Abstract
Blautia is a genus of anaerobic microbe extensively present in the intestine and feces of mammals. This study aims to investigate the influence of Blautia producta to prevent lipopolysaccharide (LPS)-induced acute liver injury (ALI) and elaborate on its hepatoprotective mechanisms. B. producta D4 and DSM2950 pretreatment decreased the activities of serum aspartate transferase (AST), and alanine transaminase (ALT) in mice with LPS treatment significantly decreased the levels of inflammatory tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6) and increased the activities of antioxidative superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-Px). Compared with the model group, B. producta D4 and B. producta DSM2950 pretreatment slightly increased the levels of cecal propionic acid, isobutyric acid, butyric acid, valeric acid, and isovaleric acid (p > 0.05). Metagenomic analysis showed that B. producta D4 and DSM2950 pretreatment remarkably increased the relative abundance of [Eubacterium] xylanophilum group, Lachnospira, Ruminiclostridium, Ruminiclostridium 9, Coprococcus 2, Odoribacter, Roseburia, Alistipes, and Desulfovibrio in ALI mice, and their abundance is negatively related to the levels of inflammatory TNF-α, IL-1β, and IL-6 as revealed by Spearman’s correlation analysis. Moreover, transcription and immunohistochemistry analysis revealed that B. producta D4 and B. producta DSM2950 intervention remarkably suppressed the transcription and expression levels of hepatic Tlr4, MyD88, and caspase-3 (p < 0.05). These data indicated that B. producta may be a good candidate for probiotics in the prevention of ALI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The human gastrointestinal tract is a complex microbial ecosystem that harbors an enormous diversity of commensal microbes which contribute to regulating the gut barrier, performing resistance to pathogenic bacteria, and modulating the immune system [1]. It is accepted that probiotics improve the ecological balance of intestinal microbiota and regulate the host glucolipid metabolism by secreting antibacterial substances and producing short-chain fatty acids (SCFAs). Blautia is a novel genus of anaerobic microorganisms widely present in the intestine and feces of mammals [2]. In recent years, the antibacterial activity of Blautia and its potential ability to regulate host health and alleviate metabolic syndrome have gradually attracted people’s attention, which was considered to possess the potential to become a probiotic [3]. Blautia glucerasei sp. nov. HFTH-1 T produces extracellular glucosylceramidase that promotes the conversion from glucosylceramide of the plant into ceramide, a functional substance with a specific preventive effect against colon cancer [4]. Blautia obeum A2-162, isolated from the human intestine, is reported to inhibit the growth of Clostridium because it possessed the antibiotic nisin O [5]. The correlation analysis from several reports has shown that the relative abundance of Blautia was negatively related to the inflammatory cytokines, the body mass index, visceral fat accumulation, and type 2 diabetes [6,7,8]. Previous studies also exhibited that the relative abundance of Blautia was remarkably reduced in patients with liver cirrhosis [9]. However, little is known about the mitigative influences of Blautia on liver function injury and inflammation in vivo.
The liver takes a vital role in energy metabolism and biotransformation, but it is susceptible to being affected and stimulated by a variety of pathogenic factors, which could cause liver function damage and inflammation [10]. Accumulating evidence indicates that acute liver injury (ALI) has an extremely poor prognosis and the mortality rate of ALI patients is up to 50% [11]. According to the previous investigation, ALI is the result of many cellular responses caused by infectious and non-infectious inflammation and is characterized by impaired liver function, coagulation disorders, and liver failure [12]. Although the molecular mechanisms of ALI include many aspects, the inflammatory response takes a vital role in accelerating the pathogenesis and development of ALI [13]. A previous study suggested that inflammation of ALI results from various pathogen-related molecules from microbial organisms, for example, lipopolysaccharide (LPS) [14]. LPS is a major endotoxin produced by commensal Gram-negative bacteria in the intestinal lumen, which has been widely used to establish the ALI model [15]. Numerous reports indicated that LPS treatment can stimulate excessive count of neutrophils and promote inflammatory cytokines secretion, for example, tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6 [16]. Elevated inflammatory cytokine secretion is associated with the occurrence of ALI. Therefore, LPS-induced ALI models in mice have been widely applied to explore the potential mechanisms and promising therapeutic drugs for ALI.
Blautia producta D4 was previously isolated from the healthy mouse feces and Blautia producta DSM2950 was procured from the BeNa Culture Collection (Shanghai, China), respectively. So far, the efficacy of B. producta in preventing or treating some diseases has been unclear. Therefore, the purpose of this research was to explore whether B. producta isolated from mouse feces can ameliorate the symptoms of ALI in vivo, and reveal its potential mechanism using high-throughput sequencing and RT-qPCR.
Materials and Methods
Materials and Strain Preparation
B. producta DSM2950 was procured from the BeNa Culture Collection (Shanghai, China). B. producta D4 was deposited in the Culture Collection of Food Microorganisms (CCFM) in Jiangnan University (Wuxi, China). LPS were procured from Sigma-Aldrich (Saint Louis, USA, Lot No.: L2630). Test kits for the determination of TNF-α, IL-1β, and IL-6 were obtained from R&D Systems (Minneapolis, MN). Commercial antioxidant assay kits for analysis of superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-Px) and malondialdehyde (MDA) were procured from Nanjing Jiancheng Co., Ltd. (Nanjing, China).
