Abstract
The evidence available for risk reduction of lymphedema after breast cancer treatment is sparse and inconsistent. It is limited by confounding factors such as axillary disease burden, number of lymph nodes harvested, and radiation treatment. However, there are several strategies for prevention and risk reduction prior to the onset of lymphedema. Techniques such as sentinel lymph node biopsy, axillary reverse mapping, lymphatic anastomosis, and lymphovenular anastomosis are aimed at preventing or minimizing the disruption of lymphatic flow from the upper extremity. Few surgical procedures, such as the historical Charles procedure, as well as newer techniques including distal lymphaticovenular anastomosis, lymph node transfer, suction-assisted protein lipectomy, and low-level laser therapy exist. Nonsurgical treatments include complete decongestive therapy, pneumatic compression, Kinesio tape, and exercise. These have varying degrees of effectiveness but have limitations in patient compliance or availability of certified therapists.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to the World Health Organization, as many as 170 million people worldwide and 3–5 million people in the USA suffer from secondary lymphedema. Current rates of lymphedema in patients being treated for breast cancer depend not only on what surgery was performed [∼16–25 % following axillary lymph node dissection (ALND) versus ∼5–8 % following sentinel lymph node biopsy (SLNB)] but also variations with the surgeon, which emphasizes the fact that ALND and even SLNB are not standardized (Table 1) [1–13]. While ALND clearly has a two- to three-fold greater rate of lymphedema as SLNB, three times as many patients undergo SLNB as ALND. Thus, it is important to find strategies to improve surgical lymphadenectomy and reduce lymphedema, especially when lymphedema results from a negative SLNB. Lymphedema represents one of the most feared complications with associated psychological distress ranging from 17 to 50 % [14, 15] and is an independent predictor of decreased quality of life [16], yet lymphedema is one of the most under-recognized, miscomprehended, relatively unassessed, and under-researched complications of breast cancer (http://www.cancer.gov/cancertopics/pdq/supportivecare/lymphedema/Patient/page1).
Because as many and varied procedures have failed to resolve lymphedema, new strategies for preventing its development are needed. The procedures currently used for breast cancer staging involve surgical removal of some (SLNB) or all (ALND) lymph nodes in the axilla below the vein from the serratus anterior to the latissimus dorsi and posteriorly to the teres major. Surgery can disrupt the drainage of arm lymphatics coursing through the axilla, leading to lymphedema. When radiation and chemotherapy are added, further damage to the lymphatics is incurred.
Lymphedema (elephantiasis chirurgical) occurs when regional lymphatic flow is insufficient and the lymphatic system cannot perform its role of maintaining tissue fluid homeostasis. This condition causes variable amounts of swelling of the extremity as a result of accumulation of protein-rich interstitial fluid, and chronic lymphedema and chronic infections lead to fibrosis over time [17]. Symptoms can be mild (reversible), moderate (a heavy limb that impairs physical activity and interferes with clothing), or severe (accompanied by extreme fibrosis, massive girth, and skin/tissue alterations). Secondary-acquired lymphedema can be caused by infection, solid tumors, lymphoma, radiation, or chronic venous insufficiency, but the most common cause of lymphedema is surgical intervention for cancer during which lymphatic tissue draining the arm is damaged or removed.
Anatomy of the Lymphatic System of the Axilla
Until recently, an understanding of lymphatic drainage of the arm was based on extensive lymphatic mapping that included only the arm but not the axilla. Foldi and others have carefully catalogued the drainage of various parts of the arm, but a lymph node identified in the axilla is categorized by its position or level in the axillary bed, not by its source of drainage [17]. The upper extremity itself has most of its drainage coming into the upper inner volar surface of the arm, with some draining posteriorly, even bypassing the axilla and draining directly into the subclavian vein more medially [17]. Recent reports have characterized lymphatic routes draining the arm through the axilla [18–20] (Fig. 1). About one third of the routes are seen low enough in the axilla as it can be seen from a SLNB incision, and about 8 % is separate but juxtaposed to the sentinel lymph node (SLN) itself. About two thirds of lymphatics can be seen from a typical ALND incision [19•]. This has led to techniques to identify, preserve, and/or reanastomose/reapproximate lymphatics draining the arm resulting in dramatic decreases in lymphedema in a short-term follow-up for SLN as well as ALND [20].
