Abstract
Parasitized animals may alter their life histories to minimize the costs of parasitism. Organisms are predicted to decrease investment in current reproduction when parasitism has the greatest impact on current reproductive ability. In contrast, if parasitism decreases residual reproductive value, hosts should increase current reproductive investment, referred to as fecundity compensation or terminal investment. In mammalian hosts, parasitic infection most often leads to reductions in current host reproduction, perhaps attributable to the emphasis on parasites that are unlikely to impact the host’s residual reproductive value. In this study, the life history response of a rodent, Peromyscus maniculatus, to infection with a parasite that should strongly impact the residual reproductive value of its host (Schistosomatium douthitti, Trematoda) was examined. Infection decreased survival for hosts exposed to a high dose of parasites and was chronic in survivors, confirming that infection had strong impacts for the residual reproductive value of the host. As predicted, infected mice increased their reproductive output, producing litters of greater mass due to heavier offspring. However, this increased output was observed after a greater delay to begin breeding in infected mice and was not observed in animals that suffered early mortality. The deer mouse S. douthitti system may provide a rare example of fecundity compensation in mammals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Organisms have limited resources and must trade off between investment options such as reproduction and survival (Stearns 1992). The balance of this trade-off may vary among individuals of the same species as expressed through condition-dependent phenotypic plasticity (Stearns 1992; Schlichting and Pigliucci 1998). When residual reproductive value, which is a measure of future reproductive opportunities, declines for an individual, life history theory predicts an increase in current reproductive investment (Williams 1966; Pianka and Parker 1975). Recently, it has become apparent that parasites and the host’s immune system play an important role in individual-level trade-offs between reproduction and survival (Forbes 1993; Sheldon and Verhulst 1996; Norris and Evans 2000; Zuk and Stoehr 2002). Indeed, empirical research has shown that infection with a parasite can lead to plasticity in the reproductive investment of hosts (Agnew et al. 2000; Hurd 2001).
Plasticity in host reproduction should depend on the parasite’s impact on the host’s current reproductive ability and its residual reproductive value (Forbes 1993; Perrin et al. 1996; McCurdy et al. 2001; Gandon et al. 2002; Schwanz 2006a). Parasitic infection may directly impact a host’s reproductive ability (the relationship between reproductive effort and reproductive output) or the host’s survival. Hosts are predicted to maximize their fitness by altering their reproductive investment in response to the impacts of infection. When infection negatively impacts only the current reproductive ability of the host, hosts should invest less in current reproductive effort and more in survival to increase their chances of reaching the next parasite-free reproductive opportunity (Schwanz 2006a). In this case, residual reproductive value is not impacted. In contrast, if a host is infected with a parasite that negatively impacts its survival or is chronic, the residual reproductive value of the host declines. Here, current reproductive effort is predicted to increase because future reproductive opportunities have decreased, a response termed fecundity compensation or terminal investment (Clutton-Brock 1984; Minchella and LoVerde 1981; Forbes 1993; Schwanz 2006a). It is important to note, however, that an increase in reproductive effort may not lead to increased reproductive output if the parasite additionally impacts current reproductive ability (Perrin et al. 1996; Schwanz 2006a).
Reduced current reproduction in response to parasitic infection has been recorded as reduced probability of reproducing, delayed reproduction, smaller clutch sizes, and reduced male mating effort (Boonstra et al. 1980; de Lope and Moller 1993; Hurd 2001; Kolluru et al. 2002; Telfer et al. 2005). This may be due to indirect effects through host reallocation of resources (i.e., plasticity) or to direct costs of the parasite (i.e., energy reduction). Infected hosts have also shown the opposite response, increasing investment in reproduction, measured as increased reproductive output (Minchella 1985; Minchella and LoVerde 1981; Agnew et al. 2000). This fecundity compensation has been recorded in snails infected with castrating trematodes (Minchella 1985; Thornhill et al. 1986) and also in parasitized insects (Polak and Starmer 1998; Adamo 1999), crustaceans (McCurdy et al. 1999; Chadwick and Little 2005;), birds (Allander and Bennett 1995; Richner 1998; Sanz et al. 2001), and lizards (Sorci et al. 1996).
