Abstract
Purpose of Review
Breast cancer is the most common malignancy diagnosed in women worldwide. As the average age of child-bearing increases, more women will not have started or completed their families at the time of a breast cancer diagnosis. The scope of this review is to present current practices for fertility preservation, evidence for such practices, and future directions for fertility counseling and treatment for women with breast cancer.
Recent Findings
In the face of multimodality treatment for breast cancer including surgery, gonadotoxic chemotherapies, and radiation, women who desire to become biological mothers face complex decisions, including the pursuit of fertility preservation prior to treatment which may be dictated by age, ovarian reserve, and the choice of systemic therapy.
Summary
Several considerations impact the decision to pursue fertility preservation, and practices are continually advancing. This discussion is aimed at improving access and information on fertility preservation methods in breast cancer patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Fertility and Breast Cancer
Breast cancer (BC) is the most common malignancy diagnosed in women of child-bearing age, accounting for approximately 5% of new cancer cases in the US each year [1]. With the steady increase of childbearing age in the last two decades, approximately 50% of young women with BC will not have started or completed their families at the time of diagnosis [2]. In the face of a BC diagnosis, women who desire to become a biological mother in the future face the complex decision of whether to undergo fertility preservation before treatment. Fertility following BC treatment will be dictated by many factors including ovarian reserve, the age of the patient, the choice of systemic treatment, and the interventions pursued prior to gonadotoxic therapy [3, 4].
Patient Counseling at the Time of Diagnosis
Following an abnormal mammogram, ultrasound, or MRI, breast cancer can be diagnosed with a percutaneous biopsy. Once the patient is diagnosed, the patient is often referred to a breast surgeon or medical oncologist. While cancer diagnosis and treatment are the primary focus of a patient’s initial consultation, the treating clinician should also prioritize the discussion of future fertility with appropriate premenopausal patients. Given the timing to breast cancer treatment, in most cases, fertility preservation can be safely performed prior to the initiation of any oncologic management. In the setting of fertility preservation, overall survival, disease free survival, and local recurrence do not appear to be impacted by the time to initiation of first oncologic treatment [5].
Breast surgeons with knowledge of oncofertility are more likely to discuss their patients’ fertility treatment plans, resulting in more referrals to specialty care [6]. A study published by Letourneau et al. in 2012 showed that BC patients who received counseling focused on fertility preservation and future pregnancy experienced less regret and a better quality of life [2]. Studies have also shown that when providers discuss fertility options with their premenopausal cancer patients, a large majority (up to 89%) of those patients seek further information [7••]. Another study by Jeruss et al. demonstrated that BC patients may choose not to initiate treatment or experience decreased treatment adherence because of fertility concerns [8]. Oncofertility decision aids and success rate calculators have been created to assist patients in the decision-making process surrounding fertility and cancer and are available online at the following websites:
Breast Surgery and Fertility
Breast surgery itself will not affect future fertility but may affect lactation. Bilateral mastectomy will impede breastfeeding, but if the surgery is a unilateral mastectomy, segmental mastectomy, or a lumpectomy, the patient may still be able to lactate sufficiently [9]. Most breast conservation surgery will involve only one quadrant of the breast, sparing the majority of the architectural structure of the milk ducts, though radiation treatment could impact milk production. In rare instances, such as in Paget’s disease, central lumpectomy is the treatment of choice and lactation will not be possible.
Radiation and Fertility
Several trials have demonstrated the importance of radiation therapy after breast conservation surgery for the treatment of breast cancer. The National Surgical Adjuvant Breast Project (NSABP) B-06 study twenty-year follow-up revealed lower recurrence rates when comparing breast conservation surgery followed by breast irradiation (BI) (14.3%) with breast conservation surgery alone (39.2%) [10]. Breast irradiation is therefore an important aspect of breast cancer therapy. BI is a localized modality of treatment and generates minimal exposure to intrathoracic organs and very minimal to no exposure to the abdominal cavity. Targeted BI should not significantly affect ovarian reserve and future fertility [11], though it may affect lactation. New radiation accelerators also allow computed tomography planning integration to minimize radiation injury to adjacent structures.
Lactation can be successful after BI [12] and when the contralateral (non-irradiated) breast is used. After BI, 50% of patients still are able to use the ipsilateral (radiated) breast for breastfeeding, but 80% reported a comparative decrease in milk output [12].