Anatomical variations of the lymphatics as they course through the axilla. 1 The pattern hugging the vein that was thought to be the usual route of arm lymphatics. 2 The sling pattern that is commonly seen even during SLNB. 3, 4 A lateral and medial apron pattern. 5 A twine pattern made up of multiple lymphatics and small nodes. This can also be a chain of nodes that runs across the axilla in harm’s way
Prevention and Risk Reduction Procedures
The evidence-based methods of risk reduction for breast cancer-related lymphedema are scant and contradictory, and most studies in the area are limited by methodological problems such as small sample size or retrospective design. Existing studies of lymphedema are complicated by inconsistent relationships in a number of personal, disease, and treatment-related risk factors (Table 1) that include numbers of positive lymph nodes removed, postoperative infection, radiation to the axilla, younger age at diagnosis, history of hypertension, body mass index of >30 kg/m2, and length of follow-up.
SLN Biopsy: Reducing Lymphedema Resulting from Surgical Resection of Breast Cancer
While breast cancer outcomes heavily rely on thoroughly evaluating axillary lymph nodes, the procedure can result in lymphedema and other morbidities (Table 1) [14, 15, 21–24]. Until the recent release of new guidelines by the American Society of Clinical Oncology (ASCO) and National Comprehensive Cancer Network (NCCN), ALND was the recommended procedure for the approximately 30 % of women with histologically positive lymph nodes (clinically positive or via SLNB), and ∼16–25 % of these women developed lymphedema. SLNB alone is an option for women with negative SLNB and recently advocated for some women with positive nodes (those undergoing lumpectomy and whole breast radiation with one or two positive SLNs) and results in lymphedema in only 5–8 % of cases (Table 1). This is a fraction of the incidence resulting from ALND, but because so many more women undergo SLNB (∼70 %), the number of women who have developed lymphedema after a negative SLNB approximates those who have developed the condition after ALND. In combining outcomes from both ALND and SLNB, there are an estimated 11,000 new cases of lymphedema per year in the USA. Furthermore, lymphedema is considered to be underreported because postsurgical arm volumes are not routinely measured, and swelling is usually not clinically detectable unless ≥200 mL of fluid has been retained in the extremity [25]. Further, the presentation can be latent and treatment delays are common, often resulting in a chronic debilitating disease from an otherwise successful treatment of the breast cancer. New approaches are needed to prevent this significant morbidity and improve survivorship.
In a purely anatomical dissection that has changed little over the last several decades, ALND includes the level I and II nodes with level III nodes removed only for grossly palpable disease. ALND does not take into account the lymphatic drainage from the breast versus that of the arm because drainage from the arm into the axilla was only recently described by our group [18–20]. Thus, lymphedema from surgical treatment is most likely caused by a disruption of lymph vessels from the arm that course through the axilla [19•]. Reports of lymphedema rates in patients undergoing ALND vary, depending on how closely lymphedema was monitored, length of follow-up [26], number of positive lymph nodes [27], use of postoperative irradiation [28], extent of surgery, body habitus, and a number of other patient characteristic [29]. SLNB for breast cancer was first described by Krag and colleagues to prevent the high morbidity seen with ALND. Multiple comparison studies, several of which were randomized, have confirmed that SLNB consistently has lower morbidity and lymphedema rates than ALND (Table 1). Several cooperative group trials have shown lymphedema rates of approximately 5–8 % with SLNB alone, but reports vary from 0 to 13 % (Table 1). The variability in reported lymphedema rates again emphasizes the variability in surgical technique as well as detection, emphasizing the need to further define and protect the anatomy of lymphatic drainage from the arm within the axilla.
Axillary Reverse Mapping
Axillary reverse mapping (ARM) is a surgical technique which is available to assist in reducing the risk of lymphedema [18–20]. ARM defines the “functional” anatomy of the axilla, and in so doing, a methodology (ARM) to be used during SLNB and/or ALND may not only predict lymphedema risk but also prevent lymphedema from developing (Fig. 1). This technique as initially described uses a technetium sulfur colloid (∼4 mL) injected in the subareolar location and ∼5 mL of isosulfan blue dye injected subcutaneously in the ipsilateral upper volar surface of the upper extremity. The use of “split” injections is crucial in identifying crossover between arm and breast lymphatic drainage as well as juxtaposition of blue ARM nodes to a SLN. This technique can be applied in both SLNB and ALND to allow for visualization of lymphatics draining the upper arm which may otherwise not be spared (Fig. 2). Its use has been shown in a short-term follow-up (20 months) to significantly reduce the incidence of lymphedema from SLNB or ALND to 2 % overall [30].