In rodents, parasitic infection typically leads to no change or a decrease in reproduction (e.g., Boonstra et al. 1980; Bindseil and Hau 1991; Neuhaus 2003; Burns et al. 2005; Telfer et al. 2005). However, many of these studies involve parasites from which the host recovers relatively quickly (∼1 month; Boonstra et al. 1980; Burns et al. 2005; Telfer et al. 2005). Thus, the impact on residual reproductive value may be small. In contrast, the two studies that record increased reproductive investment in female rodents utilize parasites that can remain in the host for the natural life span of the host and, therefore, may strongly impact the host’s residual reproductive value (Willis and Poulin 1999; Kristan 2004). This study examines the reproductive response of a naturally short-lived mammal (Peromyscus maniculatus; average life span in the wild is 6 months, Fairbairn 1977; Millar and Innes 1983; Millar et al. 1992) when infected with a parasite which should have a strong impact on residual reproductive value due to its potential for being chronic and decreasing host survival (Schistosomatium douthitti, Trematoda; Kagan and Meranze 1957; Zajac and Williams 1981).
Deer mice, P. maniculatus, are ideal mammals on which to conduct experiments of reproductive investment due to the wealth of information on this species (e.g., Millar 1979, 1985; Hayes 1989; Hammond and Kristan 2000; Meagher and O’Connor 2001; Kalcounis-Rueppell et al. 2002). Peromyscus spp. are natural hosts of the blood fluke S. douthitti, with adult parasites living in the mesenteric veins of rodents (Price 1931; Malek 1977). Parasites cannot be transmitted between rodents or between mother and offspring because they require an intermediate host (freshwater snails) to complete their life cycle. The pathology of S. douthitti in rodents arises from parasite eggs, which either pass into the lumen of the intestine to be released in host feces or are moved by blood flow into the host liver. In the liver and intestines, parasite eggs elicit host immune cell recruitment and cause tissue damage (Kagan and Meranze 1957; Zajac and Williams 1981). In P. maniculatus, infection leads to reduced liver function and altered thermoregulation (Schwanz 2006b).
In this study, the effect of parasitic infection on the survival and reproductive investment of deer mice over 6 months following exposure was examined. This time frame was chosen because it approximates the natural life span of an adult deer mouse in the wild (Fairbairn 1977; Millar and Innes 1983; Millar et al. 1992; in the lab, mice can live >2 years, Botten et al. 2001). Liver and spleen masses were examined to confirm primary infection pathology (Schwanz 2006b). The potential impact of the parasite on the host’s residual reproductive value was assessed by confirming whether infection was chronic (i.e., remains for the 6-month experimental period) and determining the degree of infection-induced lab mortality. If infection with S. douthitti is chronic and has an impact on survival, deer mice are predicted to increase investment in current reproduction.
Materials and methods
Study system
Deer mice were fifth- to ninth-generation lab-born animals from a larger colony collected from New Mexico (Botten et al. 2001). Mice were kept in plastic rodent cages (48 × 27 × 16 cm L × W × H) and maintained at room temperature (22–24°C) under a 12:12 light/dark cycle throughout the year. Food (Formulab Diet 5008) and water were provided ad libitum.
S. douthitti and the intermediate snail host (Stagnicola elodes, Lymnaeidae) were from lines originally collected in Indiana (provided by D. Daniell, Butler University). At the University of New Mexico, the parasite was cycled through S. elodes and hamsters (Mesocricetus auratus) or lab mice (Mus musculus). The life stage of the parasite which is infective to rodents (cercariae) was collected by placing infected snails in Artificial Spring Water (ASW) in the dark for approximately 1.5 h to induce cercarial shedding. Cercariae were collected under a dissecting scope with a small (3 mm) metal loop. The mouse was allowed to lick the loop to ensure transfer of the cercariae to the mouth of the mouse. This method allowed approximate counts of the number of cercariae to which a mouse was exposed. Control mice were provided uncontaminated ASW using a different metal loop.
