Abstract
The bacterial isolate Bacillus thuringiensis TS110 was isolated from the rice field soil of Burdwan district, West Bengal, India. Bioassay test of the bacteria TS110 against 3rd, 4th and 5th instar larvae of Cnaphalocrocis medinalis was carried out. Cut leaf assay, potted plant assay and field assay were done. During filed assay, it has been observed that the LC50 (×107) values of TS110 against 3rd, 4th and 5th instar larvae of C. medinalis were 3.77, 5.29, 4.83 and 4.93, 4.42, 4.72 in dry and wet season, respectively. The morphological, biochemical and phylogenetic analysis of the isolate TS110 were done. TS110 was positive for catalase, nitrate reductase, methyl red, voges-proskauer, oxidase, urease, indole, citrate utilization, arginine dihydrolase test, starch, lipid, gelatin, casein, and lecithin hydrolysis test. TS110 showed fermentation test positive for glucose, fructose, mannose, arabinose and trehalose in nutrient broth medium. The antibiotic sensitivity test showed that the bacterial isolate was sensitive to kanamycin (30 µg/disc), nalidixic acid (30 µg/disc), rifampicin (5 μg/disc), doxycycline (30 µg/disc), gatifloxacin (10 µg/disc), vancomycin (30 µg/disc), gentamycin (10 µg/disc), ampicillin (10 µg/disc), ofloxacin (5 μg/disc), levofloxacin (5 μg/disc), streptomycin (10 µg/disc), gentamycin (10 µg/disc). The phylogenetic analysis revealed that TS110 was closely related to different species of B. thuringiensis submitted to the GenBank. On the basis of morpho-physiological and molecular characterization, the bacterial isolate was identified as B. thuringiensis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Insects of different orders constitute the majority of pests infesting different plants. Almost 128 species of insects have been reported to cause damages of rice crop and out of these, 15–20 species are most important in relation to economic loss (Kalode et al. 1995). Insect pests caused extensive damages in rice crop at different developmental stages and among them leaf feeding pests are the important ones due to their ability of decomposing chlorophyll or defoliating that led to yield loss (Kaur et al. 2008). The lepidopteran insect rice leaffolder (Cnaphalocrocis medinalis) is one of the major pests of rice plants accounting great economic loss in rice cultivating countries (Riley et al. 1995). In India, the infestation of rice leaffolder has been increased after late 80’s and became the major cause of economic loss in rice cultivation (Nanda and Bisoi 1990). Chemical insecticides applied against this rice pest caused environmental hazards and the insects also developed resistance against them (Shahid et al. 2003). In view of these, use of Bt as pesticide became the need of the day. Bacillus thuringiensis (Bt) is a gram-positive, rod-shaped, spore-forming bacterium producing different crystal proteins due to which it is widely used in biocontrol of different insect pests (Shishir et al. 2012). They are eco-friendly, do not have a negative effect on nontarget animals, including vertebrates (Joung and Cote 2000). B. thuringiensis produce Cry and Cyt proteins that are effective against different insect pest (Ibrahim et al. 2010). Till now 68 groups of Cry toxin and 3 groups of Cyt toxins have been isolated from different subspecies of Bt (Crickmore et al. 1995). Proteins coded by genes cry1, cry2, cry7, cry8, cry9, cry15, cry22, cry51,and cyt1 are effective against Lepidopteran pests (Frankenhuyzen 2009). Several Bt strains have been used against different lepidopteran insects all over the world; and wild strains of B. thuringiensis isolated from different environmental samples can show higher activity against different insect pests in comparison to already available B. thuringiensis formulated as pesticides (Konecka et al. 2012). On the view of this, the aim of the present study was to isolate and characterize novel Bt strain from the rice field soils of Burdwan district, West Bengal, India and evaluate its pathogenicity against rice leaffolder C. medinalis.
Materials and methods
The soil samples were collected from rice fields of different village areas of Burdwan district, West Bengal. The top most soil (1 cm) was scrapped off and then about 100 g soils (pH 6.9–7.1) from each area were collected in sterile polythene bags, sealed with rubber bands.
Bacteria isolation
Soil samples were collected from the rice fields of Burdwan and taken to the laboratory for further analysis. The soil samples were blotted and diluted serially up to 10−3 level. A 100 μl suspension was mixed with 100 ml Nutrient Agar (NA) (g/l: peptone 5, beef extract 3, agar 2, pH7), distributed in five plates and incubated at 30 ± 0.1 °C in the BOD incubator for 24 h. The colonies were checked under a phase-contrast microscope and those having spores were purified on NA plates and the pure cultures were maintained at 4 ± 0.1 °C on NA slants.