Lymphatic Anastomosis
Lymphatic anastomosis is another technique that is being used in the prevention and treatment of lymphedema. As a preventative measure, the transected ends of a lymphatic channel are anastomosed or reapproximated at the time of lymphadenectomy during SLNB or ALND (Fig. 3). This technique is based on experiments conducted by Guthrie and Gagnon in 1946 where transection of the hind limb lymphatics caused lymphedema, but when simply transected and then sutured back together, new lymphatics were subsequently seen to cross the suture line without the occurrence of lymphedema [31]. This technique is an area of ongoing research, but in a short-term follow-up (20 months), reanastomosis has been associated with no cases of lymphedema in the first 80 patients with known lymphatic transection [30].
Lymphovenular Anastomosis
Lymphatic microsurgical preventing healing approach (LYMPHA) is a procedure involving anastomosis of arm lymphatics to a collateral branch of the axillary vein. This technique has demonstrated patency of anastomoses postoperatively and reduced lymphedema rates in patients undergoing axillary lymph node dissection. One potential limitation of this approach arises in the setting of high venous pressures proximally. Results also suggest that the learning curve may play a role in outcomes. Still, this technique appears to have promising results in select populations with reduced lymphedema rates with up to 4 years of follow-up [32].
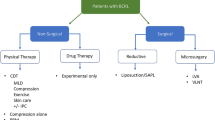
Treatment
Surgical
Charles Procedure
Historically, the Charles procedure was one of the first techniques developed in the treatment of lymphedema. Described in 1912, it entails radical resection of skin and subcutaneous tissue down to the level of fascia with skin grafts then used for tissue coverage. Throughout the years, this technique has undergone multiple variations but has fallen out of favor due to concerns over cosmesis as well as its associated morbidity and risk of complications. The Charles procedure has recently resurfaced and is again being used in extreme cases of severely limiting lymphedema where other methods of treatment have failed. Recent modifications on the approach now entails the addition of liposuction techniques or a staged approach and are showing encouraging results for these extreme cases [33].
Distal Lymphaticovenular Anastomosis
Distal lymphaticovenular anastomosis through the use of a microscope is a technique for the treatment of lymphedema which may be used in recent-onset lymphedema [34•]. This approach must be employed prior to the onset of fibrosis earlier in the clinical course of lymphedema to have the greatest opportunity for success. It may as well be used in combination with other methods, such as the use of compression postoperatively to increase lymphatic return and subsequently reduce the resultant lymphedema.
Lymph Node Transfer
Lymph node transfer involves the use of lymph nodes harvested from various donor sites for the treatment of postmastectomy lymphedema [35]. Results using this technique have varied but have seen improved outcomes when applied to early-stage lymphedema. Although no clear consensus has been reached, some variations include donor sites from inguinal lymphatic basins to wrist, forearm, and axillary recipient sites [36]. This approach as well seems to be better suited for the treatment of lymphedema while in its earlier stages. Risks include lymphedema to areas draining from the donor site.
Suction-Assisted Protein Lipectomy
Suction-assisted protein lipectomy is a specialized type of liposuction. In contrast to other surgical treatments of lymphedema that target the fluid component, it targets the solid component of chronic non-pitting edema. The proteinaceous subcutaneous tissue is suctioned out of the edematous limb using liposuction cannulas. This technique should be used in conjunction with postoperative compression garments and other surgical approaches which aim to address the pathophysiology of lymphedema [37].
Low-Level Laser Therapy
Low-level laser therapy is a therapeutic option that is designed to promote lymphagiogenesis and macrophage activity. It results in softening of fibrosis associated with lymphedema and, when used in combination with compressive therapy, has been found effective in reducing sequelae of lymphedema in small trials [38].
Nonsurgical Treatment
Complementary, Alternative, and Other Nonsurgical Treatments
Cormier and colleagues have recently published a comprehensive literature search on nonsurgical lymphedema management and categorized these according to the standard of putting evidence to practice (PEP) [39, 40].