Experimental design
Females used for this study were sexually mature, parasite-naïve virgins (70–159 days old at infection). Three treatments were established for the female mice: control (N = 21), exposure to 30–50 cercariae [low dose (LD), N = 20], and exposure to 100–150 cercariae [high dose (HD), N = 10]. These infection doses are similar to natural intensities of adult worm infection in wild rodents (Zajac and Williams 1981). Because the parasite requires ∼30 days to begin inflicting damage on the host (Kagan and Meranze 1957; Zajac and Williams 1981), females were paired with randomly chosen, uninfected males (100–346 days old) 30 days after infection (DAI). The pairs were subsequently allowed to breed freely for 150 days. At 180 DAI, females were euthanized with CO2 and dissected. The livers from females were homogenized in ASW with a Waring blender, and infection status was confirmed by searching the homogenate for S. douthitti eggs or hatched parasites using a dissecting scope. Numbers of eggs and larvae were not quantified systematically enough to estimate infection intensity. The experiment was conducted during 2 years. In 2004, females were infected in February (control and LD only). In 2005, females were infected in April (control, LD, and HD).
Experimental measurements
Females were weighed at infection and at pairing. Following introduction of the male, females were weighed at least weekly, and the cages of pregnant females (determined by weight gain) were checked daily (2005) or every other day (2004) for new litters. Females that did not show weight gain typical of pregnancy were checked every other day. Pups were weaned at 21 days old by removing them from the maternal cage (normal age at independence is ∼3 weeks, Millar 1985).
Maternal mass before and during breeding was examined to assess its potential for influencing treatment-specific reproduction. At euthanasia, wet liver and spleen masses were recorded as a measure of organ pathology. The effects of parasitism on life history were first assessed by comparing the survival of adult female mice in each treatment. To examine reproductive effects, multiple measures of reproductive output were used: (1) the percent of pairs that successfully weaned at least one litter, (2) the time between pairing and the first litter birth (including litters which were recorded as born and subsequently were completely cannibalized), and (3) the average interbirth interval (IBI), excluding intervals after cannibalized litters because gestation time is shorter when females are not simultaneously lactating. Finally, for each litter at weaning, the litter mass, litter size (number of offspring in litter), offspring mass (to the nearest 0.1 g), and sex ratio were recorded. All animal procedures were approved by the Institutional Animal Care and Use Committee at the University of New Mexico (protocol #20305).
Statistical analysis
Treatment effects on survival were examined with Kaplan–Meier survival analysis, using the log rank test statistic. Differences among treatments in the probability of breeding were tested for using Zar’s test of multiple proportions (Zar 1999, p. 562). Where possible, variables were compared for equal variances among treatments with Levene’s test. Where variances did not significantly differ, parametric statistics were used. Where variances did differ, nonparametric statistics were employed because they are less sensitive to differences in variance. Treatment effects on maternal mass at infection and pairing and the change in weight between these two times were analyzed with analysis of variance (ANOVA). Differences in maternal mass during reproduction (mass at each litter birth) were tested with unbalanced repeated measures analysis of covariance (ANCOVA). Liver and spleen masses were examined with ANCOVA, using as a covariate the body mass of the female without liver and intestines. Time until first litter and average IBI were compared among treatments with ANOVA. Unbalanced repeated measures ANCOVA [factors (F) and covariates (C) presented in the results] were used for all measurements made on a per-litter basis (litter size, litter mass, and sex ratio). Differences in offspring mass were tested using nested ANCOVA, with breeding pair as a factor nested within parasite treatment. In the repeated measures and nested ANCOVA models, I included interactions that were relevant to the model: parity × treatment, maternal age × treatment, and year × treatment (not in the nested ANCOVA). Interactions were removed from the model if p > 0.10. For most analyses, infection groups (LD and HD) did not differ, so the LD and HD data for 2005 were combined in a single ‘Infected’ treatment group and compared to the control group with pairwise statistics (t tests and Mann–Whitney U tests). Statistics were performed using SPSS 12.0 (except for proportions tests which were calculated by hand).
Results
Infection duration and organ pathology
Two of the 21 control mice had eggs in their liver homogenate. However, their livers had a healthy appearance (pink and unpitted, see Schwanz 2006b for more description of liver appearance of infected animals) so they were retained in the control treatment. In these cases, the presence of eggs was likely due to contamination during liver homogenization. If the mice were indeed infected, their inclusion in the control treatment would diminish rather than exaggerate the differences between treatments. Infection was confirmed in 16 of the 20 LD mice and 9 of the 10 HD mice. Mice in the infection treatments for which infection was not confirmed were discarded from analysis. Due to mortalities, not all confirmations of infection were made at 180 DAI. Infection was confirmed at 180 DAI for 14 of 18 LD mice and 5 of 6 HD mice, indicating that infection was typically chronic.