Ovarian Function and Assessing Ovarian Reserve
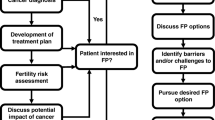
In order to understand ovarian reserve and its impact on fertility, it is important to understand folliculogenesis and ovulation. Folliculogenesis—the development of a follicle in preparation for ovulation—is a complex physiologic process. During fetal development, a woman forms a limited number of primordial follicles, which consist of a single oocyte surrounded by a single layer of flattened granulosa cells. At birth, the ovary contains approximately 1 million oocytes, dropping to 300,000-500,000 at puberty and 1000 by menopause [13, 14]. Once menarche has occurred, a limited number of primordial follicles are triggered to mature at the start of each menstrual cycle. Through timed patterns of hormonal release, a primordial follicle transitions into a mature ovarian follicle or Graafian follicle. This mature ovarian follicle will then take one of two paths: ovulation in anticipation of fertilization or atresia. It takes approximately one year for a primordial follicle to develop to the ovulatory stage [15] (see Fig. 1).
There is typically one dominant follicle that completes maturation and releases an ovum, and the others regress and eventually deteriorate. Pituitary follicle stimulating hormone (FSH) stimulates a single follicle to outcompete the other developing follicles. The dominant follicle rapidly grows into a secondary follicle with a defined outer layer called the theca interna, which contributes to the production of estradiol. Rising estradiol levels ultimately trigger a surge in luteinizing hormone (LH) and the release of the ovum from the dominant follicle. After release, the follicle regresses into a steroidogenic complex known as the corpus luteum. The corpus luteum secretes important hormones, particularly progesterone in anticipation of supporting a developing pregnancy.
Any rapidly dividing cell is harmed by chemotoxic agents, and ovarian follicles are particularly sensitive to chemotherapy. Prior to therapy, ovarian reserve is extremely variable among patients, and there is no direct way to measure how many follicles remain in an ovary. Two indirect ways to measure ovarian reserve include antral follicle count and anti-mullerian hormone (AMH) levels. Antral follicle counts can be done with a trans-vaginal ultrasound. While primordial follicles are not visible to the naked eye, follicles recruited for maturation can be visualized on ultrasound as fluid filled antral follicles measuring 2-10mm. This method indicates ovarian activity, but does not fully estimate future ovarian function.
AMH is a hormone that rises at the beginning of follicular development. Granulosa cells and antral follicles are mainly responsible for the production of AMH. AMH is used to estimate ovarian reserve and could be considered a marker of ovarian function before and after chemotherapy [16, 17]. During chemotherapy, AMH levels fall steadily becoming undetectable in 50% of the patients. For therapies with lower gonadotoxicity, AMH may recover, and women may resume normal menses. However, the resumption of menses does not entirely indicate return of ovarian function nor does it predict future fertility [18]. Although it is not a perfect test, AMH is the preferable laboratory test to measure ovarian function recovery following treatment. AMH does not fluctuate with the menstrual cycle or hormonal manipulation [19]. For chemotherapies that have high levels of gonadotoxicity, AMH will often become undetectable and will not recover [16].
Ovarian Injury in Chemotherapy
First line systemic BC therapy damages the DNA of the oocytes, impairing cell repair and leading to apoptosis [20]. Unfortunately, there are no first-line chemotherapy regimens for BC that completely spare the ovaries from toxicity. Each class of antineoplastic agent has a distinct action on cancer cells resulting in the arrest of cell division (Table 1). The National Surgical Adjuvant Breast and Bowel Project (NSABP) B-15 [28] and B-16 [28] studies established current preferred first lines regimens including doxorubicin and cyclophosphamide in BC with metastatic nodal disease. These agents had equivalent results and were better tolerated than cyclophosphamide, methotrexate, and 5-fluorouracil. The addition of paclitaxel to doxorubicin and cyclophosphamide was evaluated and proved to increase disease free survival and also overall survival [27]. All of these agents have varying levels of gonadotoxicity (see Table 1).
Apoptosis, caused by DNA damage, is the most common mechanism of ovarian cell demise caused by DNA damage. Double-stranded breaks are the most harmful type of injury to the ovarian cells and are common results of gonadotoxic therapies. The oocyte initially attempts to repair the DNA through the ataxia telangiectasia mutated (ATM)-mediated DNA damage repair pathway. If the cell cannot be repaired, apoptosis will occur [32]. The administration of antineoplastic agents is also associated with a sharp reduction in ovarian blood volume and spasm of small vessels in the ovary [33]. Chronic spasm and vascular flow deregulation ultimately lead to fibrosis of the ovarian cortex [32].
Anti-Hormonal Therapy and Fertility
One important agent for breast cancer treatment, trastuzumab, does not appear to affect ovarian reserve. This monoclonal antibody targets breast cancers with HER2/neu receptor over-expression. Treatment with trastuzumab is contraindicated in pregnancy as this agent is considered a teratogen [29].