B. producta D4 and B. producta DSM2950 were first activated in GAM broth and grown anaerobically at 37 °C for 16 h. L. rhamnosus GG was first activated in MRS medium at 37 °C for 18 h. Afterwards, the bacteria were collected by centrifugation at 8000 g for 20 min after sub-culturing, then rinsed twice with sterile PBS (0.01 M, pH 7.4). The samples were resuspended in 13% (m/v) of skim milk and preserved at – 80 °C. The concentration of gavage bacteria was adjusted to 5 × 109 CFU/mL for B. producta D4 and B. producta DSM2950.
Experimental Design
Forty male C57BL/6 J mice (6 weeks old, 18 ± 2 g) were procured from Charles River (Beijing, China). All mice were admitted to adaptation for 1 week. All mice were stochastic assigned into 4 groups (n = 10) as follows: (1) Control group: intragastric administration of 0.2 mL of sterile skim milk (13%, w/v); (2) Model group: intragastric administration of 0.2 mL of sterile skim milk(13%, w/v); (3) D4 group: intragastric administration of 0.2 mL of B. producta D4 (5 × 109 CFU/mL); (4) DSM2950 group: intragastric administration of 0.2 mL of B. producta DSM2950 (5 × 109 CFU/mL). The intervention period lasted for 2 weeks. At the end of the experiment, mice in the Control group were intraperitoneally injected with 0.2 mL of sterile normal saline. Others were intraperitoneally injected with the same volume of 0.2 mL of LPS (5 mg/kg). After 4 h of LPS injection, all mice were euthanized, and tissues were harvested to further analysis. The whole experiment was approved by the Ethics Committee of Jiangnan University (No20201115c0701240[309]).
Analysis of Serum Biochemical Parameters
Blood from all mice was collected and placed at 25 ℃ for 90 min. Serums from each mouse were collected by centrifugation at 6000 rpm/min for 10 min at 25 °C. The serum glucose (Glu), total cholesterol (TC), triglyceride (TG), alanine transaminase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), creatine kinase (CK), and lactic dehydrogenase (LDH) levels were analyzed by an automatic hematology analyzer Mindray BC-5000 (Shenzhen, China). The serum TNF-α, IL-1β, and IL-6 were determined using R&D Systems (Minneapolis, MN).
Analysis of Hepatic Oxidative Stress Parameters
The partial liver was harvested, cleared, and preserved at – 80 ℃. According to the instructions of commercial kits, a high-speed homogenizer was used to prepare a 10% liver tissue homogenate for the detection of liver biochemical parameters [malondialdehyde (MAD), superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-Px)].
Histological Examination
Live sections were fixed with 4% paraformaldehyde, dehydrated with a series of ethanol and xylene solutions, paraffin-embedded, and cut into 2–4-μm-thick sections for histopathological analysis. After hematoxylin–eosin (H&E) staining, liver, spleen, and kidney sections were observed with a light microscope and photographed with a digital camera, respectively (Nikon, Tokyo, Japan).
Analysis of Cecal Short-Chain Fatty Acids
Cecal SCFA concentrations were obtained and analyzed following a previously published method [17]. A total of 100 mg of feces was homogenized in 800 μL of saturated NaCl solution and placed at 4 ℃ for 0.5 h. Then, 1000 μL of diethyl ether and 40 μL of 10% sulfuric acid were mixed into the solution and centrifuged at 4 °C at 12,000 rpm for 10 min. The water in the samples was removed using anhydrous Na2SO4. The concentrations of cecal SCFAs were detected using a gas chromatograph equipped with an Rtx Wax column (Kyoto, Japan).
Quantitative Real-Time qPCR
Total hepatic RNA was obtained using commercial kits (Takara, Dalian, China), and RNA reverse transcription was carried out using a commercial cDNA kit (Takara, Dalian, China). RT-qPCR was implemented in StepOnePlus Real-Time PCR System (AB, Foster City, CA, USA) with SYBR Premix Ex Taq II (Takara, Dalian, China). The hepatic mRNA expression level was analyzed by the β-actin.
Immunohistochemical Analysis
Immunohistochemical analysis was carried out following a previous report [18]. Briefly, the liver tissues were harvested and fixed with 4% paraformaldehyde. The tissue was pressed with paraffin and cut into uniform pieces with a thickness of 2–4 µm. Paraffin sections were mixed with xylene, deparaffinized, gradually rehydrated with alcohol, and blocked with 5% bovine serum albumin for 10–30 min. Tissue sections were then incubated overnight with appropriate primary antibodies (Tlr4, MyD88, caspase-3), rinsed with phosphate-buffered saline (PBS), and incubated with secondary antibodies. Average densities of TLR4, MYD88, and caspase-3 were analyzed using 1.8.0 images.
16S rDNA Amplicon High-Throughput Sequencing
Total bacterial DNA of feces was obtained using a fecal DNA extraction kit (MoBio, USA) and amplified the 16S rDNA V3-V4 sequencing with 341F-806R primers based on the Illumina MiSeq platform of Tianhao Co., Ltd. (Shanghai, China). The sequences were classified into operational taxonomic units (OTUs) with a divergence of 3% by Xshell (Ver.7.0). Hierarchical clustering analysis was carried out and visualized by SIMCA-14.1 software (UMETRICS, Sweden). Differential OTU abundance analyses based on negative binomial distribution were performed using STAMP software (Ver. 2.1.3). The correlations between the key bacterial OTUs with biochemical parameters were revealed by Spearman’s correlation analysis, visualized by heatmap and network through R software (Ver. 4.3.0) and Cytoscape software (Ver. 3.9.0), respectively.
Statistical Analysis
All data are presented as mean ± standard deviation. Data analysis was performed using SPSS 22.0 statistical software (IBM Corporation, Chicago, IL, USA), and one-way ANOVA between groups was performed using Tukey's multiple comparison test.