Maternal mass did not differ at infection (t = 0.87, p = 0.38) or at pairing (t = −0.64, p = 0.52), but infected females gained more mass during this time interval (U = 161, p = 0.025; N Control = 21, N Infected = 25 for all mass comparisons). For females that bred, mass at each litter birth was not greater for infected females but increased in both treatments through the third parity (repeated measures ANOVA: treatment, F 1,58.4 = 1.40, p = 0.24; parity, F 3,33.2 = 3.23, p = 0.035; treatment × parity, F 3,33.2 = 0.10, p = 0.96; N Control = 11, N Infected = 14). Wet liver mass was greater for infected mice compared to controls after accounting for body mass (treatment, F 1,36 = 12.07, p = 0.001; body mass, F 1,36 = 66.61, p < 0.001; N Control = 20, N Infected = 19). Wet spleen mass was unrelated to body mass and was not significantly greater in infected mice (t = −1.14, p = 0.27; N Control = 10, N Infected = 12; spleen mass was only recorded in the second year of the experiment).
Survival
Within 65 DAI, no control, one LD, and three HD mice died. All dead mice had parasites present in their liver. One additional mouse in each treatment died during the experiment (undetermined cause), all after 130 DAI. Survival differed significantly among treatments (Fig. 1; p = 0.004). Post hoc pairwise comparisons revealed that HD mice had reduced survival compared to control mice (Bonferroni-adjusted α = 0.017: control vs. LD, p = 0.36; control vs. HD, p = 0.002; LD vs. HD, p = 0.04).
Reproductive success and output
The percentage of pairs (alive past 65 DAI) that successfully weaned at least one litter did not differ among treatments [control, 57% (12/21); LD, 73% (11/15); HD, 50% (3/6); χ 2 = 1.39, p = 0.50) or between control and infected mice with the two 2005 infection treatments combined [infected, 67% (14/21); χ 2 = 0.40; p = 0.53). Only three mice from the HD treatment group bred (contributing only four litters). There were no substantial differences between HD and LD mice in reproductive measures, so LD and HD mice were combined in 2005 for the reproductive analyses. The results are qualitatively the same if the HD data are excluded from analyses.
Of the mice that bred, the time until first litter birth was greater for infected mice compared to control mice (Fig. 2a; U = 44.00, p = 0.039, N Control = 12, N Infected = 14). The variance in this variable was substantially greater in infected mice, indicating that some infected mice bred soon after pairing (8 of 14 within 60 days after pairing), whereas other infected mice delayed breeding. Treatment had no effect on the average IBI (Fig. 2b; t = 0.82, p = 0.42, N Control = 10, N Infected = 13). Whole- and partial-litter cannibalism occurred, but did not differ between treatments (data not presented).
The number of litters weaned per pair ranged from one to five, with 284 total offspring weaned. Few mice weaned a fifth litter (only two control and two infected pairs), so repeated measures analyses were performed using only the first four litters (parities) of each pair.
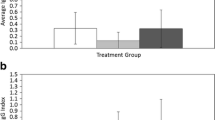
Litter mass differed significantly between treatments (Table 1; Fig. 3a), with infected mice producing litters of 6% greater total mass compared to control mice. Litter size and maternal mass (at litter birth) had positive effects on litter mass, and maternal age had a negative effect. Litter size was positively influenced by maternal mass but was not greater in infected mice (Table 1; Fig. 3b). Infected mice had offspring of greater mass than control mice (Table 2; Fig. 3c). Offspring mass at weaning was positively associated with parity and negatively associated with litter size and maternal age but did not differ between male and female offspring. Treatment did not affect litter sex ratio [predictors: treatment (F), parity (F), treatment × parity (F), litter size (C), maternal mass (C), maternal age (C), and year (F); all p > 0.10).
