Abstract
Purpose of Review
Young women represent a minority of breast cancer patients for which fertility, family planning, and pregnancy represent unique vulnerabilities. This review intends to discuss recent published evidence regarding treatment-related infertility, fertility counseling, and preservation.
Recent Findings
Fertility concerns are common among young women with breast cancer and may negatively affect treatment decisions. Data is available to aid providers in approximating odds of post-treatment amenorrhea and infertility. Multiple fertility preservation techniques are available. While embryo preservation is most commonly used, recent guidelines endorse oocyte preservation and support for ovarian tissue cryopreservation is increasing. Most recently, the contribution of ovarian suppression during chemotherapy to ovarian function preservation has been established. Germline BRCA mutations may impact fertility potential and challenge fertility preservation and preimplantation genetic testing should be discussed with this subset.
Summary
Fertility counseling and preservation have become an integral part of the multidisciplinary care for breast cancer at diagnosis and throughout survivorship. Efforts to further individualize recommendations are necessary.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the most common female cancer worldwide [1]. In 2018 an estimated 417,091 women < 45 years of age were diagnosed with breast cancer around the world and nearly one million prevalent cases were diagnosed within the previous 5 years [2]. Thus, while young women account for only a minority of the breast cancer population, due to the high incidence of the disease worldwide, this translates into a considerable global disease burden superseding that of many other malignancies. Young women with breast cancer have been identified as a subset with unique concerns and outcomes compared to older woman, and international consensus guidelines for breast cancer in young women have been established to optimize their management [3••]. Fertility, family planning, and pregnancy stand out as issues of importance for these patients given treatment may threaten or impair fertility. Further, while available evidence suggests that pregnancy after breast cancer does not increase risk of recurrence or death, feasibility and optimal timing of pregnancy after treatment are major issues for this population and the subject of ongoing research [4••]. It is imperative that multidisciplinary teams including oncology, reproductive, and psychosocial providers are knowledgeable about the latest data and provide adequate counseling and support for young patients who face these issues when dealing with a cancer diagnosis and treatment.
Fertility Concerns
The role of fertility preservation in cancer care and survivorship is amplified by the fact that more women are postponing childbirth to later ages. Since 1995, the mean age of women at first childbirth has increased by at least 2 years in most countries [5], and more women may experience breast cancer prior to initiating or completing childbirth plans. Surveys in the USA and Europe show that over 50% of women diagnosed ≤ 40 years of age report concern regarding future fertility [6•, 7, 8]. Unsurprisingly, women with childbearing plans or without any children at diagnosis have consistently been found to have higher fertility concerns [6•, 7,7,9]. Increased concerns have also been associated with receipt of chemotherapy, younger age, nonwhite race, a history of problems conceiving, and preferring more information [6•, 7, 9].
Fertility concerns may contribute to the greater level of distress among younger breast cancer survivors [10]. Concerns may also affect women’s treatment decision making. In a cohort of 620 women diagnosed ≤ 40 years of age, 26% reported that fertility affected their treatment decisions including the following: declining chemotherapy, preferring one regimen over another, declining endocrine therapy, and/or considering discontinuing endocrine therapy before 5 years [7]. Adherence to adjuvant endocrine therapy, consistently shown to be particularly poor in younger women, has also been associated with fertility concerns [11]. Thus, fertility concerns may compromise the curative treatment of young women with early breast cancer.
Treatment-Related Gonadotoxicity
Oncologic treatments may impair fertility either by direct gonadotoxicity, most commonly associated with chemotherapy, or by delaying conception to a later age after adjuvant endocrine therapy, and consequently exposing women to the natural age-related fertility decline. Chemotherapy-induced ovarian toxicity is primarily mediated by direct damage to oocyte DNA, triggering apoptosis, cell death, and the dwindling of a finite population of primordial follicle oocytes [12]. Female aging is associated with decreased ovarian reserve and diminished oocyte quality resulting in a reduced reproductive capacity [13]. Clinically, the probability of pregnancy for women aged 19–26 years has been estimated to be twice as high compared with women aged 35–39 years [14].
Determining the actual risk of infertility after breast cancer treatment however may be challenging. First, outcome measures reflecting the risk of infertility are not regularly recorded or reported in early breast clinical trials. Second, several different outcomes have been used, including amenorrhea, menopause, pregnancy, and birth rates, each reflecting different aspects of ovarian function [15•]. Treatment-related amenorrhea (TRA), or absence of menses in previously menstruating premenopausal women, is a commonly used surrogate marker of ovarian function and associated infertility in survivors. However, women who resume menstruation may still have a diminished ovarian reserve, with manifestations of menstrual irregularity, infertility, and premature ovarian failure [16,16,17,19]. Conversely, some women may retain ovarian function, as evidenced by estradiol secretion and occasionally subsequent pregnancy despite TRA [20].
The risk of developing TRA is related to age, chemotherapy regimen, and adjuvant endocrine therapy [21]. Additional variation is introduced by the timepoint at which TRA is measured. An extensive review including 75 retrospective and prospective studies reporting TRA rates after adjuvant chemotherapy for breast cancer summarized increasing TRA rates of 26, 39, and 77% for women < 35, 35–40, and > 40 years old, respectively [21]. In a recent meta-analysis of five prospective, randomized trials evaluating Gonadotropin-releasing hormone (GnRH) agonists during breast cancer chemotherapy, the rate of TRA in the control (chemotherapy alone) arms was 36.8% at 1 year and 30.0% at 2 years, and younger age at diagnosis was associated with reduced risk of TRA [22••]. Most women who resume menstruation do so within a year of treatment, and survivors of breast cancer may be more likely resume menses later than a year from treatment compared with survivors of other cancer types [16].
Beyond age, other patient-specific determinants of TRA are lacking. Anti-Mullerian hormone (AMH), a hormone produced by the granulosa cells of the preantral and antral follicles of the ovary, correlates with primordial follicle counts, and has emerged as an important biomarker of ovarian reserve [23]. In women treated with chemotherapy, higher pretreatment AMH is associated with a lower risk of TRA and higher chance of return of menstruation [24]. The relation between AMH levels and fertility outcomes however is less clear, and currently cannot reliably predict neither natural conception in healthy women or in cancer survivors [24].
Germline BRCA mutation carriers have been found to have lower measures of ovarian reserve, lower ovarian response rates to hyperstimulation, and earlier onset of menopause compared to non-carriers [25,25,26,28]. It has been hypothesized that BRCA carriers may experience larger declines in ovarian reserve after chemotherapy. However, data in this regard have been mixed. Valentini et al. could not find an increased risk of TRA among BRCA carriers compared to controls; however, TRA risk after chemotherapy was significantly higher for BRCA2 carriers compared with BRCA1 carriers (46.8 vs. 32.7%; P < 0.001) [29]. Lambertini et al. observed no difference in AMH levels between carriers and non-carriers treated with anthracycline- and cyclophosphamide-based chemotherapy (AC) before treatment and at 1 and 3 years post diagnosis [30]. In contrast, Oktay et al. recently presented data in abstract form showing significantly decreased AMH recovery in BRCA carriers undergoing AC with paclitaxel (AC-T) chemotherapy for breast cancer [31]. However, both the latter studies included only small numbers of BRCA carriers (n = 35 and n = 14, respectively). Further studies of the gonadotoxic effects of cancer treatments in patients with germline BRCA mutations or other DNA-repair impairing cancer-susceptibility genes are warranted.