Results
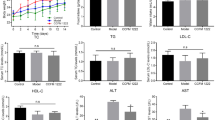
Influences of B. producta on Body Weight and Serum Lipid Profile of Mice
As shown in Fig. 1, there was no remarkable difference in the body weight of mice among the four groups after 17 days of intervention (p > 0.05). Interestingly, the serum ALT and AST activities in the model group were remarkably higher than that in the control group (p < 0.05), indicating the ALI model was successfully established. B. producta D4 and B. producta DSM2950 pretreatment could reduce the serum ALT, and AST activities in LPS-treated mice, especially B. producta DSM2950. Nevertheless, there was no remarkable difference in the serum ALP activity of mice among the four groups (p > 0.05).
B. producta Improved the Inflammatory Response and Anti-Oxidative Enzymes in LPS-Treated Mice
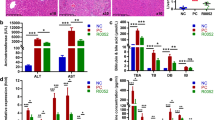
As compared with the control group, the serum TNF-α, IL-6, and IL-1β levels were remarkably increased in the model group (p < 0.05) (Fig. 2A). B. producta D4 and B. producta DSM2950 pretreatment remarkably reduced the serum TNF-α and IL-1β levels compared with the model group (p < 0.05). However, B. producta D4 pretreatment remarkably decreased the serum IL-6 levels in ALI mice (p < 0.05), while B. producta DSM2950 pretreatment slightly suppressed the changes in serum IL-6 levels induced by LPS (p > 0.05). In addition, excessive oxidative stress is one of the clear symptoms in patients with ALI, the hepatic MDA, SOD, GSH-Px, and CAT levels were measured (Fig. 2B). LPS treatment led to significant increases in the hepatic MDA levels, and significant decreases in the hepatic SOD, GSH-Px, and CAT activities compared with the control group (p < 0.05). As expected, B. producta D4 and B. producta DSM2950 pretreatment remarkably reduced the hepatic MDA levels and remarkably increased the hepatic GSH-Px activity in ALI mice (p < 0.05). In addition, B. producta D4 and B. producta DSM2950 pretreatment remarkably increased the hepatic SOD activity compared with the model group (p < 0.05). Interestingly, B. producta D4 pretreatment remarkably elevated the hepatic CAT activity in ALI mice (p < 0.05).
Effect of B. producta pretreatment on inflammatory cytokines and oxidative stress in LPS-treated mice (n = 10). A The serum TNF-α, IL-6, and IL-1β levels; B The hepatic MDA, SOD, GSH-Px, and CAT levels; C Representative H&E staining of liver sections. Values with different letters are significantly different (p < 0.05)
The images of histopathological examination demonstrated the large amplitude of hepatocyte swelling, hepatocyte proliferation and nuclear loss, and inflammatory cell infiltration in ALI mice (Fig. 2C). Nevertheless, B. producta pretreatment remarkably relieved these pathological changes induced by LPS to a certain extent.
Effects of B. producta on the Cecal SCFA Levels in LPS-Treated Mice
SCFAs are regarded as one of the small molecules that are involved in regulating the immune system and inflammatory response; the cecal acetic acid, propionic acid, isobutyric acid, butyric acid, valeric acid, and isovaleric acid levels were measured (Fig. 3). There was no remarkable difference in the cecal SCFA levels among the four groups (p > 0.05). Among those, B. producta D4 and B. producta DSM2950 pretreatment slightly increased the cecal propionic acid, isobutyric acid, butyric acid, valeric acid, and isovaleric acid levels compared with the model group (p > 0.05).
B. producta Pretreatment Shifted the Intestinal Microbiota Composition
The microbial community richness was measured by analysis of Chao1 and Observe indexes, but the Shannon and Simpson indexes are used to analyze the diversity and evenness of the microbial community. There was no significant difference in the Chao1, Observe, Shannon, and Simpson indexes between the control and model groups (p > 0.05) (Fig. 4A). However, B. producta D4 and B. producta DSM2950 pretreatment slightly increased these indexes of intestinal microbiota compared with the control and model groups (p > 0.05). In addition, the PCA results showed the intestinal microbiota were obviously similar between the control and model groups (Fig. 4B). However, there were dramatic changes in the intestinal microbiota composition after B. producta D4 and B. producta DSM2950 intervention, whereas the intestinal microbiota composition in the D4 and DSM2950 groups were more close, indicating that B. producta D4 and B. producta DSM2950 could induce similar intestinal microbiota composition alterations.
Effect of B. producta pretreatment on the intestinal microbiota composition in LPS-treated mice (n = 10). A Chao1, Observed, Simpson, and Shannon indexes; B PCA analysis; C Relative abundance of phylum; D Relative abundance of differential genus. Values with different letters are significantly different (p < 0.05)
At the phylum level, the same microbiota structure was performed in both the control and model groups. However, B. producta D4 pretreatment obviously elevated the relative abundance of Firmicutes, Proteobacteria, and Cyanobacteria, and obviously decreased the relative abundance of Bacteroidetes compared with the control and model groups. At the genus level, the relative abundance of Dubosiella, Family XIII AD3011 group, Desulfovibrio, Odoribacter, and [Eubacterium] ruminantium group in the model group was remarkably lower than that in the control group, while the relative abundance of Turicibacter and Muribaculaceae in the model group was remarkably higher than that in the control group (Fig. 4C). However, B. producta D4 pretreatment remarkably elevated the relative abundance of [Eubacterium] xylanophilum group, Lachnospira, Ruminiclostridium, Ruminiclostridium 9, Coprococcus 2, Odoribacter, and Roseburia in ALI mice, whereas remarkably decreased the relative abundance of Bifidobacterium, Turicibacter, Muribaculaceae, Gordonibacter, Ruminococcaceae UCG-010, Akkermansia, Prevotellaceae UCG-001, and Rikenellaceae RC9 gut group (Fig. 4D). B. producta DSM2950 pretreatment remarkably elevated the proportion of [Eubacterium] xylanophilum group, Alistipes, Lachnospira, Gordonibacter, and Desulfovibrio in ALI mice, while remarkably decreasing the proportion of Bifidobacterium, Turicibacter, Bacteroidales bacterium, Prevotellaceae UCG-001, Ruminococcaceae UCG-010, Akkermansia, Lactobacillus, and Gordonibacter.