Litter measurements for adult female deer mice in two treatments (black control; grey infected), separated by parity. Bars are estimated marginal means from repeated measures or nested ANCOVA ± 1 SE. Numbers above bars are sample sizes of pairs and vary according to successful production of a litter. For example, 11 control females bred, but 3 females cannibalized their first litter, leaving a sample size for the first parity of only 8 pairs. a Litter mass (g), b Litter size, and c offspring mass (g, average per litter)
Discussion
Life history theory predicts that organisms may maximize their fitness by altering reproductive effort in response to changes in residual reproductive value or current reproductive ability. In the present study, trematode infection in deer mice had a strong, negative effect on the residual reproductive value of the host due to its impact on survival and its duration. Deer mice infected with high doses of the parasite had ∼30% increase in mortality in a lab setting. Low doses of S. douthitti also reduce physiological performance in deer mice (Schwanz 2006b), suggesting that costs of infection may be apparent under low-dose infections in natural habitats (e.g., Fuller and Blaustein 1996). In addition, infection is maintained for the natural average life span of an adult deer mouse, indicating that future fitness components will be impacted.
In response to infection, female deer mice increased their relative reproductive output, producing litters of approximately 6% greater mass compared to uninfected mice. Because infection by S. douthitti may also damage the reproductive ability of deer mice, the increase recorded here in reproductive output indicates an equal or greater increase in reproductive effort (Perrin et al 1996; Schwanz 2006a). Increased reproductive output in response to infection-induced decreases in residual reproductive value is consistent with the prediction of fecundity compensation (Minchella and LoVerde 1981) if it is assumed that increasing reproductive output improves maternal fitness gains for that reproductive event. Infected mothers produced the same number of offspring, but each offspring was larger. The production of larger offspring may increase offspring fitness, either through (1) compensation for some other offspring fitness disadvantage caused by maternal infection (Meikle and Westberg 2001a, b; Kristan 2002) or (2) producing offspring of higher competitiveness and fitness (Fairbairn 1978; Dewsbury 1979; Millar 1983). Thus, the deer mouse–trematode system may provide a rare mammalian example of fecundity compensation.
Other aspects of deer mouse reproduction in this experiment were not consistent with fecundity compensation. First, no increase in reproductive output was observed in mice that subsequently died. This may largely be explained by the fact that mortality occurred too quickly to produce a litter (∼45 days from conception to weaning). Regardless, none of the females that died early in this study were lactating or had visible embryos. Second, some infected mice delayed initiation of breeding. This delay could have been due to a maternal delay to mate or embryo loss or due to extrinsic limitations, such as male reticence. These individual variations in life history responses to infection may reflect variation in infection intensity or immunocompetence and may indicate a complex conditional response by mice infected with S. douthitti.
Fecundity compensation in response to parasitism has been demonstrated in only a few mammals (wild-derived Mus, Kristan 2004; Apodemus, Willis and Poulin 1999), with additional examples in animals subjected to an immune challenge (Derting and Virk 2005; Weil et al. 2006). The apparent rarity of fecundity compensation in mammals may reflect that mammals have relatively long life spans. Infection may often represent a small portion of a mammal’s reproductive life span, reducing the impact on residual reproductive value (Kristan 2004; Schwanz 2006a). However, many short-lived animals do not demonstrate fecundity compensation when residual reproductive value declines (e.g., Fryer et al. 1990; Zuk 1987; Kolluru et al 2002; Branson 2003), and birds can demonstrate fecundity compensation despite having long lives (Allander and Bennett 1995; Sanz et al 2001; Bonneaud et al 2004). The diverse and often counter-intuitive empirical data on host plasticity is most likely attributable to the diversity of parasite impacts on hosts. For example, strength of the impact of a parasite on its host may be important. Infection may damage a host’s physiology, resource availability or reproductive ability so strongly that life history shifts are impossible or indiscernible. More host–parasite systems must be explored before we can validate the importance of reproductive value for host life histories.
Alternative hypotheses
Several alternative hypotheses may be considered for the life history alterations seen in infected deer mice in this study. First, offspring of infected females may have been nutritionally independent earlier than offspring of uninfected females, which may be advantageous if an infected female is at risk of dying prior to normal weaning. If infected female mice withdrew investment in offspring prior to measurement at 21 days, reproductive output would be overestimated. Second, increased reproductive output could be a non-adaptive, pathological response (e.g., altered lipid metabolism due to liver damage). Offspring of infected mothers were heavier at birth than those of control mothers, and growth rates were equivalent during lactation (Schwanz in press), indicating that any pathological effect would have to occur only during gestation.
