Abstract
Purpose of Review
Upfront fertility counseling improves quality of life for young breast cancer patients planning for pregnancy post-treatment. We reviewed the literature on the impact, if any, that breast radiation may have on post-treatment fertility and fertility preservation decisions in order to facilitate fertility counseling.
Recent Findings
While the ovaries and uterus should not receive significant doses of radiation during breast radiation, negligible radiation doses (that are too low to induce ovarian failure) may result from internal scatter. Despite a low risk of infertility from breast radiation, data suggest that women may have chosen mastectomy to avoid whole-breast radiation due to fertility concerns. Although multiple studies have provided encouraging data with successful pregnancies after breast cancer treatment, the number of patients that underwent breast radiation and required fertility preservation methods are rarely reported.
Summary
In conclusion, the impact that breast radiation appears to have on fertility is low and, in our opinion, should not result in patients choosing mastectomy over breast conservation therapy due to fears of infertility. However, further studies specifying the patients receiving breast radiation and requiring fertility preservation methods are warranted to help reassure patients and their providers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the most common cancer in women of reproductive age [1]. While most women are diagnosed with breast cancer after menopause, approximately 2% of breast cancer cases occur in young women between 20 and 34 years of age and 11% between 35 and 44 years of age [1]. Due to current trends in women postponing marriage and childbearing in developed countries, pre-menopausal women are often still planning families at the time they are diagnosed with breast cancer in their 30s and early 40s. The high success rate of breast cancer treatments also leads to many women wishing for pregnancies post-treatment. Therefore, maintaining reproductive potential after treatment should be an important consideration for physicians involved in the care of young breast cancer patients as it has been shown to be a significant factor in their quality of life after treatment [2].
Embryo and oocyte cryopreservation are commonly used for fertility preservation in breast cancer patients and are typically performed before chemotherapy [3]. In cases where it is not feasible for young breast cancer patients undergoing chemotherapy to undergo these fertility preservation techniques, patients may opt to use goserelin GnRH agonist for ovarian suppression [4, 5].While the impact of chemotherapy on fertility preservation is outside the scope of this article, there have been conflicting results regarding goserelin GnRH with no definitive data showing that this treatment increases fertility [6,7,8,9].Especially for breast cancer patients who do not need chemotherapy, it is important for patients to understand the impact, if any, that each breast cancer treatment modality may have on their fertility. We performed a comprehensive literature review on data evaluating how breast radiation may impact fertility in pre-menopausal women. While many articles discuss the impact that chemotherapies and tamoxifen have on fertility, there is a paucity of data discussing the impact, if any, of breast radiation on fertility in pre-menopausal breast cancer patients. Much of this may be explained by the negligible dose of breast radiation reaching the reproductive organs due to the large distance between the breast and the ovaries/uterus. Nevertheless, it is important to review.
The aim of this article is to review the available literature on what is known (and identify what is unknown) regarding breast radiation therapy and post-treatment fertility. It is our hope that this review may help facilitate fertility counseling and also reassure breast cancer patients and their providers on their treatment decisions.
Importance of Fertility Counseling for Breast Cancer Patients
Chemotherapy, endocrine therapy, and radiation therapy may affect fertility to varying degrees, thus emphasizing the need for fertility counseling upfront to educate breast cancer patients and encourage appropriate decision making. Many factors should be considered during fertility counseling, including the patient’s age, adjuvant treatment recommendations, timing of planned adjuvant treatments (especially timing before the start of chemotherapy), and appropriate/available fertility preservation methods. Discussing these issues upfront and before starting treatment has been shown to be associated with less regret and better quality of life for breast cancer survivors [2]. In fact, 69% of cancer patients have reported that they were not satisfied with the fertility counseling given by their providers [10], and only 50% of breast cancer survivors recollected having any discussions on cancer treatments and infertility with their providers [11]. Therefore, early referrals to fertility specialists and multi-disciplinary discussions on cancer treatment recommendations that accommodate fertility preservation and educating the patient so that she can make informed treatment decisions are critical for maximizing a young breast cancer survivor’s opportunity for pregnancy.
