Abstract
Background
The impact of chemotherapy timing on the fertility preservation (FP) decision is poorly understood. Here we evaluate factors associated with FP completion among women age ≤ 45 years with breast cancer who received chemotherapy and consulted with a reproductive endocrinology and infertility (REI) specialist, and report pregnancy and oncologic outcomes.
Patients and Methods
This retrospective review included all women age ≤ 45 years diagnosed with stage I–III unilateral breast cancer at Memorial Sloan Kettering Cancer Center between 2009 and 2015 who received chemotherapy and consulted with an REI specialist. Clinicopathologic features and factors associated with the decision to undergo FP were analyzed, and comparisons were made with the Wilcoxon rank-sum test, Chi-square test, or Fisher’s exact test. Survival curves were constructed using the Kaplan–Meier method.
Results
Among the 172 women identified, median age was 34 years (interquartile range 31–37 years). The majority of women were single (n = 99, 57.6%) and nulliparous (n = 134, 77.9%). Most women underwent FP (n = 121, 70.3%). Factors associated with the decision to undergo FP included younger median age (33 vs. 37 years, p < 0.001), having private insurance (p < 0.001), nulliparity (p < 0.001), and referral from Breast Surgery (p = 0.004). Tumor characteristics and treatments were similar between women who underwent FP and those who declined. Overall survival and recurrence-free survival were also similar between groups. Women who underwent FP were more likely to have a biological child after breast cancer treatment.
Conclusions
Women underwent FP at high rates independent of timing of chemotherapy and oncologic factors. FP is associated with having a biological child and does not compromise oncologic outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Although breast cancer is rare among young women, over 20,000 women age < 45 years are diagnosed annually, accounting for 12.5% of incident cases.1 Systemic therapy advances have translated into dramatic improvements in survival; as a result, providers are able to focus more attention on optimizing quality of life and survivorship, including fertility concerns.2
As women increasingly delay childbearing for personal and professional reasons, it is likely that many women will be diagnosed with breast cancer prior to completion of childbearing.3 For these women, breast cancer treatment-related infertility is associated with significant anxiety, emotional stress, and decreased quality of life.4 Breast cancer treatment affects fertility though direct toxicity to the ovary, and by imposing temporal delays to pregnancy to prevent pregnancy while they are at highest risk of recurrence. Many young women receive cytotoxic chemotherapy as an integral component of multimodality breast cancer treatment, and pregnancy rates remain low in the absence of assisted reproductive technology.5,6
Ovarian stimulation with oocyte/embryo cryopreservation is a highly efficacious option for fertility preservation (FP); correspondingly, the American Society of Clinical Oncology recommends early referral of young women with breast cancer to reproductive endocrinology and infertility (REI) specialists.7 Despite these recommendations, concerns regarding delays to systemic therapy, as well as potential deleterious effects of ovarian stimulation on oncologic outcomes, persist among both patients and providers, especially in the neoadjuvant setting, where the tumor remains in situ and is exposed to markedly increased levels of serum estradiol during FP.
A previous study from Memorial Sloan Kettering Cancer Center (MSKCC) showed that neoadjuvant chemotherapy (NAC) receipt was an independent predictor of declining REI referral.8 Additionally, some data suggest that NAC receipt is associated with lower FP rates among women who consult with an REI specialist, indicating that NAC may decrease the likelihood of choosing to undergo FP at multiple phases.9 Although some data suggest that ovarian stimulation results in neither delays to chemotherapy initiation nor compromised oncologic outcomes in the adjuvant setting, there is a paucity of data evaluating the oncologic safety of ovarian stimulation in the neoadjuvant setting.10,11 This study evaluates factors associated with FP completion among women age ≤ 45 years with breast cancer who received chemotherapy and consulted with an REI specialist, and reports their subsequent pregnancy and oncologic outcomes.