Correlation Between Intestinal Microbiota and ALI-Related Biomarkers
The association between the key genus and major biomarkers in ALI was carried out according to Spearman’s correlation (Fig. 5A, B). The serum AST, ALT, TNF-α, IL-6, and IL-1β levels were negatively associated with the relative abundances of Family XII AD3011 group, Prevotellaceae UCG 001, Eubacterium ruminantium group, Desulfovibrio, Roseburia, Odoribacter, Lactobacillus, Alistipes, Lachnospira, Bifidobacterium, and Gordonibacter, and positively associated with the relative abundance of Muribaculaceae, Bacteroidales bacterium, and Turicibacter. In addition, cecal SCFAs, hepatic SOD, GSH-Px, and CAT levels were positively related to the relative abundance of Alistipes, Lachnospira, Bifidobacterium, and Gordonibacter, but negatively related to the relative abundance of Muribaculaceae, Bacteroidales bacterium, and Turicibacter.
Effects of B. producta on the Expression of Genes Related to Inflammation in LPS-Treated Mice
To deeply reveal the influence of B. producta on ALI mice, the expression levels of mRNA related to ALI and inflammation were detected (Fig. 6). The transcription levels of hepatic Tlr4, MyD88, NF-κB, iNOS, COX2, TNF-α, IL-1β, and caspase-3 were significantly upregulated in the model group relative to the control group (p < 0.05). Compared with the model group, B. producta D4 and B. producta DSM2950 intervention remarkably suppressed the transcription levels of hepatic Tlr4, MyD88, COX2, TNF-α, and caspase-3 (p < 0.05), and slightly decreased the transcription levels of hepatic NF-κB, iNOS, and IL-1β (p > 0.05). In addition, the results of immunohistochemistry displayed that the expression level of hepatic Tlr4, MyD88, and caspase-3 in the model group was remarkably higher than that in the control group (p < 0.05) (Fig. 7). Nevertheless, B. producta D4 and B. producta DSM2950 treatment remarkably inhibited these changes in ALI mice (p < 0.05).
Discussion
Some reports suggested that long-time consumption of probiotics is beneficial for regulating the host glucolipid metabolism, such as Lactobacillus paracasei [19], Pediococcus acidilactici [18], and Bifidobacterium longum [19]. Body weight is one of the intuitive parameters, which is widely used in assessing the development and energy metabolism of the body [20]. In the present study, B. producta D4 and B. producta DSM2950 treatment no obviously altered the body weight of mice relative to the control and model groups, suggesting that short-term B. producta consumption could not elevate the risk of glucolipid metabolism disorder. In addition, LPS treatment elevated the serum ALT and AST activities compared with that in mice without LPS treatment, which is in agreement with this study [21]. The serum ALT and AST activities are extensively used to evaluate liver function due to they are transferred into the blood circulation when the occurrence of liver injury. According to the investigation by World Health Organization (WHO), ALT serves as the most sensitive parameter of liver structure injury, and the serum ALT mainly stemmed from the damage to the cell membrane [13]. B. producta D4 and B. producta DSM2950 pretreatment could suppress the elevation of serum ALT and AST activities induced by LPS, indicating that B. producta play a beneficial role in improving the host liver function, especially B. producta DSM2950 intervention. The major serum ALP activity stemmed from the hepatocytes, which destroys the plasma membrane [22]. The serum ALP activity was slightly reduced in ALI mice after B. producta pretreatment, which further confirmed B. producta is beneficial in preventing the development of ALI. Moreover, excessive oxidative stress is regarded as a vital symptom of ALI. LPS treatment causes the accumulation of reactive oxygen species (ROS), which can react with unsaturated fatty acids and then initiate lipid peroxidation. MDA act as the main end product of lipid peroxidation that aggravates liver function injury [23]. The function of SOD is to restrain the formation of reactive oxygen species (ROS) and promote the formation of hydrogen peroxide in vivo, which was further decomposed to non-toxic substances (oxygen and water) under the higher activity of GSH-Px [24]. In addition, CAT promotes transforming peroxides into relatively toxic hydroxyl substances, which can effectively eliminate ROS accumulation in the body [24]. In the present study, the hepatic MDA levels in B. producta groups were lower than that in the model group, and the hepatic SOD, GSH-Px, and CAT activities in B. producta groups were higher than that in the model group, indicating that B. producta prevent the development of ALI by suppressing the excessive oxidative stress.