Complete Decongestive Therapy
Any treatment strategy should be accompanied by complete decongestive therapy (CDT) as well as education about avoiding activities that could increase lymphedema [41•]. CDT involves an initial reductive phase which employs specialized manual lymphatic drainage, bandaging, and exercise which usually takes several weeks to complete. The second phase is a maintenance phase that involves compressive sleeves, exercise, and prevention and early recognition of infection which will really be used for the life of the patient. Success rates of CDT are hampered by poor patient compliance, expense, and limited availability of certified lymphedema therapists that perform and teach the specialized massage.
Pneumatic Compression
While some studies have found pneumatic compression to be effective at fluid transport, it does not seem to promote protein transport and subsequently does not appear effective as a sole modality by which to maintain control of lymphedema [42]. A prospective randomized controlled trial which involved laser versus pneumatic mechanical compression showed that while laser treatment had a 95 % reduction in volume by 12 months, pneumatic compression was ineffective [39].
Kinesio Tape
Kinesio tape is likely just as effective as the standard CDT bandaging. A randomized control trial showed Kinesio tape just as effective as compressive bandages and may be more likely to be used in patients who have poor compliance with CDT [43].
Exercise
Avoidance of exercise for patients with lymphedema is not supported by data. In fact, studies have actually supported the recommendation for resistance exercising and weight training showing a potential benefit in the risk of developing lymphedema [44]. In addition, exercise in patients with lymphedema has been shown to increase quality of life and decrease fatigue [45].
Treatments with Effectiveness Not Established
Ultrasound, electrical stimulation, electromagnetic diathermy, hyperbaric oxygen therapy, acupuncture, edermologie system, Lymphease massage unit, Sun Ancon Chi, deep oscillation therapy, aquatic lymphatic therapy, and extracorporeal shock wave therapy have all been used in the treatment of lymphedema. Their effectiveness has not been established [40].
Long-standing recommendations such as avoidance of blood pressure measurements and needle sticks in the ipsilateral arm are not evidence based. A retrospective article is one of the only available studies supporting this practice in patients, but it relied on the patient’s recall of needle sticks [46]. However, infection increases the risk of lymphedema by 50 %. In this regard, patients with needle sticks have a higher risk of infection than those without, and when possible, needle sticks should be avoided.
The use of compression garments during air travel based on a questionable risk of low cabin pressure causing a decrease in extracellular fluid pressure has not been found to be effective [44].
Conclusion
Patients fear lymphedema sometimes as much or more than a mastectomy or recurrence of breast cancer. It is not only a hindrance to most occupations but also a constant reminder to the patient and others that they are a cancer survivor. It is incumbent on surgeons to continue to educate their patients and apply new developments and improve lymphadenectomy procedures in order to reduce the incidence of lymphedema and the impact on quality of life.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Schrenk P, Rieger R, Shamiyeh A, et al. Morbidity following sentinel lymph node biopsy versus axillary lymph node dissection for patients with breast carcinoma. Cancer. 2000;88:608–14.
Haid A, Kuehn T, Konstantiniuk P, Köberle-Wührer R, Knauer M, Kreienberg R, et al. Shoulder-arm morbidity following axillary dissection and sentinel node only biopsy for breast cancer. Eur J Surg Oncol. 2002;28(7):705–10.
Swenson KK, Nissen MJ, Ceronsky C, Swenson L, Lee MW, Tuttle TM. Comparison of side effects between sentinel lymph node and axillary lymph node dissection for breast cancer. Ann Surg Oncol. 2002;9(8):745–53.
Blanchard DK, Donohue JH, Reynolds C, et al. Relapse and morbidity in patients undergoing sentinel lymph node biopsy alone or with axillary dissection for breast cancer. Arch Surg. 2003;138:482–8.
Schijven MP, Vingerhoets AJ, Rutten HJ, et al. Comparison of morbidity between axillary lymph node dissection and sentinel node biopsy. Eur J Surg Oncol. 2003;29:341–50.
Ronka R, von Smitten K, Tasmuth, et al. One-year morbidity after sentinel node biopsy and breast surgery. Breast. 2005;14:28–36.
Leidenius M, Leivonen M, Vironen J, von Smitten K. The consequences of long-time arm morbidity in node-negative breast cancer patients with sentinel node biopsy or axillary clearance. J Surg Oncol. 2005;92(1):23–31.
Mansel R, Fallowfield L, Kissin M. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer. The ALMANAC Trial: JNCI. 2006;98(9):599–609.