Patients and Methods
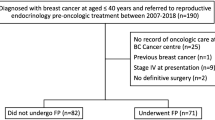
This institutional review board-approved retrospective review of a prospectively maintained breast cancer database identified all women with stage I–III breast cancer between 2009 and 2015 who consulted with an REI specialist after meeting with a fertility nurse specialist from the Cancer and Fertility Program at MSKCC. Clinicopathologic features, FP procedures, and pregnancy and oncologic outcomes were recorded. Women who underwent FP were compared with those who consulted with an REI specialist and declined FP. Women with bilateral cancers and those who did not receive chemotherapy were excluded. Timely initiation of systemic therapy was defined as 6 weeks from time of diagnosis for NAC and 12 weeks from time of surgery for adjuvant chemotherapy.
Statistical Methods
All statistical analyses were conducted in R software version 3.6.3 (R Core Development Team, Vienna, Austria). Continuous characteristics were summarized by median and interquartile range (IQR), whereas categorical characteristics were summarized by frequency and percentages. Comparisons between those women who underwent FP and those who declined were made with the Wilcoxon rank-sum test for continuous variables, and the chi-square test or Fisher’s exact test, as appropriate, for categorical variables. A p value ≤ 0.05 was considered statistically significant.
Overall survival (OS) was computed as time from histologic diagnosis to date of death or last known follow-up. Recurrence was defined as any locoregional or distant recurrence. Recurrence-free survival (RFS) was calculated from time of histologic diagnosis to date of clinically diagnosed recurrence or last known follow-up. Survival curves were constructed using the Kaplan–Meier method.
MSKCC Cancer and Fertility Program
The MSKCC Cancer and Fertility Program was established in 2009 utilizing fertility nurse specialists (FNSs) who provide comprehensive education to men and women with cancer about treatment-related fertility risks as well as fertility-preservation and family-building options. Additionally, FNSs facilitate REI referrals. Patients are referred to the FNS program from breast surgery, medical oncology, and medical genetics.
Results
Clinicopathologic Features
A total of 172 (49%) of 349 women consulted with an REI specialist after meeting with an FNS. Patient demographics are detailed in Table 1. Median age was 34 years (IQR 31–37 years). Most women were Caucasian (n = 112, 65.1%) and had private insurance (n = 153, 89.0%). The majority of women were single (n = 99, 57.6%) and nulliparous (n = 134, 77.9%).
Stage II was the most common stage at diagnosis (n = 90, 52.3%). Most tumors were invasive ductal carcinoma (n = 168, 97.7%), high grade (n = 144, 83.7%), and estrogen receptor (ER)/progesterone receptor (PR) positive and human epidermal growth factor receptor 2 (HER2) negative (n = 95, 55.2%). HER2 overexpressing tumors (n = 41, 23.8%) and triple-negative tumors (n = 36, 20.9%) were less common.
The most common surgical approach was mastectomy (n = 115, 66.9%). Of women treated with mastectomy, 46.5% (n = 80) elected to undergo contralateral prophylactic mastectomy for risk reduction. All patients underwent axillary staging, most with sentinel lymph node biopsy alone (n = 106, 61.6%). Most also underwent radiation therapy (RT) (n = 113, 65.7%). Three patients who underwent lumpectomy subsequently declined adjuvant radiation therapy. All women received cytotoxic chemotherapy; most received adjuvant chemotherapy (n = 147, 85.5%), whereas a neoadjuvant approach was less common (n = 25, 14.5%). Most women received dose-dense doxorubicin and cyclophosphamide followed by paclitaxel (n = 126, 73.3%). Endocrine therapy receipt was common (n = 133, 77.3% overall and 129 of 130, 99.2%, with hormone receptor (HR) positive tumors). One patient with an HR positive tumor declined endocrine therapy.
Factors Associated with Fertility Preservation
A total of 121 (70.3%) women underwent FP. Patients who underwent FP were younger than those who declined (33 vs. 37 years, p < 0.001). A comparison of women who underwent FP with those who declined is presented in Table 1. Race was associated with decision to pursue FP; while the majority of White women (n = 86, 76.8%) completed FP, most Black women declined (n = 10, 58.9%, p = 0.015). Insurance type was also associated with decision to undergo FP, with 74.5% (n = 114) of women with private insurance completing FP compared with 26.7% (n = 4) of women with government insurance (p < 0.001).