Two studies conducted in Europe and the US showed that fertility discussions before treatment influenced treatment decision making in 26% and 29% of women enrolled, respectively [10, 12, 13•]. In the US study, 1% of women enrolled chose not to get chemotherapy, 3% chose not to get endocrine therapy, and 11% shortened the duration of endocrine therapy treatment due to fertility-related concerns. Interestingly, 1% of women enrolled in the study chose to undergo mastectomy rather than breast conservation therapy [10]. While this study did not specify why 1% of women chose mastectomy over breast conservation therapy, the results suggest that these women may have avoided radiation therapy after lumpectomy due to fertility concerns, further emphasizing the need for patient education. Moreover, the percentage of patients choosing mastectomy over breast conservation therapy due to fertility concerns was equivalent to the percentage of patients declining cytotoxic chemotherapy, a treatment modality known to cause ovarian toxicity in breast cancer patients. The European study did not mention the percentage of patients, if any, choosing mastectomy and opting out of breast radiation due to fertility concerns [13•]. In our opinion, a patient’s fear of infertility from breast radiation should not result in the patient undergoing mastectomy. In fact, patients who undergo lumpectomy and radiation for breast cancer can still successfully breastfeed from the affected breast after treatment (reviewed in [14]).
Mechanisms of Radiation-Induced Infertility in Cancer Patients
In the treatment of cancers other than breast, radiation can reduce fertility by inducing ovarian failure, damaging the structure and function of the uterus, or disrupting the hypothalamic-pituitary-gonadal axis. The risk of radiation-induced infertility by each of these mechanisms depends on radiation field location and trajectory, cumulative dose, and patient age.
Women undergoing radiation to the pelvis for treatment of cancers, such as cervical cancer, rectal cancer, and sarcoma, as well as those undergoing total body irradiation before bone marrow transplantation for lymphoma are at the highest risk of radiation-induced infertility due to ovarian failure and uterine damage. The most commonly known mechanism of radiation-induced infertility involves ovarian failure after radiation damage of oocyte DNA. While there is a natural decline in oocyte numbers that occurs between a woman’s birth and the time of menopause, this natural decline can be further accelerated by radiation to the pelvis, thus leading to premature menopause and subsequent infertility.
On average, a 20 Gy dose of pelvic radiation increases the risk of premature ovarian failure (POF) in women less than 35 years old; however, even lower doses of radiation can induce POF in women older than 35 years of age due to their naturally reduced oocyte reserve [15]. Moreover, Chiarelli et al. reported that radiation increases the risk of POF in a dose-dependent manner with 20–35 Gy causing a 22% risk of infertility and doses > 35 Gy leading to a 32% risk of infertility [16]. More recently, Wallace et al. showed that oocytes are even more sensitive to radiation than previously believed. They used mathematical modeling in lymphoma patients undergoing total body irradiation to estimate the LD50 of oocytes (the dose required to reduce the oocyte reserve by 50%) to be as low as < 2 Gy [17, 18].
Reducing radiation dose to the ovaries by ovarian transposition to a location outside of the radiation field is an option for patients undergoing pelvic radiation; however, the success of these procedures has been variable due to radiation scatter and the risk of remigration of the ovaries [19, 20••].
In addition to premature ovarian failure, radiation may also lead to infertility by directly damaging the structure and function of the uterus in patients undergoing pelvic radiation. Irreversible radiation damage to the musculature and vasculature of the uterus leads to endometrial dysfunction and later obstetrical complications [21], including miscarriage, pre-term labor, low birthweight, and placental abnormalities (reviewed in [22]). While the threshold radiation dose to the uterus that would prevent pregnancy is uncertain, one study reported that pregnancy has not been successful in cases receiving a whole pelvis radiation dose > 45 Gy [21]. In order to reduce the risk of obstetrical complications from these radiation-related structural and functional changes to the uterus, cancer survivors who undergo pelvic radiation are often discouraged from attempting pregnancy less than a year after pelvic radiation.