The influence of probiotics on liver function injury is accompanied by alteration in the diversity and structure of intestinal microbiota [25]. Some reports affirmed that probiotic intake maintains the balance of intestinal microbiota and elevates the intestinal microbiota diversity in animal experiments [26]. Our data suggested that B. producta treatment slightly elevated the intestinal microbiota diversity, indicating B. producta can help to prevent the development of ALI. At the phylum level, Bacteroidetes, Firmicutes, Proteobacteria, Verrucomicrobia, and Cyanobacteria were the major phylum in four groups. Firmicutes and Bacteroidetes are the main phyla that play the most beneficial role in the maintenance of host health by processing and scavenging dietary polysaccharides on the basis of carbohydrate-active enzymes [27]. Our results showed that the relative abundance of Firmicutes and Bacteroidetes was obviously changed in ALI mice after B. producta treatment, indicating B. producta improve the host energy metabolism by altering the proportion of Firmicutes and Bacteroidetes. At the genus level, [Eubacterium] xylanophilum group, Ruminiclostridium, Ruminiclostridium 9, Lachnospira, Coprococcus 2, and Roseburia are regarded as the butyrate-producing bacterium, and its abundance was positively related to the serum antioxidant activities and negatively related to the concentrations of MDA and inflammatory cytokines, which is in agreement with this study [28,29,30,31]. Butyric acid promotes IL-22 production in the intestines by CD4+ T cell and ILCs via combining the G-protein receptor 41 (GPR41) and activating aryl hydrocarbon receptor (AhR) and hypoxia-inducible factor 1α (HIF-1α) [32]. Previous reports have shown that gut-derived IL-22 maintained the integrity of the intestinal barrier and elevated the host immune system, and then improve liver function injury [33]. Odoribacter and Lachnospira, another SCFAs-producing bacterium, play a pivotal role in maintaining a healthy gut and lowering systolic blood pressure in pregnant women [34, 35]. Alistipes are anaerobic bacteria discovered in the healthy human gastrointestinal tract [36]. A previous study exhibited that Alistipes have protective effects against the secretion of TNF-α, IL-6, and IL-1β, which is in agreement with this study [37]. These results suggested that B. producta induced selective increase and reduces of intestinal microorganism may be contributed to the hepatoprotective effects of B. producta.
To further reveal the underlying mechanism whereby B. producta pretreatment prevents liver function injury and inflammation in ALI mice, the transcriptional level of genes related to ALI and inflammation were measured by RT-qPCR, such as Tlr4, MyD88, NF-κB, iNOS, COX2, TNF-α, IL-1β, and caspase-3. A previous study exhibited that intraperitoneal injection of LPS activated the expression of Tlr4 which is a type I transmembrane protein. TLR4 activates two different signaling pathways, namely the MyD88 and TRIF pathways [38]. Deficiency Myd88 is beneficial for hampering the development of inflammation. In addition, overexpression of hepatic Tlr4 causes the secretion of pro-inflammatory cytokines and neutrophil transmigration into the liver by activating the NF-ҡB and other transcription factors [39]. NF-κB is regarded as a central governor, which takes a vital role in the survival of lymphocytes and activation of innate immune cells [40]. Activation of hepatic NF-κB is frequently accompanied by high levels of TNF-α, IL-6, and IL-1β. TNF-α serves as a vital regulator of immune regulation and inflammation and will exert a direct cytotoxic effect and induce hepatocyte necrosis. The secretion of TNF-α is monitored by the levels of IL-1β that one of the most cell cytokines and plays an important role in the so-called cytokine storm. IL-6 is traditionally considered a regulator of acute-phase responses, and its levels are strongly associated with cardiovascular disease, type 2 diabetes, and liver functional decline [41]. In addition, the transcription of hepatic iNOS and COX2 was regulated by the NF-κB transcription. Overexpression of iNOS destroys liver function and leads to large quantities of nitric oxide production in the liver [42]. The accumulation of nitric oxide is reported to promote the occurrence and development of some diseases, such as liver injury, brain inflammation, and cancer [43]. Moreover, COX2 serves as an inducible enzyme responsible for the development of many inflammatory diseases [44]. Moreover, caspase-3 is a vital apoptotic effector, which is reported to accelerate DNA damage and cell death [45]. In the present study, B. producta pretreatment decreased the hepatic Tlr4, MyD88, NF-κB, iNOS, COX2, TNF-α, IL-1β, and caspase-3 transcriptions in ALI, implying that B. producta serve as a candidate for preventing/treating ALI.
Conclusions
The potential influence of B. producta on LPS-induced ALI and their possible mechanism were firstly investigated. B. producta D4 and B. producta DSM2950 pretreatment remarkably not only suppressed the excessive inflammatory response and oxidative stress, but also regulated the intestinal microbiota composition in ALI mice. The transcription levels of genes related to inflammation of ALI mice were remarkably ameliorated after B. producta treatment. This may be the first study to confirm a direct effect of Blautia producta to ameliorate systemic inflammation. These results exhibited that Blautia producta may be a good candidate for probiotics in the prevention of ALI.
Data Availability
The data generated in the current study are available from the corresponding author on reasonable request.