Although relationship status was not associated with decision to undergo FP, nulligravid status and nulliparity were both strongly associated with FP completion (p = 0.004 and p < 0.001, respectively). Additionally, referral from the MSKCC Breast Surgery service to the MSKCC Cancer and Fertility Program was associated with completing FP (p = 0.004). Tumor and treatment factors, including timing of chemotherapy and stage, were not associated with undergoing FP.
On multivariate analysis, younger age (p = 0.003), nulliparity (p = 0.001), referral from Breast Surgery (p = 0.009), and private insurance (p < 0.001) remained associated with FP completion.
Impact of FP on Time to Systemic Therapy
FP was not associated with breast cancer treatment delays. In the adjuvant chemotherapy setting, FP was performed between surgery and the start of adjuvant chemotherapy, with a median interval of 7 weeks both for women who underwent FP and for those who declined (p = 0.9). Delays past 12 weeks were uncommon in both groups. (FP, n = 3, 2.9% vs. declined FP, n = 2, 4.7%, p = 0.63).
In the NAC setting, a median interval of 3 weeks (range 1–8) between time of diagnosis and start of NAC was noted for women who underwent FP (before NAC start) and those who declined (p = 0.29). Delays past 6 weeks were uncommon regardless of whether FP was performed (FP, n = 2, 11.8% vs. declined FP, n = 1, 12.5%, p > 0.99).
Factors Associated with Pregnancy After Breast Cancer Treatment
A total of 25.6% (n = 44) of women in the study had a biological child following breast cancer treatment, including 20.3% (n = 35) who had a successful pregnancy themselves, and 5.2% (n = 9) who used surrogates with a median follow-up of 70 months (range 4–127 months). A comparison of women who had a biological child after breast cancer treatment and those who did not is presented in Table 2. Women who underwent FP were more likely to have a biological child (p = 0.001); 91% (n = 40) of women who had a biological child after breast cancer treatment had undergone FP. Race and insurance type were also associated with a biological child, with White women and women with private insurance being most likely to have a biological child (p = 0.029 and p = 0.020, respectively).
Married women (n = 30, 41.1%) were more likely to have a biological child compared with unmarried women (n = 14, 14.1%, p < 0.001). Multivariate analysis revealed that undergoing FP, being married, and being Caucasian were associated with having a biological child.
Oncologic Outcomes
OS and RFS Kaplan–Meier curves are shown in Fig. 1. The 5-year OS was 97.5% (95% confidence interval [CI] 93.5–99.1), and the 5-year RFS was 91.4% (95% CI 85.9–94.8). There was no difference in RFS between women who underwent FP (92.1%, 95% CI 85.4–95.8) and those who declined (89.7%, 95% CI 76.9–95.6, p = 0.43). Disease stage, race, pregnancy, and insurance type were not associated with RFS. Among tumor factors, lymphovascular invasion presence was associated with increased recurrence risk (hazard ratio 3.69, 95% CI 1.21–11.2, p = 0.022) (Table 3). Among treatment factors, RT receipt was associated with lower risk of recurrence (hazard ratio 0.31, 95% CI 0.12–0.80, p = 0.015).
OS was similar between women who underwent FP (98.2%, 95% CI 92.9–99.5) and those who declined (95.9%, 95% CI 84.6–98.9, p = 0.21). While disease stage, race, and pregnancy were not associated with OS, private insurance (hazard ratio 0.06, 95% CI 0.01–0.36, p = 0.002) and RT were associated with improved OS (hazard ratio 0.14, 95% CI 0.02–0.82, p = 0.029) (Table 4).
Discussion
Among women who met with an REI, 70% underwent FP, and of the 121 women who underwent FP, 40 (33.3%) had a biological child. Although women who completed FP tended to be younger than those who declined, no tumor or treatment factors were associated with the decision to undergo FP, including disease stage or timing of chemotherapy.
A previous study from MSKCC showed that, among premenopausal women, NAC receipt was an independent predictor of declining REI referral.8 The present study finds that women with invasive breast cancer who require chemotherapy as part of oncologic treatment and choose to consult with an REI specialist are likely to proceed with FP independent of whether an adjuvant or neoadjuvant approach is recommended. This differs from the findings reported by Kim et al., who found that NAC was an independent negative predictor of completing ovarian stimulation following REI consultation among 185 women with breast cancer who consulted with an REI specialist between 2005 and 2010.9 They found that, while 58.4% of women underwent FP, only 1/19 (5.3%) patients who received NAC completed FP. Receipt of NAC remained significantly associated with declining FP after controlling for cancer stage and other predictive factors from their univariate analysis; however, with women with stage III disease accounting for only 9% (n = 16) of their study population, it is difficult to draw definitive conclusions.