Anti-hormonal therapies including tamoxifen and aromatase inhibitors have also become mainstays of adjuvant treatment for hormone receptor positive breast cancer. In premenopausal women, a five- to ten-year tamoxifen treatment regimen is recommended. Tamoxifen is considered a teratogen, and its use is contraindicated during pregnancy [34]. Patients receiving a recommendation for tamoxifen therapy may desire to delay their treatment or take a closely monitored hiatus from treatment in order to pursue pregnancy, but the safety of such approach is unknown. The IBCSG 48-14 POSITIVE trial is evaluating the safety of interruption in anti-hormonal treatment in order to pursue pregnancy [35]. Clarification of the temporary hiatus will be available with the POSITIVE trial results.
Fertility Preservation Options
Options are available for cancer patients who desire to pursue pregnancy after treatment (see Fig. 1). Many of these options require fertility preservation prior to systemic therapy and should be discussed as early as possible in the patient’s treatment course. Women of advanced reproductive age or limited ovarian reserve should be carefully counseled before the application of any fertility preserving or fertilization techniques. The rates of successful live birth drop significantly after the age of 42 (24% success rate) compared with patients younger than 35 years of age (45% success rate) [36]. Realistic expectations must be set to avoid frustration, disappointment, and unnecessary costly procedures.
Oocyte or Embryo Cryopreservation
Oocyte/embryo cryopreservation is considered the gold standard for BC patients trying to achieve fertility preservation. In recent years, advances in the rapid vitrification process have led to outcomes similar to fresh embryos used for traditional in vitro fertilization procedures (IVF) [37]. In order to harvest oocytes for preservation, patients typically undergo controlled ovarian stimulation (COS). COS can be started at any point in the menstrual cycle, known as random start protocol, minimizing the time needed for fertility preservation and the delay in systemic therapy or breast surgery [38,39,40,41]. Successful COS and oocyte harvesting can be performed over a two-week period. In non-BC patients, ovarian stimulation protocols induce high levels of circulating estrogen. COS protocols for patients with BC include a gonadotropin (recombinant follicle-stimulating hormone or urinary human menopausal gonadotropin) combined with an aromatase inhibitor (AI), most commonly letrozole. The use of an aromatase inhibitor results in lower levels of circulating estrogen with similar ovarian stimulation results to traditional ovarian stimulation modalities. These agents stimulate multiple fluid-filled ovarian follicles to form without releasing their oocytes [37]. Following stimulation, oocytes are retrieved transvaginally using ultrasound guidance. Mature oocytes are frozen without being fertilized or are fertilized with a partner or donor sperm to create embryos. Embryos may be used or frozen at fertilization, day three, or day five. Live birth rates and perinatal outcomes are the equivalent between frozen embryos and frozen oocyte-derived embryo transfers (≅25%) [42]. Embryos five days and older can be genetically analyzed to rule out genetic mutations, such as BRCA, PALB2, and ATM. Among different biological tumor profiles, triple-negative breast cancer patients have lower numbers of mature oocytes when compared with hormonal positive patients after COS [43••].
Ovarian Tissue Cryopreservation and Transplantation
Ovarian tissue cryopreservation (OTC) is an active area of research and may be a reasonable option for selected patients. It is also the only option for fertility preservation in prepubertal patients with cancer. The procedure to harvest ovarian tissue is typically performed laparoscopically. This tissue may comprise the entire ovary or just strips of tissue. The tissue is subsequently cryopreserved offering the potential for thousands of follicles to be fertilized in the future. Whenever motherhood is desired, autologous transplantation of the tissue can be performed in order to mature the oocytes within the ovarian tissue in preparation for subsequent fertilization. In a recently published meta-analysis, a cumulative clinical birth of 57.5% has been reported for cryopreserved ovarian tissue [44••].
Aside from the invasiveness of this process, the biggest disadvantage is the risk of reseeding of potential malignant cells during autologous transplantation, especially in patients with oncogenic genetic mutations. The use of retrievable hydrogels may be a novel way to reduce the likelihood that malignant cells will be re-seeded [45]. This promising, experimental technique involves the encapsulation of nascent follicles from ovarian tissue using alginate hydrogels. Future heterotopic transplantation of the encapsulated follicles is performed in order to allow the nascent follicles to be exposed to the hormonal milieu necessary for follicular maturation and subsequent fertilization. The use of these hydrogels, which separate nascent follicles from harvested ovarian tissue, decreases the risk of re-introducing malignant cells upon reimplantation [45].
Encapsulated In Vitro Follicle Growth (eIVFG)
Encapsulated in vitro follicle growth (eIVFG) is the harvest of immature oocytes transvaginally or from OTC material for later use in in vitro fertilization process [46••]. The growth of ovarian follicles in biomaterials such as alginate is important to the provision of supporting matrices that allow follicles to mature outside the body. Live birth has been accomplished in mice from eIVFG and from follicles enclosed in an ovarian bioprosthetic. These future uses for the ovarian tissue that has been cryopreserved provide hope for patients, especially pediatric patients, for fertility restoration in future years.