References
Wang R, Yang X, Liu J, Zhong F, Zhang C, Chen Y, Sun T, Ji C, Ma D (2022) Gut microbiota regulates acute myeloid leukaemia via alteration of intestinal barrier function mediated by butyrate. Nat Commun 13(1):2522. https://doi.org/10.1038/s41467-022-30240-8
Ricaboni D, Mailhe M, Labas N, Vitton V, Raoult D, Million M (2016) Draft genome sequence of Blautia faecis strain marseille-P328, isolated from the human ascending colon. Genome Announc 4(6). https://doi.org/10.1128/genomeA.01383-16
Liu X M, Mao B Y, Gu J Y, Wu J Y, Cui S M, Wang G, Zhao J X, Zhang H, Chen W (2021) Blautia-a new functional genus with potential probiotic properties?. Gut Microbes 13(1). https://doi.org/10.1080/19490976.2021.1875796
Furuya H, Ide Y, Hamamoto M, Asanuma N, Hino T (2010) Isolation of a novel bacterium, Blautia glucerasei sp. nov., hydrolyzing plant glucosylceramide to ceramide. Arch Microbiol 192(5):365–72. https://doi.org/10.1007/s00203-010-0566-8
Hatziioanou D, Gherghisan-Filip C, Saalbach G, Horn N, Wegmann U, Duncan SH, Flint HJ, Mayer MJ, Narbad A (2017) Discovery of a novel lantibiotic nisin O from Blautia obeum A2–162, isolated from the human gastrointestinal tract. Microbiology (Reading) 163(9):1292–1305. https://doi.org/10.1099/mic.0.000515
Liu Y, Jiang Q, Liu Z, Shen S, Ai J, Zhu Y, Zhou L (2021) Alteration of gut microbiota relates to metabolic disorders in primary aldosteronism patients. Front Endocrinol 12:667951. https://doi.org/10.3389/fendo.2021.667951
Ozato N, Saito S, Yamaguchi T, Katashima M, Tokuda I, Sawada K, Katsuragi Y, Kakuta M, Imoto S, Ihara K, Nakaji S (2019) Blautia genus associated with visceral fat accumulation in adults 20–76 years of age. NPJ biofilms and microbiomes 5(1):28–28. https://doi.org/10.1038/s41522-019-0101-x
Inoue R, Ohue-Kitano R, Tsukahara T, Tanaka M, Masuda S, Inoue T, Yamakage H, Kusakabe T, Hasegawa K, Shimatsu A, Satoh-Asahara N (2017) Prediction of functional profiles of gut microbiota from 16S rRNA metagenomic data provides a more robust evaluation of gut dysbiosis occurring in Japanese type 2 diabetic patients. J Clin Biochem Nutr 61(3):217–221. https://doi.org/10.3164/jcbn.17-44
Kakiyama G, Pandak WM, Gillevet PM, Hylemon PB, Heuman DM, Daita K, Takei H, Muto A, Nittono H, Ridlon JM, White MB, Noble NA, Monteith P, Fuchs M, Thacker LR, Sikaroodi M, Bajaj JS (2013) Modulation of the fecal bile acid profile by gut microbiota in cirrhosis. J Hepatol 58(5):949–955. https://doi.org/10.1016/j.jhep.2013.01.003
Wang W, Zhang Y, Li H, Zhao Y, Cai E, Zhu H, Li P, Liu J (2018) Protective effects of sesquiterpenoids from the root of panax ginseng on fulminant liver injury induced by Lipopolysaccharide/d-Galactosamine. J Agric Food Chem 66(29):7758–7763. https://doi.org/10.1021/acs.jafc.8b02627
Williams R (1996) Classification, etiology, and considerations of outcome in acute liver failure. Semin Liver Dis 16(4):343–348. https://doi.org/10.1055/s-2007-1007247
Walesky CM, Kolb KE, Winston CL, Henderson J, Kruft B, Fleming I, Ko S, Monga SP, Mueller F, Apte U, Shalek AK, Goessling W (2020) Functional compensation precedes recovery of tissue mass following acute liver injury. Nat Commun 11(1):5785. https://doi.org/10.1038/s41467-020-19558-3
Guo W, Xiang Q, Mao B, Tang X, Cui S, Li X, Zhao J, Zhang H, Chen W (2021) Protective effects of microbiome-derived inosine on lipopolysaccharide-induced acute liver damage and inflammation in mice via mediating the TLR4/NF-κB pathway. J Agric Food Chem 69(27):7619–7628. https://doi.org/10.1021/acs.jafc.1c01781
Yang JX, Hsiung TC, Weng FC, Ding SL, Wu CP, Conti M, Chuang TH, Catherine Jin SL (2018) Synergistic effect of phosphodiesterase 4 inhibitor and serum on migration of endotoxin-stimulated macrophages. Innate Immun 24(8):501–512. https://doi.org/10.1177/1753425918809155
Xiang Y, Zhang H, Xu Zhang Z, Yang Qu X, Xia Zhu F (2022) Dihydrosanguinarine based RNA-seq approach couple with network pharmacology attenuates LPS-induced inflammation through TNF/IL-17/PI3K/AKT pathways in mice liver. Intern Immunopharmacol 109:108779. https://doi.org/10.1016/j.intimp.2022.108779
Dhar R, Rana MN, Zhang L, Li Y, Li N, Hu Z, Yan C, Wang X, Zheng X, Liu H, Cui H, Li Z, Tang H (2021) Phosphodiesterase 4B is required for NLRP3 inflammasome activation by positive feedback with Nrf2 in the early phase of LPS- induced acute lung injury. Free Radical Biol Med 176:378–391. https://doi.org/10.1016/j.freeradbiomed.2021.10.007
Guo WL, Cao YJ, You SZ, Wu Q, Zhang F, Han JZ, Lv XC, Rao PF, Ai LZ, Ni L (2022) Ganoderic acids-rich ethanol extract from Ganoderma lucidum protects against alcoholic liver injury and modulates intestinal microbiota in mice with excessive alcohol intake. Curr Res Food Sci 5:515–530. https://doi.org/10.1016/j.crfs.2022.02.013