It is important to note that all women in this study met with an FNS in the MSKCC Cancer and Fertility Program prior to REI consultation, and that this may have resulted in improved selection of women who were likely to complete FP following REI consultation. Additionally, changes in opinions regarding oncologic safety of FP in the NAC setting among both providers and patients, as well as the evolving indications for NAC, may have contributed to the increased rate of pursuit of FP among women receiving NAC in the present study. Finally, because the study period spanned 2009–2015, predating the publication of multiple practice-changing studies that increased indications for NAC among women with operable breast cancers, only a small percentage of women received NAC (n = 25, 14.5%) in this study. These small numbers limit the ability to draw definitive conclusions regarding the relationship between NAC and FP.
Concerns regarding potential delays to initiation of systemic therapy, which could translate to worse oncologic outcomes, are shared among patients and providers, and may lead to reluctance to pursue FP. Indeed, traditional ovarian stimulation protocols were timed with the follicular phase of the menstrual cycle, which could result in women requiring 6 weeks to complete an FP cycle.12 Random-start protocols have eliminated the need to synchronize the stimulation with the natural menstrual cycle, and can allow women to complete an FP cycle within 2 weeks.13
Optimal timing for initiation of systemic therapy, in both the adjuvant and neoadjuvant settings, remains elusive. While deleterious effects on survival are not observed when chemotherapy is initiated within 3 months of definitive surgery, more recent series have suggested that receptor profiles and tumor stage may modulate the impact of systemic therapy delays on oncologic outcomes.14,15,16 In the present study, median time to adjuvant chemotherapy was 7 weeks, with < 5% of women experiencing delays over 12 weeks. FP was not associated with an increased time interval to chemotherapy or likelihood for experiencing a delay over 12 weeks in the adjuvant setting.
Similarly, FP did not have a deleterious effect on the interval between diagnosis and initiation of NAC. The median time to NAC was 3 weeks both among women who underwent FP and those who declined. The optimal window for initiation of NAC remains poorly defined, with recent studies demonstrating mean time intervals of approximately 6 weeks.17,18 The complexity of diagnostic workups and the need for consultations with multiple specialists have been cited as potential causes for this protracted time course. Our previous study showed similar time to treatment between women who consulted with REI and those who declined consultation.8 The present study’s data parallel those of other recent studies that have shown that completion of FP is not associated with delays to initiation of NAC, though it is important to know that these studies may be underpowered to detect small differences in time to treatment.17,18
Breast surgical oncologists are often the first doctors who women with breast cancer meet, and are uniquely positioned to facilitate timely referrals for FP in both the adjuvant and neoadjuvant settings. While a previous study from MSKCC found that referral from the Breast Surgery service was associated with acceptance of REI referral after FNS consultation,8 this study demonstrates that referral from Breast Surgery is also an independent predictor of FP completion, highlighting the important role that breast surgical oncologists play in the education and timely referral of women with breast cancer who are interested in FP. It is also important to note that patients referred to REI by surgeons may be those who have the most interest in FP, while others may be referred by other providers after further discussion.
Both OS (97.5% at 5 years) and RFS (91.4% at 5 years) were excellent in the present study, confirming the importance of addressing quality-of-life and survivorship concerns. Although studies reporting survival outcomes following FP are limited, existing studies have failed to show a difference in RFS or OS between patients who underwent FP and those who declined.11,17,18,19,20 The finding of similar RFS and OS after 70 months median follow-up between women who underwent FP and those who declined is consistent with those studies and adds to the growing body of literature on oncologic outcomes following FP. It is important to note that survival was not associated with disease stage, suggesting that the survival analyses may be underpowered.