Ovarian Suppression
Another method of ovarian preservation includes ovarian suppression with gonadotropin-releasing hormone agonists (GnRHa), such as leuprolide. Using GnRHa for chemical ovarian protection in cancer patients has been studied and extensively debated. The 2020.1 National Comprehensive Cancer Network (NCCN) and The American Society of Clinical Oncology (ASCO) Guidelines endorsed the use of GnRHa [24, 47] to preserve ovarian function and diminish the likelihood of chemotherapy induced amenorrhea. Recent studies suggest a 16.8% absolute reduction in premature ovarian failure when GnRHa was administered concomitantly with chemotherapy [48]. However, many of these patients will still experience ovarian failure, and GnRHa’s should not be used with the intent to ensure future fertility. These drugs are a tool that can be used to protect the remaining ovarian tissue during systemic treatment, but they do not support normal reproductive function after treatment.
A variety of different mechanisms of GnRHa have been hypothesized to contribute to ovarian protection. There is some thought that GnRHa recreates the prepubertal hypogonadotropic milieu, leading the ovary to a quiescent prepubertal state [49, 50]. GnRHa also decreases estrogen levels and decreases ovarian perfusion, limiting ovarian exposure to chemotherapeutic agents [51]. GnRHa may also have a direct effect mediated through receptors in the ovary [52, 53]. An anti-apoptotic molecule, sphingosine-1-phosphate (S1P), is upregulated with GnRHa administration. This molecule inhibits the ceramide pathway and is implicated in chemotherapy induced apoptosis of the ovary [52,53,54]. GnRHa may also protect the ovarian germinative stem cells. These ovarian stem cells may be able to reconstitute the primordial follicle pool following the administration of gonadotoxic agents [55, 56]. Another mechanism may involve the antiapoptotic action that GnRHa have been shown to have on cumulus cells [57••].
The suppression ovarian function trial (SOFT) and the Tamoxifen and Exemestane Trial (TEXT) demonstrated superior cancer outcomes when ovarian suppression was added to anti-hormonal therapy for premenopausal patients with breast cancer [58]. Disease free survival was 83.2% for the group that received tamoxifen and ovarian suppression, 85.9% for the group that received exemestane, and ovarian suppression versus 78.9% for the group that received tamoxifen alone [58]. While ovarian suppression was shown to be a tool in patients with hormonal positive cancers, fertility preservation was not a major outcome of this trial. Ovarian suppression was performed with triptorelin 3.75 mg by intramuscular injection, or bilateral oophorectomy, or ovarian irradiation. Thus, the mechanism of action and indeed the value of GnRHa remain to be proven as a categorical way in which fertility can be spared.
Future Perspectives
Sphingosine-1-phosphate (S1P) is an important cell mediator and functions through cellular proliferation, angiogenesis, and cytoskeleton reordering [59, 60]. This protein may hold potential for ovarian protection and preservation in cancer patients receiving systemic therapy. S1P promotes corpus luteum development and steroid synthesis and also plays a major role as a cytoprotective for ovarian follicles by protecting luteinized granulosa cells from apoptosis [61]. Studies have shown that S1P treatment of human ovarian tissue transplanted to mouse ovaries reduces the number of apoptotic cells when exposed to cyclophosphamide or doxorubicin and may be useful during cryopreservation and chemotherapy [61,62,63,64,65]. Rodent studies demonstrated that the administration of S1P intravenously decreases the effects of ovarian toxicity when receiving cyclophosphamide and cisplatin. Pre-treatment with S1P in mice receiving dacarbazine increases preantral follicle count and the number of pregnancies [66]. In another study, S1P-treated vitrified ovaries have a lower mRNA expression of caspase 3 and c-myc. Caspase 3 is considered an “executioner” caspase, coordinating DNA fragmentation during apoptosis. Decreasing c-myc production and therefore decreasing apoptosis enzymes leads to increased primordial follicles during the vitrification process [67,68,69]. S1P-treated ovaries of bovine, sheep, and rhesus monkeys had reverse radiation effects, increased the activation of primordial follicles, and promoted the survival of granulosa cells [70,71,72]. Unfortunately, S1P is oncogenic and has also been implicated on the migration, proliferation, and vascular development of tumor cells [73,74,75]. Its concentration has been noted to be increased in patients with ovarian cancer, and levels drop when the cancer is removed [76]. So, while S1P is not a good therapeutic, these studies point toward a mechanism that could be utilized in the future.
Conclusion
Breast cancer can alter the course of a woman’s life, but in premenopausal women desiring fertility, there are tools available to mitigate the negative impact of this diagnosis. Strategies for fertility preservation in women diagnosed with BC are available and continue to advance. Breast surgeons and oncologists should become comfortable with the options available to their patients, and their counseling and treatment algorithms should include oncofertility as an essential component. Fertility preservation options should be discussed with the patient at the time of the diagnosis, and hospitals should seek to ensure the availability of these services to patients in need.
