Abstract
In the current study, we investigated the effect of a probiotic bacterium (Lactobacillus rhamnosus ATCC 7469) microencapsulated with alginate and hi-maize starch and coated with chitosan on improving growth factors, body composition, blood chemistry, and the immune response of rainbow trout (initial weight: 18.41 ± 0.32 g). Four experimental diets were formulated to feed fish for 60 days. They were control diet without any additive (C), diet added with beads without probiotic (E), a probiotic sprayed to the diet (L.r), and encapsulated probiotic supplemented diet (E-L.r). The results indicated that feeding with E-Lr significantly improved weight gain (84.98 g) and feed conversion ratio (0.95) compared to the other groups (P < 0.05). Also, fish fed E-Lr diet had a significantly higher value of whole-body protein (17.51%), total protein in the blood (4.98 g/dL), lysozyme (30.66 U/mL), alternative complement pathway hemolytic activity (134 U/mL), superoxide dismutase (203 U/mg protein), and catalase (528.33 U/mg protein) (P < 0.05) as compared to those fed the control diet. Similarly, a higher relative expression of immune-related genes such as interleukin-1 (Il-1) and tumor necrosis factor-alpha (TNF-1α) were reported in those fed E-L.r and L.r diets respectively. Interestingly, the fish fed dietary E-L.r had a significantly lower value of lipid in the whole body (4.82%) and cholesterol in the blood (160.67%) in comparison with those fed the control diet (P < 0.05). At the end of the experiment, all groups were challenged by Yersinia ruckeri where the survival rate of rainbow trout fed dietary E-L.r (70.36%) was statistically higher than that of the others (P < 0.05). Overall, the results suggested that encapsulated probiotic Lact. rhamnosus ATCC 7469 acted better than unencapsulated probiotic and has a potential to improve growth performance, flesh quality, and the immune response of rainbow trout.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Increasing population and the need to provide food for them has attracted the attention of the different countries to increase the number of aquaculture products in their food basket. Furthermore, the high quality of aquaculture products for human nutrition has made the aquaculture industry the fast growing sector of food industry with 8% growing each year [1]. Farmers tend to increase the stocking density in some species such as rainbow trout (Oncorhynchus mykiss) in order to boost the fish production. However, high density adversely affects health, survival ratio, growth performance, and product quality of rainbow trout [2, 3]. One of the worst consequences of high density has been an increase in the population of pathogenic microorganisms. In the last decades, irresponsible use of antibiotics for killing these microorganisms has brought the emergence of numerous resistant bacteria [4, 5]. In this sense, supplementing fish diets with some alternative immunostimulants, such as probiotic for boosting the immune system, are an appropriate substitution. Probiotics can help to replace or minimize the application of antibiotics and chemotherapeutics in fish farming [6,7,8]. The inclusion of probiotics in the diets is a key step to protect farmed fish against stress or infection under farming conditions [9]. Probiotics benefit the host by improving disease resistance, health status, growth performance, feed utilization, and stress response when administered in the feed or in the rearing water [10]. Among probiotics, different strains of Lactobacillus spp. have been added to farm animals’ diet for a long time and still is one of the most common probiotics in animal farming [11]. Different strains of Lactobacillus rhamnosus had various health effects such as the prevention of acute diarrhea, allergies, and lowering cholesterol levels and immune stimulation in humans [12]. In aquaculture studies, some investigators reported the potential of different Lact. rhamnosus strains administration against Aeromonas salmonicida, Vibrio anguillarum, and Flavobacterium psychrophilum in rainbow trout [13]; and Edwardsiella tarda [14] and Streptococcus agalactiae [15] in tilapia (Oreochromis niloticus). Many studies also indicated the role of different strains of Lact. rhamnosus in improving the growth performance and the immune response in different fish species [16,17,18,19,20,21,22]. However, there is no study investigating the effect of Lact. rhamnosus ATCC 7469 on aquaculture species.
Although probiotics such as Lact. rhamnosus ATCC 7469 provide a healthy condition for the host, it is important to supplement in appropriate ratios [23, 24]. It was recommended that minimum level of probiotic in food product to be106 colony-forming unit gram−1 (CFU/g) [25], or 107 CFU/g at the point of delivery or to be eaten in appropriate quantities (daily intake of 108–109 cells) [26]. Most probiotics species such as Lact. rhamnosus ATCC 7469 can be destroyed by harsh processing and gastrointestinal conditions, such as low pH, salinity, temperature, or oxidative stress [27]. Protecting probiotic living cells against adverse environmental conditions using a physical barrier is an approach currently receiving great considerations in order to maintain the viability of probiotics during the preparation, storage, and consumption stages [28, 29]. The encapsulation technique is a simple, safe, and reliable method, which increases the survival of bacteria. This method also results in the controlled release of probiotics at the targeted region (posterior intestinal tract, as they are supplied orally) [30, 31].