Lucci A, McCall LM, Beitsch PD, et al. Surgical complications associated with sentinel lymph node dissection (SLND) plus axillary lymph node dissection compared with SLND alone in the American College of Surgeons Oncology Group Trial Z0011. J Clin Oncol. 2007;25:3657–63.
McLaughlin SA, Wright MJ, Morris KT, et al. Prevalence of lymphedema in women with breast cancer 5 years after sentinel lymph node biopsy or axillary dissection: objective measurements. J Clin Oncol. 2008;26(32):5213–9.
Ashikaga T, Krag DN, Land SR, et al. Morbidity results from the NSABP-B32 trial comparing sentinel lymph node dissection versus axillary dissection. J Surg Oncol. 2010;102:111–18.
Wernicke AG, Goodman RL, Turner BC, et al. A 10-year follow-up of treatment outcomes in patients with early stage breast cancer and clinically negative axillary nodes treated with tangential breast irradiation following sentinel lymph node dissection or axillary clearance. Breast Cancer Res Treat. 2011;125(3):893–902.
Giuliano AE1, Hunt KK, Ballman KV, Beitsch PD, Whitworth PW, Blumencranz PW, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA. 2011;305(6):569–75.
Passik SD, McDonald MV. Psychosocial aspects of upper extremity lymphedema in women treated for breast carcinoma. Cancer. 1998;83:2817–20.
Tobin MB, Lacey HJ, Meyer L, Mortimer PS. The psychosocial morbidity of breast cancer-related arm swelling. Psychological morbidity of lymphedema. Cancer. 1993;72:3248–52.
Rowland JH, Hewitt M, Ganz PA. Cancer survivorship: a new challenge in delivering quality cancer care. J Clin Oncol. 2006;24(32):5101–4.
Foldi M. Remarks concerning the consensus document (CD) of the International Society of Lymphology “The diagnosis and treatment of peripheral lymphedema”. Lymphology. 2004;37(4):168–73.
Thompson M, Korourian S, Henry-Tillman RS, Adkins L, Mumford S, Westbrook K, et al. ARM (axillary reverse mapping): a new concept to identify and enhance lymphatic preservation. Ann Surg Oncol. 2007;14(2):84–5.
Boneti C, Korourian S, Bland K, Cox K, Adkins LL, Henry-Tillman RS, et al. Axillary reverse mapping: mapping and preserving arm lymphatics may be important in preventing lymphedema during sentinel lymph node biopsy. J Am Coll Surg. 2008;206(5):1038–42. This article describes the anatomical variations of the lymphatics draining the arm within the axillary boundaries and a way of mapping them and preserving them.
Boneti C, Badgwell B, Robertson Y, Korourian S, Adkins L, Klimberg V. Axillary reverse mapping (ARM): initial results of phase II trial in preventing lymphedema after lymphadenectomy. Minerva Ginecol. 2012;64(5):421–30.
Ivens D et al. Assessment of morbidity from complete axillary dissection. Br J Cancer. 1997;66:136.
Petrek JA, Senie RT, Peters M, et al. Lymphedema in a cohort of breast carcinoma survivors 20 years after diagnosis. Cancer. 2001;92:1368–77.
Silberman AW, McVay C, Dohen JS, et al. Comparative morbidity of axillary lymph node dissection and the sentinel lymph node technique: implications for patients with breast cancer. Ann Surg. 2004;240:1–6.
Nesvold IL, Dahl AA, Løkkevik E, Marit Mengshoel A, Fosså SD. Arm and shoulder morbidity in breast cancer patients after breast-conserving therapy versus mastectomy. Acta Oncol. 2008;47(5):835–42.
Rockson SG. Tissue changes, bioimpedance, and acquired lymphedema. Lymphat Res Biol. 2013;11(4):195.
Hayes SC, Janda M, Cornish B, Battistutta D, Newman B. Lymphedema after breast cancer: incidence, risk factors and effect of upper body. J Clin Oncol. 2008;26:3536–42.
Albert US, Koller M, Kopp I, Lorenz W, Schulz KD, Wagner U. Early self-reported impairments in arm functioning of primary breast cancer patients predict late side effects of axillary lymph node dissection: results from a population-based cohort study. Breast Cancer Res Treat. 2006;100(3):285–92.
Larson D, Weinstein M, Goldberg I, et al. Edema of the arm as a function of the extent of axillary surgery in patients with stage I-II carcinoma of the breast treated with primary radiotherapy. Int J Radiat Oncol Biol Phys. 1986;12:1575–82.