Chemotherapy
Table 1 summarizes TRA rates after treatment with standard (neo)adjuvant chemotherapy protocols. Cyclophosphamide (C) is incorporated into most regimens and appears to be an important driver of TRA. For example, in a randomized trial comparing 3 anthracycline (A) and docetaxel (T) containing regimens of different durations with or without C, TRA rates at 12 months were lower with the 12-week AT regimen (38%), compared to 12-week TAC (58%), and compared to 24-week sequential AC-T (70%) [38•]. Furthermore, AC compared to standard cyclophosphamide-methotrexate-5-fluorouracil (CMF) differ in the route of C administration (IV vs. PO) and cumulative dose (600 mg/m2 × 4 vs. 1400 mg/m2 × 6) and both parameters appear to further modulate the risk of TRA. In a prospective observational study, 12-month TRA rates were 19 and 30% for AC and CMF, respectively, and an increased percentage of women who developed TRA after AC went on to resume menstruation (42 vs. 16%) [32]. Regarding taxanes, available data are mixed. A 2016 meta-analysis could not find an increased risk for TRA; however, the level of evidence was considered weak [21]. In a recent unplanned analysis of the ALTTO trial comprising a larger population than the prior meta-analysis, increased TRA was observed when a taxane was added to A-based chemotherapy (OR 2.24, 95% CI 1.18 to 4.27) [36]. Effects were similar for paclitaxel and docetaxel. Ruddy et al. published the only report on the risk of TRA after a taxane in the absence of other gonadotoxic treatments [37]. In a population of 64 premenopausal women participating in a phase 2 single-arm trial of adjuvant paclitaxel-trastuzumab (APT trial) at a median follow-up of 51 months from first infusion, 28% were amenorrheic. Among a small subgroup of 11 patients who were diagnosed at age ≤ 40, only a single patient (9%) remained amenorrheic.
Anti-HER2 and Other Modern Adjuvant Breast Cancer Therapy
As we incorporate new treatments in the adjuvant setting, it is imperative that we consider the potential effects on TRA and fertility in our young patients. To date, data regarding the gonadotoxicity of anti-HER2 targeted therapy on TRA are limited, though suggest trastuzumab does not increase risk of TRA [21, 33]. The risk of TRA associated with other anti-HER2 therapy including pertuzumab and trastuzumab-emtansine, as well as anti-HER2 combinations, has yet to be reported. In current practice, most women with HER2 positive breast cancer are treated with dual HER2 blockade incorporating trastuzumab and pertuzumab. Lambertini et al. showed that dual blockade, albeit with trastuzumab and lapatinib, did not increase TRA (OR 1.19; 95% CI 0.94–1.51; P = 0.14) compared to trastuzumab alone [36]. Their analysis was also able to add to our understanding of the gonadotoxic potential of carboplatin which is increasingly incorporated in regimens for HER2 positive and triple negative breast cancers. Docetaxel-carboplatin-trastuzumab (TCH) was associated with a 75.6% TRA rate and a higher risk as compared with anthracycline-based chemotherapy (OR 2.24, 95% CI 1.18–4.27) [30]. Cyclin-dependent-kinase (CDK) 4/6 inhibitors may have a future role in the treatment of early breast cancer. Preliminary observations in rats show preserved estrous cyclicity or functional fertility however data in humans is unavailable [39]. Similarly, poly-(adenosine diphosphate [ADP]-ribose) polymerase (PARP) inhibitors are being evaluated in BRCA carriers with no published reliable data on gonadotoxicity. In a recent study of talazoparib in the neoadjuvant setting, no menstrual changes were noted by the 20 participating patients; however, no objective measures were collected [40].
Endocrine Therapy
Tamoxifen is consistently reported to increase TRA rates by about two-fold following chemotherapy [21, 22••, 41]. This effect may also vary by age and in one study was observed to be significant only in women aged ≥ 40 (OR 2.51; 95% CI 1.20–5.24) compared to younger women (OR 1.89; 95% CI 0.52–6.89) [33]. The mechanism for TRA, while poorly understood, appears to be driven by hypothalamic-pituitary interactions rather than direct gonadotoxicity, and thus is likely reversible when tamoxifen is discontinued. Nevertheless, tamoxifen is teratogenic and contraindicated in pregnancy. Aromatase inhibitors must be administered in premenopausal women with concomitant ovarian function suppression which by mechanism of action leads to reversible TRA. Accordingly, both drugs must be discontinued before conception. Current guidelines call for 5–10 years of adjuvant endocrine therapy consequently forcing women to postpone childbearing by several years in order to complete an optimal course of therapy. Thus, while not directly gonadotoxic, and effects on TRA are generally reversible, use of endocrine therapies can have a profound impact on future fertility and family planning given the natural decline in oocytes with age, combined with potential subfertility even in young women who remain premenopausal after adjuvant chemotherapy [17].
Fertility Counseling
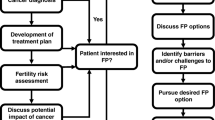
There is wide agreement among professional organizations, including the American Society for Clinical Oncology (ASCO) [42] and European Society for Medical Oncology [43], regarding the responsibility of health care providers to address the potential risks to fertility as a result of anticancer treatment as early as possible, discuss fertility preservation options, and refer interested patients to appropriate reproductive specialists. Nevertheless, rates of discussion and referral for fertility preservation have been lower than expected [44, 45]. In one multicenter study of women ≤ 40 years of age diagnosed with breast cancer between 2006 and 2012, only 68% reported discussing fertility with their providers prior to starting therapy [7]. Fertility counseling in survivorship may also be lacking and contributing to observations that some women previously interested in childbearing are not seeking fertility care upon completion of treatment [46].
Quality fertility counseling should be available to all young breast cancer patients from diagnosis through long-term survivorship. Key themes to be discussed are listed in Table 2. It should be noted that TRA, premature menopause, and/or infertility may become manifest in an individual and personalized estimates taking into consideration a women’s age, fertility history, cancer type, and treatment plan should be provided. Providers should be able to discuss the different available methods of fertility preservation and parenthood after cancer options in the context of patient preferences and cancer treatment recommendations. A systematic review has indicated that fertility preservation counseling reduces long-term regret and dissatisfaction with fertility with improved physical quality of life [47]. Providers should also be aware of the significant impact of fertility counseling on emotional health and utilize the opportunity to offer psychological supportive care [48].
Fertility Counseling in BRCA Carriers
Fertility is a major issue for BRCA1/2 carriers given their high risk of early onset breast cancer, and, high lifetime ovarian cancer risk, with standard recommendation for risk reducing bilateral salpingo-oopherectomy (RRBSO) typically between age 35 and 40 years [49]. Many carriers will consider childbearing earlier rather than later, pursue fertility treatment, or decline to have any additional children [50]. While some physicians have advocated fertility consultations for all BRCA carriers prior to any cancer diagnosis, many will present for initial consultation at time of breast cancer diagnosis, often because of lack of awareness until cancer diagnosis. After a diagnosis of breast cancer, the potential need to delay pregnancy for treatment, and related gonadotoxicty, further narrow the window of opportunity for these patients.
