(6):1308–1316. https://doi.org/10.1645/0022-3395(2001)087[1308:EOSSAA]2.0.CO;2
Steel A, Jacob J, Klasner I, Howe K, Jacquier S, Pitt W, Hollingsworth R, Jarvi S (2021) In vitro comparison of treatments and commercially available solutions on mortality of Angiostrongylus cantonensis third-stage larvae. Parasitology 148(2):212–220. https://doi.org/10.1017/S0031182020001730
Tew TE, Macdonald DW (1994) Dynamics of space use and male vigour amongst wood mice, Apodemus sylvaticus, in the cereal ecosystem. Behav Ecol Sociobiol 34(5):337–345. https://doi.org/10.1007/BF00197004
Tseng M, Myers JH (2014) The relationship between parasite fitness and host condition in an insect-virus system. PLoS One 9(9):e106401. https://doi.org/10.1371/journal.pone.0106401
Tujan MA, Fontanilla IKC, Paller VGV (2016) Vectors and Spatial patterns of Angiostrongylus cantonensis in selected rice-farming villages of Muñoz, Nueva Ecija, Philippines. J Parasitol Res 2:1–7
Wang QP, Lai DH, Zhu XQ, Chen XG, Lun ZR (2008) Human angiostrongyliasis. Lancet Infect Dis 8(10):621–630
Acknowledgements
The author would like to express the most profound appreciation and gratitude to the locals of Ampayon, Butuan City, for assistance during the collection of samples and to the Department of Biology, College of Mathematics and Natural Sciences, Caraga State University, for allowing the researcher to conduct the processing of samples and laboratory analysis. To: Celestial, H., Aro, K.L., Casindac, K.I., Andress, P.K., and Sevandal, E.B., for assistance collecting samples. The 6th International Angiostrongyliasis and Angiostrongylus workshop for the available information and data presentations to make this study attainable. Paller, V.G., for the completed research works in Angiostrongylus in Northern Philippines and information integration for this study conducted in Southern Philippines.
Funding
This work was supported by the Philippine Commission of Higher Education (CHED) for the research fund granted to L.A. Estaño.
Author information
Authors and Affiliations
Contributions
Conceived and designed sampling: LAE performed the collection of samples and laboratory analysis as well as the writing of the paper.
Corresponding author
Ethics declarations
Conflict of interest
The author declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Estaño, L.A. Prevalence of Angiostrongylus cantonensis in definitive and intermediate hosts collected from agricultural areas in Ampayon, Butuan City, Southern Philippines. J Parasit Dis 47, 807–814 (2023). https://doi.org/10.1007/s12639-023-01626-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12639-023-01626-2