Bio assay of the bacterial isolates against rice leaffolder
Cut leaf assay
Bioassay was carried out on 3rd, 4th and 5th instar larvae of C. medinalis against the bacterial isolates. Rice leaf pieces were surface sterilized and soaked separately in each bacterial suspension (of different concentrations; 104, 105, 106, 107, 108 cfu/ml) for 15–20 min. Then different larval instars of rice leaffolders (10 larae/test tube) were placed in test tubes and covered with an insect proof cloth. The mortality of the larvae were recorded up to 72 h.
Potted plant assay
Pots containing freshly cultivated 30-day old rice plants (5 tillers/pot) of insect’s favourable variety was infested with C. medinalis larvae (10 larvae/pot) before 2 weeks of spraying. Then the infested potted plants with folded leaves were treated separately with different concentrations (104, 105,106, 107, 108 cfu/ml) of the bacterial isolates. The control plants were sprayed with water.
Characterization of bacterial isolate
The bacterial isolate giving best mortality of rice leaffolder was then characterized on the basis of morphological, physiological and biochemical and molecular aspects (Collee and Miles 1989; Smibert and Krieg 1994; Lacey 1997; Logan and de Vos 2009; Janssen 1994; Thompson et al. 1994). Scanning electron micrographs of the isolates were taken to study the morphology of the bacterial isolates. Antibiotic sensitivity was tested using different antibiotic discs viz. amoxycilin (10 µg/disc), kanamycin (30 µg/disc), nalidixic acid (30 µg/disc), rifampicin (5 μg/disc), doxycycline (30 µg/disc), gatifloxacin (10 µg/disc), vancomycin (30 µg/disc), gentamycin (10 µg/disc), ampicillin (10 µg/disc), ofloxacin (5 μg/disc), levofloxacin (5 μg/disc), streptomycin (10 µg/disc), penicillin G (10 U/disc) and gentamycin (10 µg/disc) following Brown (2004). Phylogenetic tree of the potential bacterial isolate TS 110 was constructed following Tamura et al. (2007).
Field assay of spore crystal formers
The isolate TS110 was grown on Nutrient Broth (NB) for 10 d on a rotary shaker (200 rpm, 30 ± 0.1 oC). After that the culture was centrifuged at 10,000 rpm for 10 min and washed three times with distilled water by centrifugation and then preserved at −20 oC. Suspension of the isolate was made in distilled water containing 0.001 % APSA (commercial sticker, 1 μl/100 ml) and diluted logarithmically to obtain 0–108 bacteria/ml and APSA sprayed in the LF infected rice field twice a year i.e. in dry and wet season, respectively, from 2012 to 2014. The mortality was recorded daily.
Result
The bacterial isolate TS110 formed filamentous, off-white, elevated colony ranging 2.45–3.87 mm in diameter. The isolate was rod shaped having spores and crystals (Fig. 1). TS110 was found to be positive for catalase, oxidase, citrate, nitrate, urease, methyl red, voges-proskauer and arginine dihydrolase test. TS110 had the ability to hydrolyse starch, gelatin, casein, lipid and lecithin. The isolate could ferment glucose, fructose, mannose, arabinose and trehalose but could not ferment sucrose, xylose and mannitol present in the medium. TS110 showed growth at pH 5.7–6.8 and 4 –50 °C temperature; this isolate could tolerate up to 10 % NaCl in the Nutrient Broth (NB) medium (Table 1). The total DNA and protein content of the isolate were 7.6 µg/ml and 7.8 µg/ml respectively. SDS-PAGE protein profiling showed that TS110 contained 18 bands of protein having molecular weight of 85.101 to 11.00 kDa (Fig. 2). Antibiotic sensitivity test showed that the isolate was sensitive to kanamycin (30 µg/disc), nalidixic acid (30 µg/disc), rifampicin (5 μg/disc), doxycycline (30 µg/disc), gatifloxacin (10 µg/disc), vancomycin (30 µg/disc), gentamycin (10 µg/disc), ampicillin (10 µg/disc), ofloxacin (5 μg/disc), levofloxacin (5 μg/disc), streptomycin (10 µg/disc), gentamycin (10 µg/disc) but resistant to amoxycilin (10 µg/disc) and penicillin G (10 U/disc) (Table 1). The Neighbour joining tree of TS110 revealed that the isolate Bacillus thuringiensis TS110 (KT725786) branched with the cluster containing other species of Bacillus thuringiensis, Bacillus cereus and Bacillus sp. with 33 % bootstrap value (Fig. 3).