Fertility counseling in BRCA carriers diagnosed with breast cancer should include discussion of unique issues including competing cancer risks, timing of RRBSO, ovarian function and potential decreased reserve in carriers which may impact on success of fertility preservation strategies, suitability of fertility preservation strategies and future opportunities such as preimplantation genetic testing (PGT, discussed later) [51, 52]. The relatively limited and often contradicting data to inform such conversations pose a significant challenge and should be a research priority.
Pregnancy after Breast Cancer
The safety of pregnancy after cancer, potential teratogenic effects of previous treatments, and the possibility of pregnancy causing a cancer recurrence are also major concerns shared by patients and providers [46, 53, 54]. The understanding of the impact of subsequent pregnancy on breast cancer outcomes is largely based on retrospective data. In an initial meta-analysis of 14 studies, pregnancy after breast cancer was associated with a 41% reduced risk of death [55]. In a multicenter retrospective cohort, with carefully selected controls, at a median follow-up of 7.2 years from pregnancy no adverse effect was observed for pregnancy on disease-free survival (DFS) or overall survival (OS) in both estrogen receptor (ER)-positive and ER-negative subgroups [4••]. Data supporting the safety of pregnancy in survivors with BRCA mutations was also recently presented [56]. The optimal timing for pregnancy after breast cancer is also unclear. According to expert opinion, it is reasonable to postpone pregnancy for 2 years following diagnosis thus surpassing the peak in hazard rates of recurrence [43, 57]. Women with hormone receptor-positive disease are typically recommended 5–10 years of endocrine therapy and treatment must be stopped and washed out before attempting conception. The safety of interrupting adjuvant endocrine therapy for up to 2 years to allow pregnancy, delivery, breastfeeding, or failure to conceive after completing 18–30 months of initial treatment is currently being prospectively evaluated (NCT02308085).
Fertility Preservation
Multiple methods and protocols for fertility preservation are available and selection should be individualized based on age, partner status, treatment timelines, the availability of donor sperm, and future child bearing preferences. Embryo and oocyte cryopreservation are currently the most widely used and established methods for fertility preservation, and both conventionally entail the need for ovarian stimulation. Use of ovarian suppression is widely available and evolving evidence suggest efficacy and safety. Ovarian tissue cryopreservation as well as transplantations are considered experimental, and donor oocytes, gestational carrier use, and adoption are all considerations.
Controlled Ovarian Simulation
Both oocyte and embryo cryopreservation requires women undergo a complete protocol of in vitro fertilization (IVF). Initially, controlled ovarian stimulation (COS) using exogenous hormones (gonadotropins) is employed to facilitate multiple oocyte maturation [58]. Women are cotreated with either GnRH agonists or antagonists to suppress pituitary function and prevent premature ovulation. Oocyte development is monitored with serial pelvic ultrasound. Once oocytes have achieved an appropriate size, their final maturation is triggered, and they are retrieved through a transvaginal approach.
Historically, COS cycles had to be started at the beginning of the follicular phase and spanned 12–16 days. Subsequently, potential clinically significant delays in commencing breast cancer treatment were of concern. Due to advances in fertility medicine, timing of COS to the menstrual cycle is no longer required [42]. “Random-start COS” protocols have been shown to be as effective as conventional-start COS in fertility preservation, though 2 weeks are still required for egg retrieval [59]. Ovarian hyperstimulation syndrome (OHSS) is a serious complication of COS and may be profound in cancer patients due to its potential to further delay cancer treatment. The use of a GnRH agonist instead of human chorionic gonadotropin (HCG), to trigger final oocyte maturation in women undergoing a GnRH antagonist protocol has been shown to reduce OHSS incidence and is regarded as the standard COS protocol [60, 61].
As described, some studies have indicated reduced ovarian reserve among BRCA carriers which may further complicate fertility preservation. In a retrospective analysis of 156 women diagnosed with breast cancer, including 29 with BRCA mutations, a consistent trend for higher gonadotropin dose, lower AMH levels, lower number of oocytes retrieved, and a higher rate of low ovarian response (< 4 oocytes) were observed among BRCA carriers [51]. Although feasible, the performance of interventions for fertility preservation in this setting may be inferior, and the combination of several methods may be useful.
A critical concern regarding COS in hormone receptor-positive breast cancer is that supra physiologic levels of estrogen achieved in COS may stimulate progression of these hormone-sensitive cancer cells. Modification of COS protocols including co-administration of letrozole can significantly decrease peak estrogen to near physiologic levels while maintaining oocyte yield, fertilization rates, and ultimately pregnancy outcomes [42, 62•]. Alternatively, tamoxifen, while not decreasing estrogen levels, competitively binds ER to inhibit cancer growth, has been added with preserved IVF results [62•, 63]. Limited data available on outcomes suggest safety from these strategies. A recent systematic review identified four studies, encompassing 464 women, with reports of breast cancer mortality and recurrence [64]. In the largest study, Kim et al. prospectively collected data on 120 women who underwent COS with letrozole supplementation for fertility preservation and 217 controls who underwent oncofertility consultation however declined fertility preservation [65]. Through a mean follow-up of 5 and 6.9 years, respectively, no difference in relapse-free survival was observed, including no relapse-free survival (RFS) differences according to BRCA mutation status, timing of COS before/after breast surgery, and tumor ER status. Similarly, long-term, albeit more limited, data does not suggest an increased risk of recurrence with COS and tamoxifen co-administration [63].
Patients treated with neoadjuvant chemotherapy have been significantly less likely to undergo fertility preservation and there has been concern about the safety of pursuing strategies in this setting [42, 66,66,68]. Retrospective studies suggest that for women scheduled to begin neoadjuvant chemotherapy, COS does not significantly impact the time from diagnosis to initiation of treatment [69, 70]. The largest study in this setting retrospectively examined 82 women with early stage breast cancer screened for the ISPY2 clinical trial and did not observe a significant difference in median time to recurrence or death between women who underwent COS (n = 34) and controls (n = 48) [70]. Additional reports are vital to assure the safety of COS in this setting.
Oocyte and Embryo Cryopreservation
Following COS and ultrasound-guided oocyte retrieval, unfertilized oocytes or embryos, i.e., oocytes fertilized in vitro, may be cryopreserved for future use. Cryopreservation of embryos is the most established technique for fertility preservation and can be offered to woman with a committed partner or donor sperm. Embryo cryopreservation followed by frozen-embryo transfer appears to be comparable to fresh-embryo transfer in non-cancer and breast cancer population with live birth rates in the 45–50% range [71, 72].
Due to improvement in methods of cryopreservation, specifically the adoption of vitrification over slow-freezing, cryopreservation of unfertilized oocytes is now regarded as an established fertility preservation method in recent guidelines given similar clinical outcomes for vitrified vs. fresh oocytes [42, 73]. This strategy enhances women’s reproductive autonomy allowing for fertility preservation that is independent of sperm donor or relationship status changes.