Data Availability
This is a review and no primary data is produced.
Code Availability
None.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
American Cancer Society. Breast cancer facts and figures. Atlanta: American Cancer Society; 2020.
Letourneau JM, Ebbel EE, Katz PA, et al. Pretreatment fertility counseling and fertility preservation improve quality of life in reproductive age women with cancer. Cancer. 2012;118:1710–7.
Jeruss JS, Woodruff TK. Preservation of fertility in patients with cancer. N Engl J Med. 2009;360(9):902–11. https://doi.org/10.1056/NEJMra0801454.
De Vos M, Smitz J, Woodruff TK. Fertility preservation in women with cancer. Lancet. 2014;384(9950):1302–10. https://doi.org/10.1016/S0140-6736(14)60834-5.
Bleicher RJ, Ruth MS, Sigurdson ER, Beck R, Ross E, Wong YN, et al. Egleston BL Time to surgery and breast cancer survival in the United States. Jama Oncol. 2016;2(3):330–9. https://doi.org/10.1001/jamaoncol.2015.4508.
McCray DK, Simpson AB, Flyckt R, Liu Y, O'Rourke C, Crowe JP, et al. Fertility in women of reproductive age after breast cancer treatment: practice patterns and outcomes. Ann Surg Oncol. 2016;23(10):3175–81.
Warner E, Yee S, Seminsky M, Glass K, Foong S, Kennedy E, et al. Effect of a knowledge-translation intervention on breast surgeons’ oncofertility attitudes and practices. Ann Surg Oncol. 2019;27:1645–52. https://doi.org/10.12245/s10434-019-07972-xDescribes the importance of knowledge-translation intervention in fertility preservation improving the understanding and awareness among Canadian breast oncological surgeons.
Llarnena NC, Estevez SL, Tucker SL, Jeruss JS. Impact of fertility concerns on tamoxifen initiation and persistence. J Nat Cancer Inst. 2015;107(10):djv202.
Michaels AM, Wanner H. Breastfeeding twins after mastectomy. J Hum Lact. 2013;29:20–2.
Fisher B, Stewart A, Bryant J, Margolese RG, Deutsch M, Fisher ER, et al. Twenty-year follow up of a randomized trial comparing total mastectomy, lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347(16):1233–41.
Hulvat MC, Jeruss JS. Maintaining fertility in young women with breast cancer. Curr Treat Options in Oncol. 2009;10(5-6):308–17. https://doi.org/10.1007/s11864-010-0116-2.
Moran MS, Colasanto JM, Haffy BG, Wilson LD, Lund MW, Higgins SA. Effects of breast-conserving therapy on lactation after pregnancy. Cancer J. 2005;11(5):399–403.
Baker TG. A quantitative and cytological study of germ cells in human ovaries. Proc R Soc Lond B Biol Sci. 1963;158:417–33.
Richardson SJ, Senikas V, Nelson JF. Follicular depletion during menopausal transition: evidence for accelerated loss and ultimate exhaustion. J Clin Endocrinol Metab. 1987;65:1231–7.
Erickson G. Follicle growth and development. Glob Libr Women Med. 2008. https://doi.org/10.3843/GLOWM.10289.
Brougham MF, Crofton PM, Johnson EJ, et al. Anti-Müllerian hormone is a marker of gonadotoxicity in pre- and post- pubertal girls treated for cancer: a prospective study. J Clin Endocrinol Metab. 2012;97:2059–67.
Anderson RA, Cameron DA. Pretreatment serum anti-Mülllerian hormone predicts long-term ovarian function and bone mass after chemotherapy for early breast cancer. J Clin Endocrinol Metab. 2011;96:1336–43.
Morarji K, McArdle O, Hui K, Gingras-Hill G, Ahmed S, Greenblatt EM, et al. Ovarian function after chemotherapy in young breast cancer survivors. Curr Oncol. 2017;24:494–502.
Köninger A, Kauth A, Schmidt B, Schmidt M, Yerlikaya G, Kasimir-Bauer S, et al. Anti-Mullerian- hormone levels during pregnancy and postpartum. Reprod Biol Endocrinol. 2013;11:60.
Bedoschi G, Navarro PA. Oktay.Chemotherapy-induced damage to the ovary: mechanisms and clinical impact. Future Oncol. 2012;12(20):2333–44.
Morgan S, Anderson RA, Gourley C, Wallace WH, Spears N. How do chemotherapeutic agents damage the ovary. Hum Reprod Update. 2012;18(5):525–35.
Doll DC, Ringenberg S, Yarbro JW. Antineoplastic agents and pregnancy. Semin Oncol. 1989;16:337–46.