Third, when increased mortality occurs concomitantly with increased performance in the survivors, it is possible that the mortality has selected against the poorest and left only the best individuals. This is an unlikely explanation for the differences between infected and control animals recorded in this study. Mortality was low in the low-dose females, which contribute most strongly to the reproductive differences. In addition, females in each treatment had similar masses throughout reproduction, suggesting that surviving, infected females were not of better condition. Finally, a high and equal proportion of surviving females in all three treatments did not breed, indicating that a substantial proportion of females that survived were ‘poor’ breeders.
Finally, could the results be explained by host manipulation by the parasite to benefit the propagation of the parasite (Hurd 2001)? Maternal–offspring transmission of the parasite is not possible, so it is unlikely that increased host production per se benefits that parasite. However, a parasite may increase its host’s activity or water consumption to improve its own transmission (parasite eggs in mouse feces must reach standing water to continue the life cycle). Increased host reproduction could be a by-product of this manipulation. Evidence from nonbreeding deer mice provides no support for this hypothesis—infection with S. douthitti does not elevate activity or water consumption (Schwanz 2006b).
Further insight into the fitness costs of S. douthitti infection and benefits of reproductive alteration in host deer mice would be gained from extending research into the field, quantifying parasite burdens, and monitoring immune responses. No data are available for natural burdens of S. douthitti in P. maniculatus or for the ecology of this host–parasite system. Although dosage effects are not apparent in the physiological and energetic impacts of S. douthitti in lab deer mice (Schwanz 2006b), mortality was higher for mice exposed to more parasites (shown here), suggesting that parasite burdens may be important for facultative responses in wild mice.
References
Adamo SA (1999) Evidence for adaptive changes in egg laying in crickets exposed to bacteria and parasites. Anim Behav 57:117–124
Agnew P, Koella JC, Michalakis Y (2000) Host life history responses to parasitism. Microbes and Infection 2:891–896
Allander K, Bennett GF (1995) Retardation of breeding inset in great tits (Parus major) by blood parasites. Func Ecol 9:677–682
Bindseil E, Hau J (1991) Negative effect on early postimplantation pregnancy and progesterone levels in mice infected with the intestinal trematode Echinostoma caproni. Parasitology 102:387–390
Bonneaud C, Mazuc J, Chastel O, Westerdahl H, Sorci G (2004) Terminal investment induced by immune challenge and fitness traits associate with major histocompatibility complex in the house sparrow. Evolution 58:2823–2830
Boonstra R, Krebs CJ, Beacham TD (1980) Impact of botfly parasitism on Microtus townsendii populations. Can J Zool 58:1683–1692
Botten J, Ricci R, Hjelle B (2001) Establishment of a deer mouse (Peromyscus maniculatus rufinus) breeding colony from wild-caught founders: comparison of reproductive performance of wild-caught and laboratory-reared pairs. Comp Med 51:314–318
Branson DH (2003) Effects of a parasitic mite on life-history variation in two grasshopper species. Evol Ecol Res 5:397–409
Burns CE, Goodwin BJ, Ostfeld RS (2005) A prescription for longer life? Bot fly parasitism of the white-footed mouse. Ecology 86:753–761
Chadwick W, Little TJ (2005) A parasite-mediated life-history shift in Daphnia magna. Proc R Soc Lond B Biol Sci 272:505–509
Clutton-Brock TH (1984) Reproductive effort and terminal investment in iteroparous animals. Am Nat 123:212–229
de Lope F, Moller AP (1993) Effects of ectoparasites on reproduction of their swallow hosts: a cost of being multi-brooded. Oikos 67:557–562
Derting TL, Virk MK (2005) Positive effects of testosterone and immunochallenge on energy allocation to reproductive organs. J Comp Physiol 175:543–556