Mittendorf EA, Hunt KK. Lymphatic interrupted: do we really understand the risks and consequences? Ann Surg Oncol. 2009;16(7):1768–70.
Tummel E, Ochoa D, Korourian S, et al. Does axillary reverse mapping prevent lymphedema after lymphadenectomy? Phoenix: Presented at the Society of Surgical Oncology; 2014.
Guthrie D, Gagnon G. The prevention and treatment of postoperative lymphedema of the arm. Ann Surg. 1946;123(5):925–35.
Boccardo F, Casabona F, DeCian F, et al. Lymphatic microsurgical preventing healing approach (LYMPHA) for primary surgical prevention of breast cancer-related lymphedema: over 4 years follow-up. Microsurgery. 2014;34(6):421–4.
Dosher ME, Herman S, Garfein ES. Surgical management of inoperable lymphedema: the re-emergence of abandoned techniques. J Am Coll Surg. 2012;215(2):278–83.
Yamamoto T, Yoshimatsu H, Yamamoto N, et al. Modified lambda-shaped lymphaticovenular anastomosis with supermicrosurgical lymphoplasty technique for a cancer-related lymphedema patient. Microsurgery. 2014;34(4):308–10. This article describes a new technique of microsurgical lymphovenular anastomosis which, in preliminary reports, appears to be moderately successful.
Sapountzis S, Nicoli F, Chilgar R, Ciudad P. Evidence-based analysis of lymph node transfer in postmastectomy upper extremity lymphedema. Arch Plast Surg. 2013;40(4):450–1.
Cormier JN, Rourke L, Crosby M, Chang D, Armer J. The surgical treatment of lymphedema: a systematic review of the contemporary literature. Ann Surg Oncol. 2010;19(2):642–51.
Granzow JW1, Soderberg JM, Kaji AH, Dauphine C. Review of current surgical treatments for lymphedema. Ann Surg Oncol. 2014;21(4):1195–201.
E Lima MT, E Lima JG, de Andrade MF, Bergmann A. Low-level laser therapy in secondary lymphedema after breast cancer: systematic review. Lasers Med Sci. 2014;29(3):1289–95.
Rodrick JR, Poage E, Wanchai A, Stewart BR, Cormier JN, Armer JM. Complementary, alternative, and other non-complete decongestive therapy treatment methods in the management of lymphedema: a systematic search and review. PM R. 2014;6(3):250–74.
Chang CJ, Cormier JN. Lymphedema interventions: exercise. Surg Comp Dev Sem Oncol Nurs. 2013;29(1):28–40.
Lasinski BB, McKillip Thrift K, Squire D, Austin MK, Smith KM, Wanchai A, et al. A systematic review of the evidence for complete decongestive therapy in the treatment of lymphedema from 2004 to 2011. PM R. 2012;4(8):580–601. This article describes in detail the phases of complete decongestive therapy which is considered the most reliable treatment for lymphedema.
Feldman JL, Stout NL, Wanchai A, Stewart BR, Cormier JN, Armer JM. Intermittent pneumatic compression therapy: a systematic review. Lymphology. 2012;45(1):13–25.
Tsai HJ1, Hung HC, Yang JL, Huang CS, Tsauo JY. Could Kinesio tape replace the bandage in decongestive lymphatic therapy for breast-cancer-related lymphedema? A pilot study. Support Care Cancer. 2009;17(11):1353–60.
McLaughlin SA. Lymphedema: separating fact from fiction. Oncology. 2012;26(3):242–9. This article describes some of the commonly held beliefs and recommendations that have no basis in fact.
Kwan ML, Cohn JC, Armer JM, et al. Exercise in patients with lymphedema: a systematic review of the contemporary literature. J Cancer Surviv. 2011;5:320–36.
Clark B, Sitzia J, Harlow W. Incidence and risk of arm oedema following treatment for breast cancer: a three-year follow-up study. Q J Med. 2005;98:343–8.
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
Daniela Ochoa and V. Suzanne Klimberg declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Local-Regional Evaluation and Therapy
Rights and permissions
About this article
Cite this article
Ochoa, D., Klimberg, V.S. Surgical Strategies for Prevention and Treatment of Lymphedema in Breast Cancer Patients. Curr Breast Cancer Rep 7, 1–7 (2015). https://doi.org/10.1007/s12609-014-0172-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12609-014-0172-x