Fisher B, et al. Two months of doxorubicin-cyclophosphamide with and without interval reinduction therapy compared with 6 months of cyclophosphamide, methotrexate, and fluoracil in positive-node breast cancer patients with tamoxifen-nonresponsive tumors:results from the National Surgical Adjuvant Breast and Bowel Project b-15. J Clin Oncol. 1990;8:1483–96.
The National Comprehensive Cancer Network Guidelines Version 2.2020 February 2020. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed 24 May 2020. Current guidelines in the treatment of breast cancer patients by the National Comprehensive Cancer Network.
Oktem O, Ata B, Urman B. The impact of the addition of taxane to AC regimen on ovarian function in breast cancer patients: a meta-analysis of five randomized studies. Fertil Steril. 2013;100:153–4.
Zagouri F, Sergentanis TN, Chrysikos D, Dimitrakakis C, Tsigginou A, Zografos CG, et al. Taxanes for breast cancer during pregnancy: a systematic review. Clin Breast Cancer. 2014;13(1):16–23.
Sparano JA, et al. Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med. 2008;3(58):1663–71.
Fisher B, et al. Postoperative chemotherapy and tamoxifen compared with tamoxifen alone in the treatment of positive-node breast cancer patients aged 50 years and older with tumors responsive to tamoxifen: results from the national surgical adjuvant breast and bowel Project B-16. J Clin Oncol. 1990;8:1005–18.
Samo MA, Mancari R, Azim HA Jr, et al. Are monoclonal antibodies a safe treatment for cancer during pregnancy? Immunotherapy. 2013;5:733–41.
Zagouri F, Sergentanis TN, Chrysikos D, Dimitrakakis C, Bartsch R. Platinum derivates during pregnancy in cervical cancer: a systematic review and meta-analysis. Obs Gyneco. 2013;121:337–43.
Meirow D, Biederman H, Anderson R, Wallace WH. Toxicity of chemotherapy and radiation on female reproduction. Clin Obstet Gynecol. 2010;53(4):727–39.
Soleimani R, Heytens E, Oktay K. Enhancement of neoangiogenesis and follicle survival by sphingosine-1-phosphate in human ovarian tissue xenotransplants. PLoS One. 2011;6(4):e19475.
Bar-Joseph H, Ben-Aharon I, Tzabari M, Tsarfaty G, Stemmer SM, Shalgi R. In vivo bioimaging as a novel strategy to detect doxorubicin-induced damage to gonadal blood vessels. PLoS One. 2011;6(9):e23492.
Barthelmes L, Gateley CA. Tamoxifen and pregnancy. Breast. 2004;13:446–51.
International Breast Cancer Study Group. IBCSG 48-14 positive. A study evaluating pregnancy outcomes and safety of interrupting endocrine therapy for young women with endocrine breast cancer who desire pregnancy. J Clin Oncol 2018:36(15).
Society for assisted reproductive technology clinic summary report. 2016. sartcorsonline.com/rptCSR_PublicMultYear.aspx. Accessed 24 May 2020.
Rodriguez-Wallberg KA, Oktay K. Fertility preservation in women with breast cancer. Clin Obstet Gynecol. 2010;53(4):753–62. https://doi.org/10.1097/GRF.0b013e3181f96e00.
von Wolff M, Thaler CJ, Frambach T, Zeeb C, Lawrenz B, Popovici RM, et al. Ovarian stimulation to cryopreserve fertilized oocytes in cancer patients can be started in the luteal phase. Fertil Steril. 2009;92:1360–5.
Ozkaya E, San Roman G, Oktay K. Luteal phase GnRHa trigger in random start fertility preservation cycles. J Assist Reprod Genet. 2012;29:503–5.
Sonmezer M, Turkcuoglu I, Coskun U, Oktay K. Random-start controlled ovarian hyperstimulation for emergency fertility preservation in letrozole cycles. Fertil Steril. 2011;95:2125–e9.
Practice Committee of American Society for Reproductive Medicine. Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: a committee opinion. Fertil Steril. 2013;2013(100):1214–23.
Azim AA, Costantini-Ferrando M, Oktay K. Safety of fertility preservation by ovarian stimulation with letrozole and gonadotropins in patients with breast cancer: a prospective controlled study. J Clin Oncol. 2008;26:2630–5.
Balayla J, Tulandi T, Buckett W, Holzer H, Steiner N, Shrem G, et al. Outcomes of ovarian stimulation and fertility preservation in breast cancer patients with different hormonal receptor profiles. J Assist Reprod Genet. 2020;37:913–21 Evaluation of fertility preservation among different hormonal profiles.
Ho JR, Woo I, Louie K, et al. A comparison of live birth rates and perinatal outcomes between cryopreserved oocytes and cryopreserved embryos. J Assist Reprod Genet. 2017;34:1359–66 Advancements in oocyte cryopreservation.
Rios PD, Kniazeva E, Lee HC, Xiao S, Oakes RS, Saito E, et al. Retrievable hydrogels for ovarian follicle transplantation and oocyte collection. Biotechnol Bioeng. 2018;115:2075–86.