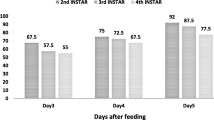
For cut leaf assay the LC50 (×107cfu/ml) values of the B. thuringiensis strain TS 110 were against 3rd, 4th and 5th instar larvae of C. medinalis in dry and wet season was 2.36, 2.37, 4.34 and 2.41,2.87, 5.25 respectively. The potted plant assay showed the LC50 (×107cfu/ml) of TS110 in dry and wet season as 4.43, 3.94, 4.89 and 4.00, 3.21, 3.98 against 3rd, 4th and 5th instar larvae of C. medinalis respectively. The field assay of the bacterial pathogen BtTS110 against 3rd, 4th and 5th instar larvae of C. medinalis showed the LC50 (×107cfu/ml) values of 3.77, 5.29, 4.83 and 4.93, 4.42, 4.72 in dry and wet season respectively (Fig. 4).
Discussion
The morphological, biochemical and phylogenetic analysis of TS110 characterized it as Bacillus thuringiensis. Phylogenetic analysis revealed very close similarity between the bacterial isolate TS110 and other B. thuringiensis sequences present in the Gen Bank. The range of LC50 in cut leaf assay was less than that of potted plant assay. This is because cut leaf assay was done in the laboratory condition which was more controlled than the net house environment where potted plant assay was performed. No such differences have been found in the effect of Bacillus thuringiensis TS110 against larvae of C. medinalis in different seasons but the LC50 value of TS110 against C. medinalis was instar dependent(p = 0.029) which was found to be similar the findings of Tabashnik and Carrie` re (2004) who also observed the same phenomenon.
The mean LC50 value (cfu/ml) of TS110 against larvae of C. medinalis were lower than the findings of Ramamourti et al. 2012 who recorded the LC50 as 0.6 × 108–22.5 × 108 of indigenous Bt isolates against rice LF. The comparatively lower LC50 value in the present study was due to the biotoxicity of the native isolate TS110. Effectiveness of the bacterial pathogen varied with concentration and the period of exposure (Savitri and Murali Mohan 2003). Application of higher concentration of Bacillus thuringiensis reduced the food consumption of C. medinalis and enhanced larval mortality (Singh et al. 2000). The insect mid gut is the main target area for Bt to infect the larvae. The crystal proteins of Bt get solubilized and activated in the mid gut at high pH and produce delta-endotoxins that showed the toxicity against the larvae (Knowles 1994). The delta-endotoxin firstly binds with the membrane glycoprotein of the midgut epithelium and disrupts it leading to cell lysis and killing the larvae ultimately (English and Slatin 1992). A wide variation in the effectiveness of B. thuringiensis isolates was found according to different target insects (Yaradoni, 1999). Environmental parameters viz; temperature, pH and light affected the pathogenicity of B. thuringiensis (Bt) in field (Somasekhar and Krishnayya 2004).
References
Brown A (2004) Benson’s microbiological applications: laboratory manual in general microbiology. McGraw-Hill, New York
Collee JG, Miles PS (1989) Tests for identification of bacteria. In: Collee JG, Duguid JP, Fraser AG, Marmion BP (eds) Practical medical microbiology, 13th edn. Churchil Livingstone, New York
Crickmore N, Zeigler DJ, Feitelson J, Schnepf E, Lambert B, Lereclus D, Baum J, Dean DH (1995) Revision of the nomenclature for the Bacillus thuringiensis pesticidal cry genes. Program and Abstracts of the 28th Annual Meeting of the Society for Invertebrate Pathology, p 14
English L, Slatin SL (1992) Mode of action of Bacillus thuringiensis dendotoxins: a comparison with other bacterial toxins. Insect Biochem Mol Biol 22:1–7
Frankenhuyzen KV (2009) Insecticidal activity of Bacillus thuringiensis crystal proteins. J Invertebr Pathol 101:1–16
Ibrahim MA, Griko N, Junker M, Bulla LA (2010) Bacillus thuringiensis: a genomics and proteomics perspective. Bioeng Bugs 1:31–50
Janssen K (1994) Current protocols in molecular biology, vol I. Greene Publ. Assoc. Inc. and John Wiley Sons Inc, New York