Although FP was not associated with worse RFS or OS, private insurance emerged as an independent predictor of improved OS. Private insurance was also an independent predictor of undergoing FP, underscoring how strongly socioeconomic factors can affect healthcare decisions and outcomes. While race was not associated with survival outcomes, it was associated with the decision to pursue FP on univariate analysis, with 76.8% of White women choosing to complete FP versus only 41.2% of Black women. Black women made up only 9.9% of the study population; given the retrospective nature of this study, we are unable to determine whether Black women received fewer referrals to the FNS program or whether they declined referral. Additionally, while race was not a predictor of pursuing FP in their multicenter study, Kim et al. demonstrated that higher income was associated with FP pursuit, findings not unexpected given that ovarian stimulation can be cost-prohibitive in the absence of insurance coverage.9
Age 45 years was selected as the upper bound of inclusion criteria on the basis of institutional data demonstrating that < 0.5% of women age > 45 years with breast cancer pursue FP. Young age and nulliparity were both independent predictors of FP completion, consistent with Kim et al., who found that age and parity were lower among women who underwent FP.9 FP, in turn, was strongly associated with having a biological child after breast cancer treatment. Race and insurance type were also associated with having a biological child after treatment, with White women and women with private insurance being more likely to have a biological child. Multivariate analysis revealed that undergoing FP, being married, and being White were independent predictors of having a biological child. These findings identify FP as a possible area of racial disparity within breast cancer care and confirm the importance of socioeconomic factors in determining fertility and pregnancy outcomes. Additionally, they serve as a reminder of the complex interplay in breast cancer treatment between socioeconomic factors, reproductive choices, and pregnancy outcomes. Counseling regarding reproductive goals, and barriers to achieving these goals, should be reviewed with women during survivorship.
Limitations to the present study include its retrospective nature, as well as the uniformity of the study population, which comprised mostly White women with private insurance. Additionally, the education and counseling from the FNS consultation may have led to improved selection of women who met with an REI specialist and increased the rate of uptake of FP, thereby potentially limiting the generalizability of this study. Additionally, MSKCC is a tertiary-care cancer center without an REI department; as such, all REI consultations and FP procedures are performed at outside institutions, and specific treatment details regarding FP cycles or number of ova harvested were not available for review. Similarly, specific details regarding pregnancies and maternal–fetal outcomes were not available for review.
Despite these limitations, this study indicates that NAC receipt does not adversely affect the FP rate among premenopausal women with breast cancer. With FP rates approaching 70% of the study cohort in both the neoadjuvant and adjuvant settings, referral to REI specialists appears to be the rate-limiting step in completion of FP. Moreover, FP was associated with having a biological child after cancer treatment and had no deleterious effect on DFS or OS. These data add to the growing evidence that FP does not delay time to systemic therapy or compromise oncologic outcomes among young women with breast cancer. Additional studies are planned to evaluate patient decision-making regarding uptake of FP options and identify barriers to FP, as well as to prospectively evaluate fertility and pregnancy outcomes.
References
Trivers KF, Fink AK, Partridge AH, et al. Estimates of young breast cancer survivors at risk for infertility in the U.S. Oncologist. 2014;19(8):814–22.
Murphy BL, Day CN, Hoskin TL, Habermann EB, Boughey JC. Adolescents and young adults with breast cancer have more aggressive disease and treatment than patients in their forties. Ann Surg Oncol. 2019;26(12):3920–30. https://doi.org/10.1245/s10434-019-07653-9.
Mathews T, Hamilton B. Mean age of mothers is on the rise: United States, 2000–2013. NCHS Data Br. 2016;232:1–8.
Howard-Anderson J, Ganz PA, Bower JE, Stanton AL. Quality of life, fertility concerns, and behavioral health outcomes in younger breast cancer survivors: a systematic review. J Natl Cancer Inst. 2012;104(5):386–405.
Moore HC, Unger JM, Phillips KA, et al. Goserelin for ovarian protection during breast-cancer adjuvant chemotherapy. N Engl J Med. 2015;372(10):923–32.
Lambertini M, Boni L, Michelotti A, et al. Ovarian suppression with triptorelin during adjuvant breast cancer chemotherapy and long-term ovarian function, pregnancies, and disease-free survival: a randomized clinical trial. JAMA. 2015;314(24):2632–40.