Radiation can also induce infertility in young patients undergoing brain radiation for primary and metastatic brain tumors. The hypothalamic-pituitary-gonadotropin hormone axis is very sensitive to radiation, and the degree of radiation toxicity depends on the biological effective dose (BED) of radiation, which is calculated from the total radiation dose, fraction size, and number of radiation treatments. Radiation injury to this neuroendocrine axis can decrease levels of gonadotropins, FSH and LH, thus leading to infertility and other issues in this patient population (reviewed in [23]). Koustenis et al. surveyed survivors who received radiation to the hypothalamic-pituitary-gonadotropin hormone axis and showed that survivors receiving ≥ 30 Gy vs. 18–29 Gy and 0–17 Gy to the pituitary gland reported less pregnancies and a higher frequency of permanent amenorrhea [24]. Moreover, Constine et al. estimated the radiation dose threshold to the pituitary gland that would disrupt the neuroendocrine axis to be 30 Gy [25]. Studies show that pre-menopausal women who wish to become pregnant after cranial irradiation should be followed long-term because there is often a latency period of several years before they experience radiation-related decreases in gonadotropin levels [26].
Breast Cancer Radiation and Fertility
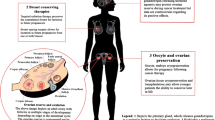
As previously discussed, the risk of radiation-induced infertility depends on radiation field location, trajectory, dose, and patient age. In cases of post-lumpectomy breast radiation or post-mastectomy radiation, the ovaries and uterus should not receive significant doses of radiation due to the typical radiation field location and trajectory. As shown in Fig. 1a, negligible doses may reach the ovaries and uterus during breast radiation through internal scatter, external scatter, and head leakage [27]. Internal scatter, however, is the main source of radiation dose to internal organs outside of the treatment field and occurs when the beam interacts with body tissue causing small amounts of radiation to be scattered to areas away from the breast. Scattered radiation typically has a much lower energy level than the initial radiation beam [27].
a Negligible doses of radiation may reach the ovaries/uterus during breast radiation through internal scatter or external scatter. Internal scatter is the main source of these negligible doses of radiation to the ovaries/uterus during breast radiation and occurs when the beam interacts with body tissue causing small amounts of radiation to be scattered to areas away from the breast, such as toward the pelvis. b A modern left breast regional nodal radiation plan to a prescribed dose of 50 Gy (5000 cGy) in 25 fractions followed by a serial boost to the lumpectomy cavity to 60 Gy. The 2 Gy (200 cGy) isodose line shown in light pink (the lowest radiation dose reported to induce ovarian failure) does not enter the abdomen from a typical regional nodal breast radiation plan. Only much lower (negligible) doses of radiation through internal scatter, if any at all, would reach the ovaries/uterus in the pelvis
Two studies have estimated the cumulative dose of radiation from internal scatter reaching the uterus during breast radiation. Mazonakis et al. estimated the radiation dose to the uterus/conceptus of a phantom that simulated each trimester of pregnancy using a breast radiation plan with tangential fields and delivering a prescription dose of 50 Gy [28]. During the first trimester of gestation, the conceptus was estimated to receive a cumulative dose of 0.021–0.076 Gy. During the second and third trimesters of pregnancy, as the uterus/conceptus expands higher into the upper abdomen and reduces the distance from breast to uterine fundus, the dose delivered to the fetus increased to 0.022–0.246 Gy and 0.022–0.586 Gy, respectively [28]. In a second study, Antypas et al. measured a 0.039 Gy cumulative radiation dose to the fetus of a 45-year-old woman who completed a prescribed dose of 46 Gy in 20 fractions of radiation therapy for her left breast cancer after realizing that she was pregnant during the second week of treatment [5]. Similar studies estimating the dose of scattering to the uterus from more modern radiation planning techniques are warranted. As shown in Fig. 1b, the 2 Gy (200 cGy) isodose line from a regional nodal breast radiation plan does not enter the abdomen, suggesting that only negligible doses of radiation, if any, reach the uterus/ovaries in the pelvis.