The in vivo maturation (IVM) of immature oocytes retrieved following minimal or no ovarian stimulation is an investigational approach which bypassing the need for COS offers several potential advantages: shorter time to oocyte retrieval and subsequent initiation of cancer treatment, reduced estradiol exposure, lower gonadotropin doses and associated costs and of OHSS [74]. In a recent report of 353 cancer patients (48.2% breast cancer), more oocytes were collected, oocytes cryopreserved, and embryos (when applicable) cryopreserved with IVF compared to IVM [75]. Cumulative pregnancy rate and live birth rate were 37 and 31% following IVF and 14 and 7% following IVM. These results are in line with previous data consistently indicating lower implantation and pregnancy rates for IVM compared to standard IVF [76].
Ovarian Tissue Cryopreservation
Ovarian tissue cryopreservation and transplantation (OTCT) involves removal of ovarian tissue in multiple biopsies or as a whole oophorectomy and cryopreservation of ovarian cortical strips before chemotherapy [77]. Subsequently, the ovarian tissue is thawed and orthotopically grafted to the ovarian medulla or re-implanted inside an artificial peritoneal window. OTCT does not require ovarian stimulation and therefore can be performed immediately without delaying chemotherapy. Furthermore, ovarian cryopreservation offers the advantage of restoration of ovarian endocrine function in ~ 95% of cases [78]. Following ovarian tissue transplantation to the pelvis, pregnancy may be spontaneous or with IVF. Meirow et al. reported results for 20 cancer survivors—16 (10 IVF, 6 spontaneous) pregnancies were achieved, resulting in 10 live births [79]. In sum, 53% of patients conceived, and 32% delivered at least once. Similar results were reported in a multicenter study including 74 cancer survivors with a pregnancy rate of 33% and a birth rate of 25% per patient [80]. A significant safety concern is the potential reintroduction of malignant cells after transplantation. Various methods including multiple section histology, immunostaining, fluorescence in situ hybridization, polymerase chain reaction, and animal studies are regularly applied to rule out the harboring of cancer cells [79]. A systematic review of all published OTCT cases including 230 women with a history of malignancy identified nine cancer recurrences, none of which were considered to be directly caused by the procedure [78]. Combination approaches entailing oophorectomy, immature oocyte retrieval from the excised ovarian tissue or aspiration from an intact ovary, and IVM have been offered as a means of increasing options and flexibility [81]. While this approach is still considered experimental, ASCO 2018 guidelines note that emerging data may prompt reconsideration of this designation in the future [42].
OTCT is suboptimal in BRCA mutation carriers due to their increased risks for development of ovarian cancer and recommendations to undergo RRBSO by age 40. Successful births have been reported in this situation and it is recommended that if OTCT is elected, candidates should be very young, and thus at lower risk for ovarian cancer [51]. A potential alternative approach in the future may be to maturate oocytes from thawed ovarian tissue and perform IVF, avoiding the need for ovarian tissue transplantation [82].
Ovarian Function Suppression
The use of ovarian function suppression with GnRH agonists during chemotherapy to reduce gonadotoxicity is controversial [83]. A 2014 meta-analysis including 9 studies found a highly significant reduction in the risk of premature ovarian failure although with statistically significant heterogeneity among studies [84]. In subgroup analyses, a significant interaction was seen between treatment arm and type of cancer; the significant protective effect was limited to women with breast cancer and was negative among those with lymphoma or ovarian cancer. These populations differ in their age at diagnosis and the gonadotoxic potential of administered chemotherapy protocols [85]. Lambertini et al. conducted an individual patient level meta-analysis of 5 randomized trials including only women receiving chemotherapy for breast cancer [22••]. A 62% reduction in the odds of ovarian failure was observed for GnRH agonists (14.1 vs. 30.9%, adjusted OR 0.38; 95% CI 0.26 to 0.57; P < 0.001). Importantly, no significant differences were seen in DFS and OS supporting the safety of this intervention irrespective of the breast cancer’s hormone receptor status. The potential role for ovarian suppression in fertility preservation was also illustrated—10.3% women treated with GnRH agonists achieved at least one pregnancy compared to 5.5% in controls (incidence rate ratio 1.83; 95% CI 1.06 to 3.15; P = 0.030). This analysis was limited by missing data regarding pregnancy intent and could not rule out a bias whereas non-blinded patients treated with GnRH may be more inclined to attempt pregnancy. Given the current state of the data, ovarian suppression is generally considered as an adjunct to the more established forms of fertility preservation and for some women, as a means of reducing the reliance on these more costly and invasive procedures [42, 86]. Moreover, the preservation of ovarian function, regardless of fertility potential, may be in itself of significant value to premenopausal women with breast cancer [3].
Preimplantation Genetic Testing
Pathogenic or likely pathogenic inherited germline mutations associated with an increased risk for breast cancer are identified in nearly 10% of patients [87]. Among women aged 40 years or younger, the rate of deleterious BRCA1/2 mutations reached 12% in one study [88]. Due to the high risk of breast, ovarian, and additional cancers associated with these mutations, and their autosomal dominant nature, women diagnosed with BRCA mutations and interested in fertility, also face a dilemma regarding the prevention of mutation transmission to their offspring. Two approaches to prevent gene transmission are currently available. Prenatal diagnosis (PND) is performed through chorionic villus sampling during pregnancy and obligates a discussion of pregnancy termination if a mutation is identified. PGT on the other hand is performed before a pregnancy is defined. Following IVF and oocyte fertilization, on day 3 of embryonal development, a single cell is extracted from a 6–8 cell embryo, DNA is analyzed for the presence of the previously characterized specific genetic mutation, and thereafter an unaffected embryo/s is transferred [89].
The ethics committee of the American Society for Reproductive Medicine supports the use of PGT for monogenic adult onset disease when “the conditions are serious and when there are no known interventions for the conditions, or the available interventions are either inadequately effective or are perceived to be significantly burdensome” [90]. They acknowledge the complexity of making such a determination in the setting of cancer disposition genes, such as BRCA, due to incomplete penetrance, established clinical screening and preventative strategies, and the availability of effective cancer treatments. The latter may be true for breast cancer, however in comparison, screening is currently inadequate and long-term outcomes are inferior for ovarian cancer. For conditions considered to be less serious or of lower penetrance, the committee find PGT “ethically acceptable as a matter of reproductive liberty.” Double testing of embryos for gender and specific mutation has also been suggested in this setting, so that if only BRCA mutated embryos are found, a couple may elect to transfer a male embryo due to a decreased cumulative cancer risk [91]. This approach may be of higher value in the case of BRCA1 mutations [92].