Loren AW, Mangu PB, Beck LN, et al. Fertility preservation for patients with cancer: American society of clinical oncology clinical practice guideline update. J Clin Oncol. 2013;31(19):2500–10.
Crown A, Muhsen S, Zabor EC, et al. Does use of neoadjuvant chemotherapy affect the decision to pursue fertility preservation options in young women with breast cancer? Ann Surg Oncol. 2020;27(12):4740–9. https://doi.org/10.1245/s10434-020-08883-y.
Kim J, Oktay K, Gracia C, Lee S, Morse C, Mersereau JE. Which patients pursue fertility preservation treatments? A multicenter analysis of the predictors of fertility preservation in women with breast cancer. Fertil Steril. 2012;97(3):671–6.
Azim AA, Costantini-Ferrando M, Oktay K. Safety of fertility preservation by ovarian stimulation with letrozole and gonadotropins in patients with breast cancer: a prospective controlled study. J Clin Oncol. 2008;26(16):2630–5.
Kim J, Turan V, Oktay K. Long-term safety of letrozole and gonadotropin stimulation for fertility preservation in women with breast cancer. J Clin Endocrinol Metab. 2016;101(4):1364–71.
Cavagna F, Pontes A, Cavagna M, et al. Specific protocols of controlled ovarian stimulation for oocyte cryopreservation in breast cancer patients. Curr Oncol. 2018;25(6):e527–32.
Cakmak H, Rosen MP. Random-start ovarian stimulation in patients with cancer. Curr Opin Obstet Gynecol. 2015;27(3):215–21.
Jara Sanchez C, Ruiz A, Martin M, et al. Influence of timing of initiation of adjuvant chemotherapy over survival in breast cancer: a negative outcome study by the Spanish Breast Cancer Research Group (GEICAM). Breast Cancer Res Treat. 2007;101(2):215–23.
Gagliato Dde M, Gonzalez-Angulo AM, Lei X, et al. Clinical impact of delaying initiation of adjuvant chemotherapy in patients with breast cancer. J Clin Oncol. 2014;32(8):735–44.
Chavez-MacGregor M, Clarke CA, Lichtensztajn DY, Giordano SH. Delayed initiation of adjuvant chemotherapy among patients with breast cancer. JAMA Oncol. 2016;2(3):322–9.
Letourneau JM, Wald K, Sinha N, et al. Fertility preservation before breast cancer treatment appears unlikely to affect disease-free survival at a median follow-up of 43 months after fertility-preservation consultation. Cancer. 2020;126(3):487–95.
Chien AJ, Chambers J, McAuley F, et al. Fertility preservation with ovarian stimulation and time to treatment in women with stage II-III breast cancer receiving neoadjuvant therapy. Breast Cancer Res Treat. 2017;165(1):151–9.
Oktay K, Buyuk E, Libertella N, Akar M, Rosenwaks Z. Fertility preservation in breast cancer patients: a prospective controlled comparison of ovarian stimulation with tamoxifen and letrozole for embryo cryopreservation. J Clin Oncol. 2005;23(19):4347–53.
Moravek MB, Confino R, Smith KN, et al. Long-term outcomes in cancer patients who did or did not pursue fertility preservation. Fertil Steril. 2018;109(2):349–55.
Acknowledgment
The preparation of this study was supported in part by NIH/NCI Cancer Center Support Grant P30CA008748 to Memorial Sloan Kettering Cancer Center, and this study was presented in poster format at the 43rd Annual San Antonio Breast Cancer Symposium Virtual Meeting, December 8–11, 2020. Dr. Shari B. Goldfarb reports research funding from Sprout Pharmaceuticals and Paxman Coolers Ltd, and consulting/medical advisory positions with Sermonix Pharmaceuticals, Procter and Gamble, NanOlogy LLC, and Ms. Medicine LLC.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All other authors have no conflict of interests or commercial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Crown, A., Muhsen, S., Sevilimedu, V. et al. Fertility Preservation in Young Women with Breast Cancer: Impact on Treatment and Outcomes. Ann Surg Oncol 29, 5786–5796 (2022). https://doi.org/10.1245/s10434-022-11910-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-022-11910-9