These studies demonstrate that the amount of radiation dose reaching the uterus appears to be much less than the 2 Gy dose previously reported to induce premature ovarian failure [15,16,17,18]; however, it is still unknown whether doses less than 2 Gy can induce premature ovarian failure in some individuals. It is important to mention that negligible doses of radiation may present a higher risk of ovarian failure in older pre-menopausal women with lower oocyte reserves. It is also important to note that, although negligible, there were detectable radiation doses from internal scatter to the uterus; therefore, patients should not undergo breast radiation therapy during pregnancy. During early embryogenesis (approximately weeks 8–15 post-conception), radiation doses of only 1 Gy have been reported to cause severe mental retardation in about 50% of cases [29].
Pregnancy After Breast Radiation
Dieci et al. recently reported that, out of 111 breast cancer patients less than 40 years old that required chemotherapy between 2014 and 2016, 64.9% used GnRH analog, 9% used GnRH analog+oocyte preservation, and 26% did not use a fertility preservation technique [13•]. Of the 26 patients who successfully became pregnant after breast cancer, 61.1% used GnRH analog for fertility preservation [13•]. Unfortunately, the number of women who received radiation was not reported in this study.
As demonstrated by the previous study, most young patients undergoing chemotherapy for their breast cancer typically choose to pursue some form of fertility preservation before chemotherapy. However, the recommendations for fertility preservation are less clear for patients with low-risk early-stage breast cancers that do not require chemotherapy before radiation therapy and endocrine therapy. An early study by Dow et al. compared treatment outcomes and quality of life among 23 breast cancer patients treated with surgery and radiation who had subsequent pregnancies compared with 23 patients without subsequent pregnancies [30]. This study showed no differences in recurrence or distant metastases between the pregnant and the non-pregnant cohorts [30]. While these results suggest that many patients are able to become successfully pregnant after breast conservation therapy without an increased risk of recurrence, it is not clear how many of these women required fertility preservation methods before pregnancy versus natural pregnancies.
Not only do many young breast cancer survivors worry about the capability of becoming pregnant after breast cancer treatment, but there is also a concern that exposure to high levels of estrogen during pregnancy may increase the risk of breast cancer recurrence, especially for estrogen receptor–positive (ER+) breast cancer. Consequently, oncologists may encourage breast cancer patients to wait at least 2 years following their diagnosis before becoming pregnant in order to allow sufficient time to take tamoxifen and ensure no recurrences. Lambertini et al. reported encouraging results that women with ER+ breast cancers at a median follow-up of 7.2 years after pregnancy showed no difference in disease-free survival or overall survival compared with ER+ breast cancer patients that did not undergo pregnancy. Moreover, the time to pregnancy and type of adjuvant therapy received (chemotherapy and/or endocrine therapy) had no impact on patients’ outcomes in the pregnant cohort. Unfortunately, there was no mention of how many patients received breast radiation therapy as part of their adjuvant breast cancer therapy in the pregnant patient cohort nor was there any data provided on how many pregnant patients required assistance from reproductive techniques (vs. natural pregnancies) [31•]. Since many women cannot wait 5–10 years to complete endocrine therapy before attempting pregnancy, the ongoing prospective POSITIVE trial (NCT02308085) is currently investigating the safety of interrupting endocrine therapy for 2 years during conception and pregnancy [32].
Summary and Conclusions
In summary, chemotherapy, endocrine therapy, and radiation therapy all affect fertility to varying degrees, therefore stressing the need for fertility counseling and multi-disciplinary discussion of care upfront before patients start treatment. Many factors need to be considered during fertility counseling, especially the patient’s age (due to lower oocyte reserves with increasing age) and adjuvant treatment recommendations (cytotoxic chemotherapies that may increase the risk of ovarian failure). While radiation can cause infertility in cancer patients undergoing radiation for cancer types other than breast, the dose reaching the ovaries and uterus from internal scatter from breast radiation is negligible (due to the large distance between the breast and ovaries/uterus) and unlikely to cause ovarian failure [5, 28]. As previously discussed, we think the low impact that breast radiation appears to have on fertility should not result in patients choosing mastectomy due to fears of infertility. In fact, lactation in the affected breast is possible after treatment with lumpectomy and breast radiation but not possible in those patients undergoing mastectomy [14].