Awareness of PGT among individuals at high risk for hereditary cancers is generally low; estimated as 35% in a 2012 systematic review [93]. Women’s interest in PGT however seems to be high. In a cross-sectional survey of 1081 BRCA carriers, a majority of respondents believed that PGT (58.7%) should be offered to BRCA mutation carriers; however, only 34.8% would consider undergoing PGT [50]. Regarding PND, 55.5% thought that it should be offered to pregnant BRCA carriers, 29.8% would consider using PND themselves, however only 4% of women stated they would consider terminating of pregnancy if a fetal mutation was identified. Notably only 8 women had undergone PGT, and none had used PND. In an Israeli hospital, when PGT was offered free of charge to a sample of 70 married female BRCA mutation carriers, only 25.7% elected to use it [94]. Only a history of infertility was significant associated with PGT uptake, possibly suggesting that for some women BRCA status is secondary to infertility in considering PGD. Less data is available to understand decision making among women with a history of breast cancer. In one focus group study, BRCA-positive breast cancer survivors emphasized their own physical and emotional experiences with breast cancer treatment as a motivation to undergo PGD [95].
Conclusions
Fertility counseling and preservation have become an integral part of the multidisciplinary care for breast cancer at diagnosis and throughout survivorship. Such discussions should include personalized estimates of infertility risk, elucidation of fertility preferences, and a tailoring of fertility preservation treatment options, particularly for women with hereditary predisposition to breast cancer. Fortunately, multiple options for fertility preservation are available today. Future research efforts should be made to further individualize recommendation and identify patients for which fertility preservation is essential and those for which it may be unnecessary.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144(8):1941–53. https://doi.org/10.1002/ijc.31937.
International Agency for Research on Cancer. Estimated number of new cases in 2018, worldwide, females, ages 0-44. Cancer Today. 2018. https://gco.iarc.fr/today/online-analysis-table?v=2018&mode=cancer&mode_population=continents&population=900&populations=900&key=asr&sex=2&cancer=39&type=0&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=8&nb_items=5&group_cancer=1&include_nmsc=1&include_nmsc_other=1. Accessed 10 Jan 2020.
Paluch-Shimon S, Pagani O, Partridge AH, et al. ESO-ESMO 3rd international consensus guidelines for breast cancer in young women (BCY3). Breast (Edinburgh, Scotland). 2017;35:203–17. https://doi.org/10.1016/j.breast.2017.07.017. Most recent consensus guidelines for the management of breast cancer in young women, highlighting unique aspects of screening, diagnosis, treatment and supportive care.
Lambertini M, Kroman N, Ameye L, et al. Long-term safety of pregnancy following breast cancer according to estrogen receptor status. J Natl Cancer Inst. 2017;110(4):426–9. https://doi.org/10.1093/jnci/djx206. This retrospective multicenter case-control study with a median follow-up of 7.2 years after pregnancy provides important reassurance regarding the long term safety of pregnancy after breast cancer, in particular for women with ER positive cancers.
OECD Family Database—OECD. http://www.oecd.org/els/family/database.htm. Accessed September 10, 2019.
Partridge AH, Gelber S, Peppercorn J, et al. Web-based survey of fertility issues in young women with breast cancer. Journal of Clinical Oncology. 2004;22(20):4174–83. https://doi.org/10.1200/JCO.2004.01.159. This was a large early study highlighting the significance of fertility concerns among young breast cancer patients, their impact on treatment decisions and the need for better communication regarding these issues.
Ruddy KJ, Gelber SI, Tamimi RM, et al. Prospective study of fertility concerns and preservation strategies in young women with breast cancer. J Clin Oncol. 2014;32(11):1151–6. https://doi.org/10.1200/JCO.2013.52.8877.
Ruggeri M, Pagan E, Bagnardi V, et al. Fertility concerns, preservation strategies and quality of life in young women with breast cancer: baseline results from an ongoing prospective cohort study in selected European centers. Breast. 2019;47:85–92. https://doi.org/10.1016/j.breast.2019.07.001.
Thewes B, Meiser B, Rickard J, Friedlander M. The fertility- and menopause-related information needs of younger women with a diagnosis of breast cancer: a qualitative study. Psychooncology. 2003;12(5):500–11. https://doi.org/10.1002/pon.685.
Howard-Anderson J, Ganz PA, Bower JE, Stanton AL. Quality of life, fertility concerns, and behavioral health outcomes in younger breast cancer survivors: a systematic review. J Natl Cancer Inst. 2012;104(5):386–405. https://doi.org/10.1093/jnci/djr541.
Llarena NC, Estevez SL, Tucker SL, Jeruss JS. Impact of fertility concerns on tamoxifen initiation and persistence. J Natl Cancer Inst. 2015;107(10). doi:https://doi.org/10.1093/jnci/djv202
Bedoschi G, Navarro PA, Oktay K. Chemotherapy-induced damage to ovary: mechanisms and clinical impact. Future Oncol. 2016;12(20):2333–44. https://doi.org/10.2217/fon-2016-0176.
Fertility and ageing. Hum Reprod Update. 2005;11(3):261–276. doi:https://doi.org/10.1093/humupd/dmi006
Dunson DB, Colombo B, Baird DD. Changes with age in the level and duration of fertility in the menstrual cycle. Hum Reprod. 2002;17(5):1399–403. https://doi.org/10.1093/humrep/17.5.1399.
Poorvu PD, Frazier AL, Feraco AM, et al. Cancer treatment-related infertility: a critical review of the evidence. JNCI Cancer Spectrum. 2019;3(1):pkz008. https://doi.org/10.1093/jncics/pkz008. An encompassing review of infertility rates associated with various specific malignancies and modern therapies. An accurate understanding of risk is critical in counseling patients on fertility preservation.
Jacobson MH, Mertens AC, Spencer JB, Manatunga AK, Howards PP. Menses resumption after cancer treatment-induced amenorrhea occurs early or not at all. Fertil Steril. 2016;105(3):765–772.e4. https://doi.org/10.1016/j.fertnstert.2015.11.020.
Partridge AH, Ruddy KJ, Gelber S, et al. Ovarian reserve in women who remain premenopausal after chemotherapy for early stage breast cancer. Fertil Steril. 2010;94(2):638–44. https://doi.org/10.1016/j.fertnstert.2009.03.045.
Partridge A, Gelber S, Gelber RD, Castiglione-Gertsch M, Goldhirsch A, Winer E. Age of menopause among women who remain premenopausal following treatment for early breast cancer: long-term results from International Breast Cancer Study Group Trials V and VI. Eur J Cancer. 2007;43(11):1646–53. https://doi.org/10.1016/j.ejca.2007.04.006.
Letourneau JM, Ebbel EE, Katz PP, et al. Acute ovarian failure underestimates age-specific reproductive impairment for young women undergoing chemotherapy for cancer. Cancer. 2012;118(7):1933–9. https://doi.org/10.1002/cncr.26403.
Krekow LK, Hellerstedt BA, Collea RP, et al. Incidence and predictive factors for recovery of ovarian function in amenorrheic women in their 40s treated with letrozole. J Clin Oncol. 2016;34(14):1594–600. https://doi.org/10.1200/JCO.2015.62.2985.
Zavos A, Valachis A. Risk of chemotherapy-induced amenorrhea in patients with breast cancer: a systematic review and meta-analysis. Acta Oncol. 2016;55(6):664–70. https://doi.org/10.3109/0284186X.2016.1155738.