Guo W, Mao B, Cui S, Tang X, Zhang Q, Zhao J, Zhang H (2022) Protective effects of a novel probiotic bifidobacterium pseudolongum on the intestinal barrier of colitis mice via modulating the Pparγ/STAT3 pathway and intestinal microbiota. Foods 11(11). https://doi.org/10.3390/foods11111551
Lv X-C, Chen M, Huang Z-R, Guo W-L, Ai L-Z, Bai W-D, Yu X-D, Liu Y-L, Rao P-F, Ni L (2021) Potential mechanisms underlying the ameliorative effect of Lactobacillus paracasei FZU103 on the lipid metabolism in hyperlipidemic mice fed a high-fat diet. Food Res Intern 139:109956. https://doi.org/10.1016/j.foodres.2020.109956
Huang Y Q, Tang Y X, Qiu B H, Talukder M, Li X N, Li J L (2022) Di-2-ethylhexyl phthalate (DEHP) induced lipid metabolism disorder in liver via activating the LXR/SREBP-1c/PPARα/γ and NF-κB signaling pathway. Food Chem Toxicol 165:113119. https://doi.org/10.1016/j.fct.2022.113119
Wu H, Wang Y, Yao Q, Fan L, Meng L, Zheng N, Li H, Wang J (2021) Alkaline phosphatase attenuates LPS-induced liver injury by regulating the miR-146a-related inflammatory pathway. Intern Immunopharmacol 101:108149. https://doi.org/10.1016/j.intimp.2021.108149
Caglayan C, Kandemir FM, Darendelioğlu E, Yıldırım S, Kucukler S, Dortbudak MB (2019) Rutin ameliorates mercuric chloride-induced hepatotoxicity in rats via interfering with oxidative stress, inflammation and apoptosis. J Trace Elem Med Biol 56:60–68. https://doi.org/10.1016/j.jtemb.2019.07.011
Lv XC, Wu Q, Cao YJ, Lin YC, Guo WL, Rao P, Zhang YY, Chen YT, Ai L, Ni L (2022) Ganoderic acid A from Ganoderma lucidum protects against alcoholic liver injury through ameliorating lipid metabolism and modulating intestinal microbial composition. Food Funct. https://doi.org/10.1039/D1FO03219D
Wang X, Yin Z, Meng X, Yang D, Meng H, Liao C, Wei L, Chen Y, Yang X, Han J, Duan Y, Zhang S (2022) Daidzein alleviates neuronal damage and oxidative stress via GSK3β/Nrf2 pathway in mice. J Funct Foods 92:105060. https://doi.org/10.1016/j.jff.2022.105060
Tian X, Li R, Jiang Y, Zhao F, Yu Z, Wang Y, Dong Z, Liu P, Li X (2020) Bifidobacterium breve ATCC15700 pretreatment prevents alcoholic liver disease through modulating gut microbiota in mice exposed to chronic alcohol intake. J Funct Foods 72:104045. https://doi.org/10.1016/j.jff.2020.104045
Llopis M, Cassard AM, Wrzosek L, Boschat L, Bruneau A, Ferrere G, Puchois V, Martin JC, Lepage P, Le Roy T, Lefèvre L, Langelier B, Cailleux F, González-Castro AM, Rabot S, Gaudin F, Agostini H, Prévot S, Berrebi D, Ciocan D, Jousse C, Naveau S, Gérard P, Perlemuter G (2016) Intestinal microbiota contributes to individual susceptibility to alcoholic liver disease. Gut 65(5):830–839. https://doi.org/10.1136/gutjnl-2015-310585
Dong YJ, Lin MQ, Fang X, Xie ZY, Luo R, Teng X, Li B, Li B, Li LZ, Jin HY, Yu QX, Lv GY, Chen SH (2022) Modulating effects of a functional food containing Dendrobium officinale on immune response and gut microbiota in mice treated with cyclophosphamide. J Funct Foods 94:105102. https://doi.org/10.1016/j.jff.2022.105102
Wang Y, Xie Q, Sun S, Huang B, Zhang Y, Xu Y, Zhang S, Xiang H (2018) Probiotics-fermented massa medicata fermentata ameliorates weaning stress in piglets related to improving intestinal homeostasis. Appl Microbiol Biotechnol 102(24):10713–10727. https://doi.org/10.1007/s00253-018-9438-y
Zong S, Ye H, Ye Z, He Y, Zhang X, Ye M (2022) Polysaccharides from Lachnum sp. Inhibited colitis-associated colon tumorigenesis in mice by modulating fecal microbiota and metabolites. Intern Immunopharmacol 108:108656. https://doi.org/10.1016/j.intimp.2022.108656
Vanegas SM, Meydani M, Barnett JB, Goldin B, Kane A, Rasmussen H, Brown C, Vangay P, Knights D, Jonnalagadda S, Koecher K, Karl JP, Thomas M, Dolnikowski G, Li L, Saltzman E, Wu D, Meydani SN (2017) Substituting whole grains for refined grains in a 6-wk randomized trial has a modest effect on gut microbiota and immune and inflammatory markers of healthy adults. Am J Clin Nutr 105(3):635–650. https://doi.org/10.3945/ajcn.116.146928
Olivares PDSG, Pacheco ABF, Aranha LN, Oliveira BDS, Santos AA, Santos PCMD, Neto JFN, Rosa G, Oliveira GMM (2021) Gut microbiota of adults with different metabolic phenotypes. Nutrition 90:111293. https://doi.org/10.1016/j.nut.2021.111293
Yang W, Yu T, Huang X, Bilotta AJ, Xu L, Lu Y, Sun J, Pan F, Zhou J, Zhang W, Yao S, Maynard CL, Singh N, Dann SM, Liu Z, Cong Y (2020) Intestinal microbiota-derived short-chain fatty acids regulation of immune cell IL-22 production and gut immunity. Nat Commun 11(1):4457. https://doi.org/10.1038/s41467-020-18262-6