Dewsbury DA (1979) Copulatory behavior of deer mice (Peromyscus maniculatus): II. A study of some factors regulating the fine structure of behavior. J Comp Physiol Psych 93:161–177
Fairbairn DJ (1977) The spring decline in deer mice: death or dispersal. Can J Zool 55:84–92
Fairbairn DJ (1978) Dispersal of deer mice, Peromyscus maniculatus: proximal causes and effects on fitness. Oecologia 32:171–193
Forbes MRL (1993) Parasitism and host reproductive effort. Oikos 67:444–450
Fryer SE, Oswald RC, Probert AJ, Runham NW (1990) The effect of Schistosoma haematobium infection on the growth and fecundity of three sympatric species of bulinid snails. J Parasitol 76:557–563
Fuller CA, Blaustein AR (1996) Effects of the parasite Eimeria arizonensis on survival of deer mice (Peromyscus maniculatus). Ecology 77:2196–2202
Gandon S, Agnew P, Michalakis Y (2002) Coevolution between parasite virulence and host life-history traits. Am Nat 160:374–388
Hammond KA, Kristan DM (2000) Responses to lactation and cold exposure by deer mice (Peromyscus maniculatus). Physiol Biochem Zool 73:547–556
Hayes JP (1989) Altitudinal and seasonal effects on aerobic metabolism of deer mice. J Comp Physiol 159:453–459
Hurd H (2001) Host fecundity reduction: a strategy for damage limitation. Trends Parasitol 17:363–368
Kagan IG, Meranze DR (1957) The histopathology of the liver in mice experimentally infected with Schistosomatium douthitti. J Infect Dis 100:23–29
Kalcounis-Rueppell MC, Millar JS, Herdman EJ (2002) Beating the odds: effects of weather on a short-season population of deer mice. Can J Zool 80:1594–1601
Kolluru GR, Zuk M, Chappell MA (2002) Reduced reproductive effort in male field crickets infested with parasitoid fly larvae. Behav Ecol 13:607–614
Kristan DM (2002) Maternal and direct effects of the intestinal nematode Heligmosomoides polygyrus on offspring growth and susceptibility to infection. J Exp Biol 205:3967–3977
Kristan DM (2004) Intestinal nematode infection affects host life history and offspring susceptibility to parasitism. J Anim Ecol 73:227–238
Malek EA (1977) Geographical distribution, hosts, and biology of Schistosomatium douthitti (Cort, 1924) Price, 1931. Can J Zool 55:661–671
McCurdy DG, Forbes MR, Boates JS (1999) Testing alternative hypotheses for variation in amphipod behaviour and life history in relation to parasitism. Int J Parasitol 29:1001–1009
McCurdy DG, Boates JS, Forbes MR (2001) An empirical model of the optimal timing of reproduction for female amphipods infected by trematodes. J Parasitol 87:24–30
Meagher S, O’Connor TP (2001) Population variation in the metabolic response of deer mice to infection with Capillaria hepatica (Nematoda). Can J Zool 79:554–561
Meikle D, Westberg M (2001a) Maternal nutrition and reproduction of daughters in wild house mice (Mus musculus). Reproduction 122:437–442
Meikle D, Westberg M (2001b) Social dominance rank and accessory glands in wild adult male house mice born to food-deprived mothers. Physiol Behav 72:359–364
Millar JS (1979) Energetics of lactation in Peromyscus maniculatus. Can J Zool 57:1015–1019
Millar JS (1983) Negative maternal effects in Peromyscus maniculatus. J Mammal 64:540–543
Millar JS (1985) Life cycle characteristics of Peromyscus maniculatus nebrascensis. Can J Zool 63:1280–1284
Millar JS, Innes DGL (1983) Demographics and life cycle characteristics of montane deer mice. Can J Zool 61:574–585
Millar JS, Derrickson EM, Sharpe STP (1992) Effects of reproduction on maternal survival and subsequent reproduction in northern Peromyscus maniculatus. Can J Zool 70:1129–1134
Minchella DJ (1985) Host life-history variation in response to parasitism. Parasitology 90:205–216
Minchella DJ, LoVerde PT (1981) A cost of increased early reproductive effort in the snail Biomphalaria glabrata. Am Nat 118:876–881
Neuhaus P (2003) Parasite removal and its impact on litter size and body condition in Columbian ground squirrels (Spermophilus columbianus). Proc R Soc Lond B Biol Sci (suppl.) 270:S213–S215
Norris K, Evans MR (2000) Ecological immunology: life history trade-offs and immune defense in birds. Behav Ecol 11:19–26
Perrin N, Christe P, Richner H (1996) On host life-history response to parasitism. Oikos 75:317–320