Lambertini M, Moore HCF, Leonard RCF, et al. Gonadotropin-releasing hormone agonists during chemotherapy for preservation of ovarian function and fertility in premenopausal patients with early breast cancer: a systematic review and meta-analysis of individual patient-level data. JCO. 2018;36(19):1981–90. https://doi.org/10.1200/JCO.2018.78.0858. A patient level metanalysis of five trials including premenopausal breast cancer patients providing quality evidence to support the use of ovarian suppression during chemotherapy to prevent premature ovarian insufficiency and potentially improve fertility outcomes.
Hansen KR, Hodnett GM, Knowlton N, Craig LB. Correlation of ovarian reserve tests with histologically determined primordial follicle number. Fertil Steril. 2011;95(1):170–5. https://doi.org/10.1016/j.fertnstert.2010.04.006.
Wong QHY, Anderson RA. The role of antimullerian hormone in assessing ovarian damage from chemotherapy, radiotherapy and surgery. Current Opinion in Endocrinology, Diabetes and Obesity. 2018;25(6):391. https://doi.org/10.1097/MED.0000000000000447.
Phillips K-A, Collins IM, Milne RL, et al. Anti-Müllerian hormone serum concentrations of women with germline BRCA1 or BRCA2 mutations. Hum Reprod. 2016;31(5):1126–32. https://doi.org/10.1093/humrep/dew044.
Oktay K, Kim JY, Barad D, Babayev SN. Association of BRCA1 mutations with occult primary ovarian insufficiency: a possible explanation for the link between infertility and breast/ovarian cancer risks. J Clin Oncol. 2010;28(2):240–4. https://doi.org/10.1200/JCO.2009.24.2057.
Finch A, Valentini A, Greenblatt E, et al. Frequency of premature menopause in women who carry a BRCA1 or BRCA2 mutation. Fertil Steril. 2013;99(6):1724–8. https://doi.org/10.1016/j.fertnstert.2013.01.109.
Ben-Aharon I, Levi M, Margel D, et al. Premature ovarian aging in BRCA carriers: a prototype of systemic precocious aging? Oncotarget. 2018;9(22):15931–41. https://doi.org/10.18632/oncotarget.24638.
Valentini A, Finch A, Lubinski J, et al. Chemotherapy-induced amenorrhea in patients with breast cancer with a BRCA1 or BRCA2 mutation. J Clin Oncol. 2013;31(31):3914–9. https://doi.org/10.1200/JCO.2012.47.7893.
Lambertini M, Olympios N, Lequesne J, et al. Impact of taxanes, endocrine therapy, and deleterious germline BRCA mutations on anti-Müllerian hormone levels in early breast cancer patients treated with anthracycline- and cyclophosphamide-based chemotherapy. Front Oncol. 2019. https://doi.org/10.3389/fonc.2019.00575.
Oktay K, Bedoschi G, Goldfarb SB, et al. Abstract PD6-06: impact of BRCA mutations on chemotherapy-induced loss of ovarian reserve: a prospective longitudinal study. Cancer Res. 2019;79(4 Supplement):PD6-06-PD6-06. https://doi.org/10.1158/1538-7445.SABCS18-PD6-06.
Sukumvanich P, Case LD, Zee KV, et al. Incidence and time course of bleeding after long-term amenorrhea after breast cancer treatment. Cancer. 2010;116(13):3102–11. https://doi.org/10.1002/cncr.25106.
Abusief ME, Missmer SA, Ginsburg ES, Weeks JC, Partridge AH. The effects of paclitaxel, dose density, and trastuzumab on treatment-related amenorrhea in premenopausal women with breast cancer. Cancer. 2010;116(4):791–8. https://doi.org/10.1002/cncr.24835.
Swain SM, Land SR, Ritter MW, et al. Amenorrhea in premenopausal women on the doxorubicin-and-cyclophosphamide-followed-by-docetaxel arm of NSABP B-30 trial. Breast Cancer Res Treat. 2009;113(2):315–20. https://doi.org/10.1007/s10549-008-9937-0.
Treatment-related amenorrhea among young women one year following diagnosis of early-stage breast cancer. | Journal of Clinical Oncology. https://ascopubs.org/doi/abs/10.1200/jco.2015.33.15_suppl.9523. Accessed November 3, 2019.
Lambertini M, Campbell C, Bines J, et al. Adjuvant anti-HER2 therapy, treatment-related amenorrhea, and survival in premenopausal HER2-positive early breast cancer patients. J Natl Cancer Inst. 2019;111(1):86–94. https://doi.org/10.1093/jnci/djy094.
Ruddy KJ, Guo H, Barry W, et al. Chemotherapy-related amenorrhea after adjuvant paclitaxel-trastuzumab (APT trial). Breast Cancer Res Treat. 2015;151(3):589–96. https://doi.org/10.1007/s10549-015-3426-z.
Ganz PA, Land SR, Geyer CE, et al. Menstrual history and quality-of-life outcomes in women with node-positive breast cancer treated with adjuvant therapy on the NSABP B-30 trial. J Clin Oncol. 2011;29(9):1110–6. https://doi.org/10.1200/JCO.2010.29.7689. This large prospective study provided important information on the risk of amenorrhea associated with Anthracycline and taxane based chemotherapy protocols commonly used in the treatment of breast cancer.
Catlin NR, Bowman CJ, Engel SM, et al. Reproductive and developmental toxicity assessment of palbociclib, a CDK4/6 inhibitor, in Sprague-Dawley rats and New Zealand white rabbits. Reprod Toxicol. 2019;88:76–84. https://doi.org/10.1016/j.reprotox.2019.07.016.
Litton JK, Scoggins ME, Hess KR, et al. Neoadjuvant talazoparib for patients with operable breast cancer with a germline BRCA pathogenic variant. JCO. 2019. https://doi.org/10.1200/JCO.19.01304.
Petrek JA, Naughton MJ, Case LD, et al. Incidence, time course, and determinants of menstrual bleeding after breast cancer treatment: a prospective study. JCO. 2006;24(7):1045–51. https://doi.org/10.1200/JCO.2005.03.3969.
Oktay K, Harvey BE, Partridge AH, et al. Fertility preservation in patients with cancer: ASCO clinical practice guideline update. JCO. 2018;36(19):1994–2001. https://doi.org/10.1200/JCO.2018.78.1914.
Peccatori FA, Azim HA, Orecchia R, et al. Cancer, pregnancy and fertility: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(suppl_6):vi160–70. https://doi.org/10.1093/annonc/mdt199.
Goossens J, Delbaere I, Van Lancker A, Beeckman D, Verhaeghe S, Van Hecke A. Cancer patients’ and professional caregivers’ needs, preferences and factors associated with receiving and providing fertility-related information: a mixed-methods systematic review. Int J Nurs Stud. 2014;51(2):300–19. https://doi.org/10.1016/j.ijnurstu.2013.06.015.
Quinn GP, Vadaparampil ST, Lee J-H, et al. Physician referral for fertility preservation in oncology patients: a national study of practice behaviors. JCO. 2009;27(35):5952–7. https://doi.org/10.1200/JCO.2009.23.0250.
Kim J, Mersereau JE, Su HI, Whitcomb BW, Malcarne VL, Gorman JR. Young female cancer survivors’ use of fertility care after completing cancer treatment. Support Care Cancer. 2016;24(7):3191–9. https://doi.org/10.1007/s00520-016-3138-x.