In conclusion, many studies have demonstrated successful pregnancies after breast cancer treatment; however, the number of patients in these studies receiving adjuvant breast radiation and endocrine therapy and requiring fertility preservation methods (versus a natural pregnancy) has not been extensively reported. The results of the ongoing POSITIVE trial (NCT02308085) should not only provide a better understanding of the safety of interrupting endocrine during pregnancy but will also evaluate how many women required fertility interventions after receiving adjuvant breast radiation and endocrine therapy [32].
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
SEER Program (National Cancer Institute (U.S.)); National Center for Health Statistics (U.S.); National Cancer Institute (U.S.). Surveillance Program; National Cancer Institute (U.S.). Cancer Statistics Branch; National Cancer Institute (U.S.). Cancer Control Research Program, SEER cancer statistics review. In: NIH publication. Bethesda: U.S. Dept. of Health and Human Services, Public Health Service, National Institutes of Health, National Cancer Institute; 1993.
Letourneau JM, Ebbel EE, Katz PP, Katz A, Ai WZ, Chien AJ, et al. Pretreatment fertility counseling and fertility preservation improve quality of life in reproductive age women with cancer. Cancer. 2012;118(6):1710–7.
Rodriguez-Wallberg KA, Oktay K. Fertility preservation in women with breast cancer. Clin Obstet Gynecol. 2010;53(4):753–62.
Loren AW, Mangu PB, Beck LN, Brennan L, Magdalinski AJ, Partridge AH, et al. Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31(19):2500–10.
Antypas C, Sandilos P, Kouvaris J, Balafouta E, Karinou E, Kollaros N, et al. Fetal dose evaluation during breast cancer radiotherapy. Int J Radiat Oncol Biol Phys. 1998;40(4):995–9.
Moore HC, Unger JM, Phillips KA, Boyle F, Hitre E, Porter D, et al. Goserelin for ovarian protection during breast-cancer adjuvant chemotherapy. N Engl J Med. 2015;372(10):923–32.
Gerber B, von Minckwitz G, Stehle H, Reimer T, Felberbaum R, Maass N, et al. Effect of luteinizing hormone-releasing hormone agonist on ovarian function after modern adjuvant breast cancer chemotherapy: the GBG 37 ZORO study. J Clin Oncol. 2011;29(17):2334–41.
Elgindy EA, El-Haieg DO, Khorshid OM, Ismail EI, Abdelgawad M, Sallam HN, et al. Gonadatrophin suppression to prevent chemotherapy-induced ovarian damage: a randomized controlled trial. Obstet Gynecol. 2013;121(1):78–86.
Lambertini M, Boni L, Michelotti A, Gamucci T, Scotto T, Gori S, et al. Ovarian suppression with triptorelin during adjuvant breast cancer chemotherapy and long-term ovarian function, pregnancies, and disease-free survival: a randomized clinical trial. JAMA. 2015;314(24):2632–40.
Ruddy KJ, Gelber SI, Tamimi RM, Ginsburg ES, Schapira L, Come SE, et al. Prospective study of fertility concerns and preservation strategies in young women with breast cancer. J Clin Oncol. 2014;32(11):1151–6.
Peccatori FA, Azim HA Jr. Pregnancy in breast cancer survivors: a need for proper counseling. Breast. 2009;18(6):337–8.
Partridge AH, Gelber S, Peppercorn J, Sampson E, Knudsen K, Laufer M, et al. Web-based survey of fertility issues in young women with breast cancer. J Clin Oncol. 2004;22(20):4174–83.
• Ruggeri M, Pagan E, Bagnardi V, Bianco N, Gallerani E, Buser K, et al. Fertility concerns, preservation strategies and quality of life in young women with breast cancer: baseline results from an ongoing prospective cohort study in selected European Centers. Breast. 2019;47:85–92. Ruggeri et al. (2019) published an ongoing prospective clinical study in 2019 evaluating how fertility discussions influence treatment decisions in young breast cancer patients.
Leal SC, Stuart SR, Carvalho Hde A. Breast irradiation and lactation: a review. Expert Rev Anticancer Ther. 2013;13(2):159–64.
Lushbaugh CC, Casarett GW. The effects of gonadal irradiation in clinical radiation therapy: a review. Cancer. 1976;37(2 Suppl):1111–25.