Joung KB, Cote JC (2000) A review of the environmental impacts of the microbial insecticide Bacillus thuringiensis. Tech Bull no. 29. Hort Res Dev Cent, Canada, p 16
Kalode MB, Pasalu IC, Krishnaiah NV, Bentur JS (1995) Changing insect pest complex in relation to cropping system of rice. In: Proceedings National Seminar on Changing Pest Situation in the Current Agriculture Scenario of India. ICAR, New Delhi
Kaur R, Virk JS, Joshi N (2008) Bio efficacy of DOR Bt, a Bacillus thuringiensis formulation against rice leaf folder Cnaphalocrocis medinalis (Guenee) in Punjab. J Biol Control 22(2):475–477
Knowles BH (1994) Mechanism of action of Bacillus thuringiensis insecticidal d-endotoxins. Adv Insect Physiol 24:275–308
Konecka E, Baranek J, Hrycak A, Kaznowski A (2012) Insecticidal activity of Bacillus thuringiensis strains isolated from soil and water. The Sci World J. doi:10.1100/2012/710501
Lacey LA (1997) Manual of techniques in insect pathology. Academic Press, New York
Logan NS, de Vos P (2009) Genus I. Bacillus. In: de Vos P, Garrity GM, Jones D, Krieg NR, Krieg NR, Ludwing W, Rainey FA, Schleifer KH, Whitman WB (eds) Bergey’s manual of systematic bacteriology, vol 3, 2nd edn. Springer, New York
Nanda VK, Bisoi RC (1990) Bionomics of rice L7 C.M. Orissa J Agrl Res 3(2):130–135
Ramamourti A, Tholkappian P, Jayachandran S (2012) In vitro studies on suitability of various natural and synthetic substrates for growth, cell biomass and crystal protein production of Bacillus thuringiensis isolate pkkvk 9. Int J Curr Agri Sci 2(1):1–8
Riley JR, Reynolds DR, Smith AD, Edwards AS, Zhang XX, Cheng XN, Wang HK, Cheng JY, Zhai BP (1995) Observations of the autumn migration of the rice leaf roller Cnaphalocrocis medinalis (Lepidoptera: Pyralidae) and other moths in eastern China. Bull Entomol Res 85:397–414
Savitri G, Murali Mohan P (2003) Pathogenicity of the bacterium Bacillus thuringiensis coagulans in silkworm, Bombyx mori (L). Indian J Sericul 42(1):4–8
Shahid AA, Nasir IA, Zafar AU, Sumrin A, Chaudhry B, Riazuddin S (2003) The use of CAMB biopesticides to control pests of rice (Oryza sativa). Asian J Plant Sci 2(15–16):1079–1082
Shishir A, Akter A, Hassan MH, Kibria G, Ilias M, Khan SN, Hoq MM (2012) Characterization of locally isolated Bacillus thuringiensis for the development of eco-friendly biopesticides in Bangladesh. J Biopest 5:216–222
Singh AP, Arora R, Battu GS (2000) Laboratory evaluation of three Bacillus thuringiensis Berliner based biopesticides against the diamondback moth, Plutella xylostella (Linnaeus). Pesti Res J 12(1):54–62
Smibert R, Krieg NR (1994) Phenotypic characterization. In: Gerhardt P, Murray RGE, Wood W, Krieg E (eds) Methods for general and molecular bacteriology. American Society for Microbiology, Washington, pp 607–654
Somasekhar MVNS, Krishnayya PV (2004) Effect of temperature, light and pH on feeding inhibition. Pupation and adult emergence of Spodoptera litura (Fabricius) feed with Bacillus thuringiensis. Indian J Plant Protec 32(1):63–66
Tabashnik BE, Carrie`re Y (2004) Bt transgenic crops do not have favorable effects on resistant insects. J Insect Sci 4:1–4
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Thompson JD, Higgins DG, Gibson TJ (1994) ClustalW: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Yaradoni S (1999) Molecular characterization of native B. thuringiensis. M.Sc. (Agri.) Thesis, University of Agricultural Sciences, Dharwad, India
Acknowledgement
The authors are grateful to The University of Burdwan for providing laboratory facilities required for the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Declared none.
Rights and permissions
About this article
Cite this article
Ghosh, T.S., Chatterjee, S., Azmi, S.A. et al. Virulence assay and role of Bacillus thuringiensis TS110 as biocontrol agent against the larval stages of rice leaffolder Cnaphalocrocis medinalis . J Parasit Dis 41, 491–495 (2017). https://doi.org/10.1007/s12639-016-0835-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12639-016-0835-9