Impact of fertility preservation counseling and treatment on psychological outcomes among women with cancer: a systematic review. Deshpande-2015-Cancer-Wiley Online Library. https://onlinelibrary.wiley.com/doi/full/10.1002/cncr.29637. Accessed September 26, 2019.
Logan S, Anazodo A. The psychological importance of fertility preservation counseling and support for cancer patients. Acta Obstet Gynecol Scand. 2019;98(5):583–97. https://doi.org/10.1111/aogs.13562.
National Comprehensive Cancer Network. Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic (Version 1.2020). https://www.nccn.org/professionals/physician_gls/pdf/genetics_bop.pdf. Published January 18, 2019.
Chan JL, Johnson LN, Sammel MD, et al. Reproductive decision-making in women with BRCA1/2 mutations. J Genet Couns. 2017;26(3):594–603. https://doi.org/10.1007/s10897-016-0035-x.
Lambertini M, Goldrat O, Ferreira AR, et al. Reproductive potential and performance of fertility preservation strategies in BRCA-mutated breast cancer patients. Ann Oncol. 2018;29(1):237–43. https://doi.org/10.1093/annonc/mdx639.
Peccatori FA, Mangili G, Bergamini A, et al. Fertility preservation in women harboring deleterious BRCA mutations: ready for prime time? Hum Reprod. 2018;33(2):181–7. https://doi.org/10.1093/humrep/dex356.
Schover LR. Motivation for parenthood after cancer: a review. J Natl Cancer Inst Monogr. 2005;2005(34):2–5. https://doi.org/10.1093/jncimonographs/lgi010.
Lambertini M, Di Maio M, Pagani O, et al. The BCY3/BCC 2017 survey on physicians’ knowledge, attitudes and practice towards fertility and pregnancy-related issues in young breast cancer patients. Breast. 2018;42:41–9. https://doi.org/10.1016/j.breast.2018.08.099.
Azim HA, Santoro L, Pavlidis N, et al. Safety of pregnancy following breast cancer diagnosis: a meta-analysis of 14 studies. Eur J Cancer. 2011;47(1):74–83. https://doi.org/10.1016/j.ejca.2010.09.007.
Lambertini M, Ameye L, Hamy A-S, et al. Safety of pregnancy following breast cancer (BC) in patients (pts) carrying a BRCA mutation (mBRCA): results of an international cohort study. JCO. 2019;37(15_suppl):11506. https://doi.org/10.1200/JCO.2019.37.15_suppl.11506.
Saphner T, Tormey DC, Gray R. Annual hazard rates of recurrence for breast cancer after primary therapy. J Clin Oncol. 1996;14(10):2738–46. https://doi.org/10.1200/JCO.1996.14.10.2738.
Gallos ID, Eapen A, Price MJ, et al. Controlled ovarian stimulation protocols for assisted reproduction: a network meta-analysis. Cochrane Database Syst Rev. 2017;3. https://doi.org/10.1002/14651858.CD012586.
Cakmak H, Katz A, Cedars MI, Rosen MP. Effective method for emergency fertility preservation: random-start controlled ovarian stimulation. Fertil Steril. 2013;100(6):1673–80. https://doi.org/10.1016/j.fertnstert.2013.07.1992.
Oktay K, Türkçüoğlu I, Rodriguez-Wallberg KA. GnRH agonist trigger for women with breast cancer undergoing fertility preservation by aromatase inhibitor/FSH stimulation. Reprod BioMed Online. 2010;20(6):783–8. https://doi.org/10.1016/j.rbmo.2010.03.004.
von Wolff M, Germeyer A, Liebenthron J, Korell M, Nawroth F. Practical recommendations for fertility preservation in women by the FertiPROTEKT network. Part II: fertility preservation techniques. Arch Gynecol Obstet. 2018;297(1):257–67. https://doi.org/10.1007/s00404-017-4595-2.
Oktay K, Buyuk E, Libertella N, Akar M, Rosenwaks Z. Fertility preservation in breast cancer patients: a prospective controlled comparison of ovarian stimulation with tamoxifen and letrozole for embryo cryopreservation. J Clin Oncol. 2005;23(19):4347–53. https://doi.org/10.1200/JCO.2005.05.037. Early study showing the feasibility of controlled ovarian stimulation with Tamoxifen and Letrozole and efficacy of the latter in reducing peak estrogen levels. More recent studies also support the safety of this approach which is now commonly used in fertility preservation in the setting of early breast cancer.
Meirow D, Raanani H, Maman E, et al. Tamoxifen co-administration during controlled ovarian hyperstimulation for in vitro fertilization in breast cancer patients increases the safety of fertility-preservation treatment strategies. Fertil Steril. 2014;102(2):488–495.e3. https://doi.org/10.1016/j.fertnstert.2014.05.017.
Rodgers RJ, Reid GD, Koch J, et al. The safety and efficacy of controlled ovarian hyperstimulation for fertility preservation in women with early breast cancer: a systematic review. Hum Reprod. 2017;32(5):1033–45. https://doi.org/10.1093/humrep/dex027.
Kim J, Turan V, Oktay K. Long-term safety of letrozole and gonadotropin stimulation for fertility preservation in women with breast cancer. J Clin Endocrinol Metab. 2016;101(4):1364–71. https://doi.org/10.1210/jc.2015-3878.
Kim J, Oktay K, Gracia C, Lee S, Morse C, Mersereau JE. Which patients pursue fertility preservation treatments? A multi-center analysis of the predictors of fertility preservation in women with breast cancer. Fertil Steril. 2012;97(3):671–6. https://doi.org/10.1016/j.fertnstert.2011.12.008.
Hershlag A, Mullin C, Bristow SL. Is fertility preservation feasible and safe with neoadjuvant therapy for breast cancer? J Glob Oncol. 2018. https://doi.org/10.1200/JGO.17.00213.
Loren AW, Mangu PB, Beck LN, et al. Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. JCO. 2013;31(19):2500–10. https://doi.org/10.1200/JCO.2013.49.2678.
Letourneau JM, Sinha N, Wald K, et al. Random start ovarian stimulation for fertility preservation appears unlikely to delay initiation of neoadjuvant chemotherapy for breast cancer. Hum Reprod. 2017;32(10):2123–9. https://doi.org/10.1093/humrep/dex276.
Chien AJ, Chambers J, Mcauley F, et al. Fertility preservation with ovarian stimulation and time to treatment in women with stage II–III breast cancer receiving neoadjuvant therapy. Breast Cancer Res Treat. 2017;165(1):151–9. https://doi.org/10.1007/s10549-017-4288-3.
Shi Y, Sun Y, Hao C, et al. Transfer of fresh versus frozen embryos in ovulatory women. N Engl J Med. 2018;378(2):126–36. https://doi.org/10.1056/NEJMoa1705334.
Oktay K, Turan V, Bedoschi G, Pacheco FS, Moy F. Fertility preservation success subsequent to concurrent aromatase inhibitor treatment and ovarian stimulation in women with breast cancer. J Clin Oncol. 2015;33(22):2424–9. https://doi.org/10.1200/JCO.2014.59.3723.