Pianka ER, Parker WS (1975) Age-specific reproductive tactics. Am Nat 109:453–464
Polak M, Starmer WT (1998) Parasite-induced risk of mortality elevates reproductive effort in male Drosophila. Proc R Soc Lond B Biol Sci 265:2197–2201
Price HF (1931) Life History of Schistosomatium douthitti (Cort). Am J Hyg 13:685–727
Richner H (1998) Host-parasite interactions and life-history evolution. Zool-Anal Complex Syst 101:333–344
Sanz JJ, Arriero E, Moreno J, Merino S (2001) Interactions between hemoparasite status and female age in the primary reproductive output of pied flycatchers. Oecologia 126:339–344
Schlichting CD, Pigliucci M (1998) Phenotypic evolution: a reaction norm perspective. Sinauer, Sunderland
Schwanz LE (2006a) Reproductive investment when condition varies: optimality models and an experiment with deer mice (Peromyscus maniculatus). Ph.D. Dissertation, University of New Mexico
Schwanz LE (2006b) Schistosome infection in deer mice (Peromyscus maniculatus): impacts on host physiology, behavior and energetics. J Exp Biol 209:5029–5037
Schwanz LE (2008) Persistent effects of maternal parasitic infection on offspring fitness: implications for adaptive reproductive strategies when parasitized. Func Ecol (in press)
Sheldon BC, Verhulst S (1996) Ecological immunology: Costly parasite defences and trade-offs in evolutionary ecology. Trends Ecol Evol 11:317–321
Sorci G, Clobert J, Michalakis Y (1996) Cost of reproduction and cost of parasitism in the common lizard, Lacerta vivipara. Oikos 76:121–130
Stearns SC (1992) The Evolution of Life Histories. Oxford University Press, New York
Telfer S, Bennett M, Bown K, Carslake D, Cavanagh R, Hazel S, Jones T (2005) Infection with cowpox virus decreases female maturation rates in wild populations of woodland rodents. Oikos 109:317–322
Thornhill JA, Jones JT, Kusel JR (1986) Increased oviposition and growth in immature Biomphalaria glabrata after exposure to Schistosoma mansoni. Parasitology 93:443–450
Weil ZM, Martin LB, Workman JL, Nelson RJ (2006) Immune challenge retards seasonal reproductive regression in rodents: evidence for terminal investment. Biol Lett 2:393–396
Williams GC (1966) Natural selection, the costs of reproduction, and a refinement of Lack’s principle. Am Nat 100:687–690
Willis C, Poulin R (1999) Effects of the tapeworm Hymenolepis diminuta on maternal investment in rats. Can J Zool 77:1001–1005
Zajac AM, Williams JF (1981) The pathology of infection with Schistosomatium douthitti in the laboratory mouse and the meadow vole, Microtus pennsylvanicus. J Comp Physiol 91:1–10
Zar JH (1999) Biostatistical Analysis, 4th edn. Prentice Hall, New Jersey
Zuk M (1987) The effects of gregarine parasites on longevity, weight loss, fecundity and developmental time in the field crickets Gryllus veletis and Gryllus pennsylvanicus. Ecol Entomol 12:349–354
Zuk M, Stoehr AM (2002) Immune defense and host life history. Am Nat 160:S9–S22
Acknowledgements
Deer mice were provided by B. Hjelle (UNM MTA). Snails and S. douthitti were provided courtesy of D. Daniell (Butler University). L. Hertel and E. S. Loker kindly provided materials and training for parasitology techniques, and R. Ricci and F. Gurule provided all animal care. I thank H. Lease, E. Schultz, D. Swenton, C. Tech, and R. Ricci for assistance with data collection. Many people provided advice and assistance on the design and implementation of this study, discussion of the results, and improvements to this manuscript including J. Bragg, J. Brown, E. Charnov, D. Daniell, M. Festa-Bianchet, A. Kodric-Brown, D. Kristan, L. Hertel, E. Loker, R. Ricci, members of the Kodric-Brown and Loker Labs, and several anonymous reviewers. Funding was provided by a National Science Foundation Graduate Research Fellowship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P. Heeb
Rights and permissions
About this article
Cite this article
Schwanz, L.E. Chronic parasitic infection alters reproductive output in deer mice. Behav Ecol Sociobiol 62, 1351–1358 (2008). https://doi.org/10.1007/s00265-008-0563-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-008-0563-y