Chiarelli AM, Marrett LD, Darlington G. Early menopause and infertility in females after treatment for childhood cancer diagnosed in 1964-1988 in Ontario, Canada. Am J Epidemiol. 1999;150(3):245–54.
Wallace WH, Thomson AB, Kelsey TW. The radiosensitivity of the human oocyte. Hum Reprod. 2003;18(1):117–21.
Wallace WH, Shalet SM, Hendry JH, Morris-Jones PH, Gattamaneni HR. Ovarian failure following abdominal irradiation in childhood: the radiosensitivity of the human oocyte. Br J Radiol. 1989;62(743):995–8.
Davis VJ. Female gamete preservation. Cancer. 2006;107(7 Suppl):1690–4.
•• Oktay K, Harvey BE, Loren AW. Fertility preservation in patients with cancer: ASCO clinical practice guideline update summary. J Oncol Pract. 2018;14(6):381–5. Oktay et al. (2018) provide guidelines and recommendations for preserving fertility in young cancer patients, including young breast cancer patients.
Teh WT, Stern C, Chander S, Hickey M. The impact of uterine radiation on subsequent fertility and pregnancy outcomes. Biomed Res Int. 2014;2014:482968.
Wo JY, Viswanathan AN. Impact of radiotherapy on fertility, pregnancy, and neonatal outcomes in female cancer patients. Int J Radiat Oncol Biol Phys. 2009;73(5):1304–12.
Vern-Gross TZ, Bradley JA, Rotondo RL, Indelicato DJ. Fertility in childhood cancer survivors following cranial irradiation for primary central nervous system and skull base tumors. Radiother Oncol. 2015;117(2):195–205.
Koustenis E, Pfitzer C, Balcerek M, Reinmuth S, Zynda A, Stromberger C, et al. Impact of cranial irradiation and brain tumor location on fertility: a survey. Klin Padiatr. 2013;225(6):320–4.
Constine LS, Woolf PD, Cann D, Mick G, McCormick K, Raubertas RF, et al. Hypothalamic-pituitary dysfunction after radiation for brain tumors. N Engl J Med. 1993;328(2):87–94.
Pai HH, Thornton A, Katznelson L, Finkelstein DM, Adams JA, Fullerton BC, et al. Hypothalamic/pituitary function following high-dose conformal radiotherapy to the base of skull: demonstration of a dose-effect relationship using dose-volume histogram analysis. Int J Radiat Oncol Biol Phys. 2001;49(4):1079–92.
Kry SF, Bednarz B, Howell RM, Dauer L, Followill D, Klein E, et al. AAPM TG 158: measurement and calculation of doses outside the treated volume from external-beam radiation therapy. Med Phys. 2017;44(10):e391–429.
Mazonakis M, Varveris H, Damilakis J, Theoharopoulos N, Gourtsoyiannis N. Radiation dose to conceptus resulting from tangential breast irradiation. Int J Radiat Oncol Biol Phys. 2003;55(2):386–91.
Streffer C. Radiation effects of exposure during prenatal development. Radiologe. 1995;35(3):141–7.
Dow KH, Harris JR, Roy C. Pregnancy after breast-conserving surgery and radiation therapy for breast cancer. J Natl Cancer Inst Monogr. 1994;16:131–7.
• Lambertini M, Kroman N, Ameye L, Cordoba O, Pinto A, Benedetti G, et al. Long-term safety of pregnancy following breast cancer according to estrogen receptor status. J Natl Cancer Inst. 2018;110(4):426–9. Lambertini et al. (2018) provide reassuring evidence that pregnancy is safe in breast cancer patients after treatment.
https://clinicaltrials.gov/ct2/show/NCT02308085. Pregnancy Outcome and Safety of Interrupting Therapy for Women With Endocrine Responsive Breast Cancer (POSITIVE). Accessed 18 July 2020.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Fertility Issues and Breast Cancer
Rights and permissions
About this article
Cite this article
Beyer, S., Sandu, A. & White, J. Impact and Timing of Breast Cancer Radiation Therapy and Fertility Preservation. Curr Breast Cancer Rep 12, 375–380 (2020). https://doi.org/10.1007/s12609-020-00394-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12609-020-00394-9