Gargus ES, Rogers HB, McKinnon KE, Edmonds ME, Woodruff TK. Engineered reproductive issues. Nat Biomed Eng. 2020. https://doi.org/10.1038/s41551-020-0525-xNovel strategies in engineered biomaterials and its use in reproductive science and medicine.
Oktay K, Harvey BE, Partridge A, et al. Fertility preservation in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol. 2018;36(19):1994–2001. https://doi.org/10.1200/JCO.2018.78.1914current guidelines of fertility preservation by the American of Clinical Oncology.
Lambertini M, Moore H, Leonard R, Loibi S, Munster P, Bruzzone M, et al. Gonadotropin-Releasing hormone agonists during chemotherapy for preservation of ovarian function and fertility in premenopausal patients with early breast cancer: a systematic review and meta-analysis of individual patient-level data. J Clin Onc. 2018;366(19):1981–90.
Xu M, Kreeger PK, Shea LD, Woodruff TK. Tissue-engineered follicles produce live, fertile offspring. Tissue Eng. 2006;12:2739–46.
Blumenfeld Z. How to preserve fertility in young women exposed to chemotherapy? The role of GnRH agonist co treatment in addition to cryopreservation of embrya, oocytes, or ovaries. Oncologist. 2007;12:1044–54. https://doi.org/10.1634/theoncologist.12-9-1044.
Chapman RM, Sutcliffe SB. Protection of ovarian function by oral contraceptives in women receiving chemotherapy for Hodgkin’s disease. Blood. 1981;58:849–51.
Kitajima Y, Endo T, Nagasawa K, et al. Hyperstimulation and a gonadotropin- releasing hormone agonist modulate ovarian vascular permeability by altering expression of the tight junction protein claudin-5. Endocrinology. 2006;147:694–9. https://doi.org/10.1210/en.2005-0700.
Whitelaw PF, Eidne KA, Sellar R, Smyth CD, Hillier SG. Gonadotropin- releasing hormone receptor messenger ribonucleic acid expression in rat ovary. Endocrinology. 1995;136:172–9. https://doi.org/10.1210/en.136.1.172.
Harrison GS, Wierman ME, Nett TM, Glode LM. Gonadotropin-releasing hormone and its receptor in normal
Morita Y, Perez GI, Paris F, Miranda SR, Ehleiter D, Haimovitz-Friedman A, et al. Oocyte apoptosis is suppressed by disruption of the acid sphingomyelinase gene or by sphingosine-1-phosphate therapy. Nat Med. 2000;6:1109–14. https://doi.org/10.1038/80442.
Sobinoff AP, Nixon B, Roman SD, McLaughlin EA. Staying alive: PI3K path- way promotes primordial follicle activation and survival in response to 3MC- induced ovotoxicity. Toxicol Sci. 2012;128:258–71. https://doi.org/10.1093/toxsci/kfs137.
Poggio F, Lambertini M, Bighin C, Conte B, Blondeaux E, D’Alonzo A, et al. Potential mechanisms of ovarian protection with gonadotropin-releasing hormone agonist in breast cancer patients: a review. Cli Med Insights Reprod Health. 2019;13:2–5. https://doi.org/10.1177/1179558119884584Reviews the potential mechanisms of ovarian protection by gonadotropin-releasing hormone in breast cancer patients.
Francis PA, Pagani O, Fleming GF, Walley BA, Colleoni M, Lang I, et al. Tailoring adjuvant endocrine therapy for premenopausal breast cancer. N Engl J Med. 2018;379:122–37.
Sanchez AM, Diaz LI. Papel de los esfingolípidos en la señalización celular. Dianas. 2006;1:3–7.
Strub GM, Maceyka M, Hait NC, Milstien S, Spiegel S. Extracellular and intracellular actions of sphingosine-1-phosphate. Adv Exp Med Biol. 2010;688:141–55. https://doi.org/10.1007/978-1-4419-6741-1-10.
Nakahara T, Iwase A, Nakamura T, Kondo M, Bayasula Kobayashi H, et al. Sphingosine-1-phosphate inhibits H2O2-induced granulosa cell apoptosis via the PI3K/Akt signaling pathway. Fertil Steril. 2012;98:1001–1008.e1. https://doi.org/10.1016/j.fertnstert.2012.06.008.
Becker S, von Otte S, Robenek H, Diedrich K, Nofer JR. Follicular fluid high-density lipoprotein-associated sphingosine 1-phosphate (S1P) promotes human granulosa lutein cell migration via S1P receptor type 3 and small G-protein RAC1. Biol Reprod. 2011;84:604–12. https://doi.org/10.1095/biolreprod.110.084152.