Nakamoto N, Amiya T, Aoki R, Taniki N, Koda Y, Miyamoto K, Teratani T, Suzuki T, Chiba S, Chu PS, Hayashi A, Yamaguchi A, Shiba S, Miyake R, Katayama T, Suda W, Mikami Y, Kamada N, Ebinuma H, Saito H, Hattori M, Kanai T (2017) Commensal Lactobacillus controls immune tolerance during acute liver injury in mice. Cell Rep 21(5):1215–1226. https://doi.org/10.1016/j.celrep.2017.10.022
González-Sarrías A, Romo-Vaquero M, García-Villalba R, Cortés-Martín A, Selma MV, Espín JC (2018) The Endotoxemia marker lipopolysaccharide-binding protein is reduced in overweight-obese subjects consuming pomegranate extract by modulating the gut microbiota: a randomized clinical trial. Mol Nutr Food Res 62(11):e1800160. https://doi.org/10.1002/mnfr.201800160
Gutiérrez-Repiso C, Hernández-García C, García-Almeida JM, Bellido D, Martín-Núñez GM, Sánchez-Alcoholado L, Alcaide-Torres J, Sajoux I, Tinahones FJ, Moreno-Indias I (2019) Effect of synbiotic supplementation in a very-low-calorie ketogenic diet on weight loss achievement and gut microbiota: A randomized controlled pilot study. Mol Nutr Food Res 63(19):e1900167. https://doi.org/10.1002/mnfr.201900167
Parker BJ, Wearsch PA, Veloo ACM, Rodriguez-Palacios A (2020) The genus alistipes: gut bacteria with emerging implications to inflammation, cancer, and mental health. Front Immunol 11:906. https://doi.org/10.3389/fimmu.2020.00906
Wang H, Huang J, Ding Y, Zhou J, Gao G, Han H, Zhou J, Ke L, Rao P, Chen T, Zhang L (2022) Nanoparticles isolated from porcine bone soup ameliorated dextran sulfate sodium-induced colitis and regulated gut microbiota in mice. Front Nutr 9:821404. https://doi.org/10.3389/fnut.2022.821404
Rosadini CV, Zanoni I, Odendall C, Green ER, Paczosa MK, Philip NH, Brodsky IE, Mecsas J, Kagan JC (2015) A single bacterial immune evasion strategy dismantles both MyD88 and TRIF signaling pathways downstream of TLR4. Cell Host Microbe 18(6):682–693. https://doi.org/10.1016/j.chom.2015.11.006
Zhou W, Pal AS, Hsu AY, Gurol T, Zhu X, Wirbisky-Hershberger SE, Freeman JL, Kasinski AL, Deng Q (2018) MicroRNA-223 Suppresses the canonical NF-κB pathway in basal keratinocytes to dampen neutrophilic inflammation. Cell Rep 22(7):1810–1823. https://doi.org/10.1016/j.celrep.2018.01.058
Álvarez K, Villar-Vesga J, Ortiz-Reyes B, Vanegas-García A, Castaño D, Rojas M, Vásquez G (2020) Induction of NF-κB inflammatory pathway in monocytes by microparticles from patients with systemic lupus erythematosus. Heliyon 6(12):e05815. https://doi.org/10.1016/j.heliyon.2020.e05815
Kimura A, Naka T, Muta T, Takeuchi O, Akira S, Kawase I, Kishimoto T (2005) Suppressor of cytokine signaling-1 selectively inhibits LPS-induced IL-6 production by regulating JAK-STAT. Proc Natl Acad Sci U S A 102(47):17089–17094. https://doi.org/10.1073/pnas.0508517102
Novianti E, Katsuura G, Kawamura N, Asakawa A, Inui A (2021) Atractylenolide-III suppresses lipopolysaccharide-induced inflammation via downregulation of toll-like receptor 4 in mouse microglia. Heliyon 7(10):e08269. https://doi.org/10.1016/j.heliyon.2021.e08269
Block ML, Zecca L, Hong JS (2007) Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci 8(1):57–69. https://doi.org/10.1038/nrn2038
Ji GQ, Chen RQ, Wang L (2016) Anti-inflammatory activity of atractylenolide III through inhibition of nuclear factor-κB and mitogen-activated protein kinase pathways in mouse macrophages. Immunopharmacol Immunotoxicol 38(2):98–102. https://doi.org/10.3109/08923973.2015.1122617
Arjumand W, Seth A, Sultana S (2011) Rutin attenuates cisplatin induced renal inflammation and apoptosis by reducing NFκB, TNF-α and caspase-3 expression in wistar rats. Food Chem Toxicol 49(9):2013–2021. https://doi.org/10.1016/j.fct.2011.05.012
Acknowledgements
The authors wish to thank the staff at the Animal Ethics Committee of Jiangnan University for their technical assistance during this study.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 31972086, 32172173, 32072197), Yongjiang Talent Introduction Programme (2021C-003-T), and Collaborative Innovation Center of Food Safety and Quality Control in Jiangsu Province.
Author information
Authors and Affiliations
Contributions
Bingyong Mao, Weriling Guo, and Xuemei Liu performed the experiments and wrote the manuscript. Shumao Cui and Qiuxiang Zhang checked the logicality and language of this manuscript. Jianxin Zhao, Xin Tang, and Hao Zhang conceived and designed the study. Xin Tang was responsible for overall study coordination of this manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mao, B., Guo, W., Liu, X. et al. Potential Probiotic Properties of Blautia producta Against Lipopolysaccharide-Induced Acute Liver Injury. Probiotics & Antimicro. Prot. 15, 785–796 (2023). https://doi.org/10.1007/s12602-023-10044-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-023-10044-y