Rienzi L, Gracia C, Maggiulli R, et al. Oocyte, embryo and blastocyst cryopreservation in ART: systematic review and meta-analysis comparing slow-freezing versus vitrification to produce evidence for the development of global guidance. Hum Reprod Update. 2017;23(2):139–55. https://doi.org/10.1093/humupd/dmw038.
Son W-Y, Henderson S, Cohen Y, Dahan M, Buckett W. Immature oocyte for fertility preservation. Front Endocrinol. 2019. https://doi.org/10.3389/fendo.2019.00464.
Creux H, Monnier P, Son W-Y, Buckett W. Thirteen years’ experience in fertility preservation for cancer patients after in vitro fertilization and in vitro maturation treatments. J Assist Reprod Genet. 2018;35(4):583–92. https://doi.org/10.1007/s10815-018-1138-0.
Fadini R, Mignini Renzini M, Dal Canto M, et al. Oocyte in vitro maturation in normo-ovulatory women. Fertil Steril. 2013;99(5):1162–9. https://doi.org/10.1016/j.fertnstert.2013.01.138.
Donnez J, Dolmans M-M. Fertility preservation in women. N Engl J Med. 2017;377(17):1657–65. https://doi.org/10.1056/NEJMra1614676.
Gellert SE, Pors SE, Kristensen SG, Bay-Bjørn AM, Ernst E, Yding Andersen C. Transplantation of frozen-thawed ovarian tissue: an update on worldwide activity published in peer-reviewed papers and on the Danish cohort. J Assist Reprod Genet. 2018;35(4):561–70. https://doi.org/10.1007/s10815-018-1144-2.
Meirow D, Ra’anani H, Shapira M, et al. Transplantations of frozen-thawed ovarian tissue demonstrate high reproductive performance and the need to revise restrictive criteria. Fertil Steril. 2016;106(2):467–74. https://doi.org/10.1016/j.fertnstert.2016.04.031.
Van der Ven H, Liebenthron J, Beckmann M, et al. Ninety-five orthotopic transplantations in 74 women of ovarian tissue after cytotoxic treatment in a fertility preservation network: tissue activity, pregnancy and delivery rates. Hum Reprod. 2016;31(9):2031–41. https://doi.org/10.1093/humrep/dew165.
Hourvitz A, Yerushalmi GM, Maman E, et al. Combination of ovarian tissue harvesting and immature oocyte collection for fertility preservation increases preservation yield. Reprod BioMed Online. 2015;31(4):497–505. https://doi.org/10.1016/j.rbmo.2015.06.025.
Lambertini M, Goldrat O, Toss A, et al. Fertility and pregnancy issues in BRCA-mutated breast cancer patients. Cancer Treat Rev. 2017;59:61–70. https://doi.org/10.1016/j.ctrv.2017.07.001.
Lambertini M, Horicks F, Mastro LD, Partridge AH, Demeestere I. Ovarian protection with gonadotropin-releasing hormone agonists during chemotherapy in cancer patients: from biological evidence to clinical application. Cancer Treat Rev. 2019;72:65–77. https://doi.org/10.1016/j.ctrv.2018.11.006.
Del Mastro L, Ceppi M, Poggio F, et al. Gonadotropin-releasing hormone analogues for the prevention of chemotherapy-induced premature ovarian failure in cancer women: systematic review and meta-analysis of randomized trials. Cancer Treat Rev. 2014;40(5):675–83. https://doi.org/10.1016/j.ctrv.2013.12.001.
Lambertini M, Partridge AH, Del Mastro L. Reply to V. Turan et al. JCO. 2018;37(1):86–8. https://doi.org/10.1200/JCO.18.00630.
Turan V, Bedoschi G, Rodriguez-Wallberg K, et al. Utility of gonadotropin-releasing hormone agonists for fertility preservation: lack of biologic basis and the need to prioritize proven methods. JCO. 2018;37(1):84–6. https://doi.org/10.1200/JCO.18.00420.
Beitsch PD, Whitworth PW, Hughes K, et al. Underdiagnosis of hereditary breast cancer: are genetic testing guidelines a tool or an obstacle? JCO. 2018;37(6):453–60. https://doi.org/10.1200/JCO.18.01631.
Copson ER, Maishman TC, Tapper WJ, et al. Germline BRCA mutation and outcome in young-onset breast cancer (POSH): a prospective cohort study. The Lancet Oncology. 2018;19(2):169–80. https://doi.org/10.1016/S1470-2045(17)30891-4.
Stern HJ. Preimplantation genetic diagnosis: prenatal testing for embryos finally achieving its potential. J Clin Med. 2014;3(1):280–309. https://doi.org/10.3390/jcm3010280.
Ethics Committee of the American Society for Reproductive Medicine. Electronic address: ASRM@asrm.org, Ethics Committee of the American Society for Reproductive Medicine. Use of preimplantation genetic testing for monogenic defects (PGT-M) for adult-onset conditions: an ethics committee opinion. Fertil Steril. 2018;109(6):989–92. https://doi.org/10.1016/j.fertnstert.2018.04.003.
Daum H, Peretz T, Laufer N. BRCA mutations and reproduction. Fertil Steril. 2018;109(1):33–8. https://doi.org/10.1016/j.fertnstert.2017.12.004.
Liede A, Karlan BY, Narod SA. Cancer risks for male carriers of germline mutations in BRCA1 or BRCA2: a review of the literature. J Clin Oncol. 2004;22(4):735–42. https://doi.org/10.1200/JCO.2004.05.055.
Quinn GP, Pal T, Murphy D, Vadaparampil ST, Kumar A. High-risk consumers’ perceptions of preimplantation genetic diagnosis for hereditary cancers: a systematic review and meta-analysis. Genet Med. 2012;14(2):191–200. https://doi.org/10.1038/gim.0b013e31822ddc7e.
Mor P, Brennenstuhl S, Metcalfe KA. Uptake of preimplantation genetic diagnosis in female BRCA1 and BRCA2 mutation carriers. J Genet Couns. 2018;27(6):1386–94. https://doi.org/10.1007/s10897-018-0264-2.
Derks-Smeets IAP, Gietel-Habets JJG, Tibben A, et al. Decision-making on preimplantation genetic diagnosis and prenatal diagnosis: a challenge for couples with hereditary breast and ovarian cancer. Hum Reprod. 2014;29(5):1103–12. https://doi.org/10.1093/humrep/deu034.
Acknowledgements
Dr. Sella is a Goldfarb Advanced Fellow in Breast Oncology at DFCI and is also supported by The American Physicians Fellowship for Medicine in Israel and the Pinchas Burstein Talpiot Medical Leadership Program, Chaim Sheba Medical Center, Tel-Hashomer, Israel.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Tal Sella reports personal fees from Roche outside the submitted work. Ann H. Partridge declares no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Breast Cancer Genetics
Rights and permissions
About this article
Cite this article
Sella, T., Partridge, A.H. Fertility Counseling and Preservation in Breast Cancer. Curr Breast Cancer Rep 12, 1–12 (2020). https://doi.org/10.1007/s12609-019-00348-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12609-019-00348-w