Jee BC, Jo JW, Suh CS, Kim SH. Dose-dependent effect of sphingosine-1-phosphate in mouse oocyte maturation medium on subsequent embryo development. Gynecol Obstet Investig. 2011;72:32–6. https://doi.org/10.1016/j.ejogrb.2010.06.019.
Meng Y, Xu Z, Wu F, Chen W, Xie S, Liu J, et al. Sphingosine-1-phosphate suppresses cyclophosphamide induced follicle apoptosis in human fetal ovarian xenografts in nude mice. Fertil Steril. 2014;102:871–7. https://doi.org/10.1016/j.fertnstert.2014.05.040.
Li F, Turan V, Lierman S, Cuvelier C, De Sutter P, Oktay K. Sphingosine-1-phosphate prevents chemotherapy-induced human primordial follicle death. Hum Reprod. 2019;29:107–13. https://doi.org/10.1093/humrep/det391.
Hancke K, Strauch O, Kissel C, Göbel H, Schäfer W, Denschlag D. Sphingosine 1-phosphate protects ovaries from chemotherapy-induced damage in vivo. Fertil Steril. 2007;87:172–7. https://doi.org/10.1016/j.fertnstert.2006.06.020.
Jee BC, Lee JR, Youm H, Suh CS, Kim SH, Moon SY. Effect of sphingosine-1-phosphate supplementation on follicular integrity of vitrified-warmed mouse ovarian grafts. Eur J Obstet Gynecol Reprod Biol. 2010;152:176–80. https://doi.org/10.1016/j.ejogrb.2010.06.019.
Tsai YC, Tzeng CR, Wang CW, Hsu MI, Tan SJ, Chen CH. Antiapoptotic agent sphingosine-1-phosphate protects vitrified murine ovarian grafts. Reprod Sci. 2014;21:236–43. https://doi.org/10.1177/1933719113493515.
Mumusoglu S, Turan V, Uckan H, Suzer A, Sokmensuer LK, Bozdag G. The impact of a long-acting oral sphingosine-1-phosphate analogue on ovarian aging in a rat model. Reprod Sci. 2018;25:1330–5. https://doi.org/10.1177/1933719117741376.
Henry L, Fransolet M, Labied S, Silvia B, Marie-Caroline M, Jean-Michel F, et al. Supplementation of transport and freezing media with anti-apoptotic drugs improves ovarian cortex survival. J Ovarian Res. 2016;9:4. https://doi.org/10.1186/s13048-016-0216-0.
Nóbrega JE, Rossetto R, Matos MHT, Magalhães DM, Lima-Verde IB, Báo SN, et al. Sphingosine 1-phosphate promotes activation of caprine preantral follicle in vitro. Arq Bras Med Vet Zootec. 2014;66:977–85. https://doi.org/10.1590/1678-6455.
Hernández-Coronado CG, Guzmán A, Rodríguez A, Mondragón JA, Romano MC, Gutiérrez CG, et al. Sphingosine-1-phosphate, regulated by FSH and VEGF, stimulates granulosa cell proliferation. Gen Comp Endocrinol. 2016;236:1–8. https://doi.org/10.1016/j.ygcen.2016.06.029.
Dai L, Xia P, Di W. Sphingosine 1-phosphate: a potential molecular target for ovarian cancer therapy? Cancer Investig. 2014;32:71–80. https://doi.org/10.3109/07357907.2013.876646.
Smicun Y, Gil O, Devine K, Fishman DA. S1P and LPA have an attachment-dependent regulatory effect on invasion of epithelial ovarian cancer cells. Gynecol Oncol. 2007;107:298–309. https://doi.org/10.1016/j.ygyno.2007.06.024.
Park KS, Kim MK, Lee HY, Kim SD, Lee SY, Kim JM, et al. S1P stimulates chemotactic migration and invasion in OVCAR3 ovarian cancer cells. Biochem Biophys Res Commun. 2007;356:239–44. https://doi.org/10.1016/j.bbrc.2007.02.112.
Sutphen R, Xu Y, Wilbanks GD, Fiorica J, Grendys EC Jr, La Polla JP, et al. Lysophospholipids are potential biomarkers of ovarian cancer. Cancer Epidemiol Biomark Prev. 2004;13:1185–91.
Funding
This review was funded in part by the Watkins Endowed Chair of Ob/Gyn.
Author information
Authors and Affiliations
Contributions
All authors wrote, reviewed, and edited this article.
Corresponding author
Ethics declarations
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of Interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Fertility Issues and Breast Cancer
Supplementary Information
ESM 1
(ZIP 82 kb)
Rights and permissions
About this article
Cite this article
Vieira, C.A., Folsom, S., Hansen, N.M. et al. Fertility and Breast Cancer. Curr Breast Cancer Rep 13, 72–80 (2021). https://doi.org/10.1007/s12609-021-00405-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12609-021-00405-3