Previous investigators found that coating alginate microcapsules with chitosan improves the stability of alginate beads and thus the viability of encapsulated probiotic organisms up to 80–95% [32, 33]. Regardless of probiotic compounds, available materials in capsules such as starch and oligosaccharides promote the survival of probiotic bacteria as well [33, 34].
Rainbow trout with 814 thousand tonnes annual production has been considered one of the most important inland aquaculture species worldwide, especially in Chile, Turkey, and Iran [1]. According to Iran Fishery Organization statistic in 2016, rainbow trout production was 166 thousand tones, being the highest consumed species nationally and it is predicted to reach 200 thousand tones soon.
Although probiotics are widely used in aquaculture, a few pieces of research have been performed on encapsulating probiotics for aquaculture application [15, 29, 35,36,37,38,39]. According to our knowledge, there is no study about the effect of Lactobacillus species encapsulation on growth performance, body composition, immunity, and resistance against pathogens in aquaculture species. Also, there is no study investigating the effect of supplementing Lact. rhamnosus ATCC 7469 on aquaculture research. To get more insight into our project about the effect of probiotic on finfishes [Montazeri et al. 2019 (unpublished), 40], we designed this research. Hence, the purpose of this work is to assess the effect of probiotic Lact. rhamnosus ATCC 7469 administered in two different ways on growth performance, body composition, immune response, blood constituents, and disease resistance against Yersinia ruckeri in rainbow trout.
Materials and Methods
We declare that all steps of this experiment were performed according to the Tarbiat Modares University protocols (The guidelines, adopted from the Declaration of Helsinki (1975) and The Society for Neuroscience Animal Care and Use guidelines (1998)) for supporting animal ethics. This guideline was approved for implementation by the Medical Ethics Committee, School of Medical Sciences of the Tarbiat Modares University on 18 April, 1385/17th April, 2006. This protocol was mentioned in our previous works [41,42,43,44].
Probiotic Preparation and Encapsulation
Pure freeze-dried probiotic culture of Lact. rhamnosus (ATCC 7469) was purchased from Persian Type Culture Collection and was cultured in the MRS-broth (de Man-Rogosa-Sharpe) at 37 °C for 48 h. The probiotic biomass was collected by centrifugation at 4000 g for 10 min at 4 °C. Then, it was washed twice with sterile saline 0.9% and collected with the same centrifugation conditions. The cell count was determined by decimal series dilution and plating on MRS agar. Culture cells were preserved with 30% glycerol at − 20 °C until the usage. All glassware and solutions used in the protocols were sterilized at 121 °C for 15 min. The microcapsule was produced by meaning the previously described emulsification method with some modifications [45, 46]. A 2% sodium alginate (medium viscosity, Sigma–Aldrich, St. Louis, USA) and 1% resistant corn starch (Sisco Research Laboratories Pvt. Ltd., India) were mixed in distilled water and then 1% of probiotic (108 CFU/mL) was added to the mixture. Next, the produced mixture was dispersed in 150 mL canola oil containing 1.5% TWEEN 80 at 400 rpm for 5 min using a magnetic stirrer (BOECO, Germany). It was followed by adding 150 mL of 0.5 M calcium chloride solution. Then, precipitation of calcium-alginate beads at the bottom of the beaker at the calcium chloride layer (water phase) was done by keeping the mixture stable for 25–30 min. They were harvested by centrifugation (750 g, 5 min) and further washed with 0.9% saline containing 5% glycerol and stored at 4 °C. Alginate beads were coated with chitosan according to previous methods [47]. In brief, 0.4 g chitosan (448869, low molecular weight, Sigma-Aldrich, St. Louis, USA) was dissolved in 90 mL distilled water acidified with glacial acetic acid to achieve a final chitosan concentration of 0.4% (w/v). The chitosan solution was autoclaved at 121 °C for 15 min. Then, the pH was adjusted to 5.7 by adding 1 M NaOH. The solution was filtered (Whatman no. 4). Alginate beads were washed with distilled water, immersed in 100 mL of chitosan solution, and gently stirred at 100 rpm for 40 min in an orbital shaker. Finally, the chitosan-coated beads were washed with distilled water and used on the same day.
Fish and Fish Rearing
The Rainbow trout (n = 180, with an average weight 18.41 ± 0.32 g and average length 12.37 ± 0.22 cm) were purchased from a private farm (Babol, Mazandaran, Iran) and transferred to the Nutrition Laboratory in Faculty of Natural Resources and Marine Sciences at Tarbiat Modares University. Before starting the experiment, acclimatization of rainbow trout was done for 15 days after which they randomly were distributed into a semi-recirculation system with 12 fiberglass circular tanks (300-L, 15 fish per tank). The fish were fed twice daily with experimental diets at satiation levels. Daily, 30–40% of the tank water was exchanged in order to keep all tanks clean. We adjusted a 12 h:12 h light:dark photoperiod for 60 days. Water temperature, dissolved oxygen, and pH were 16 ± 1.5 °C, 7.0 ± 1.6 mg/L, and 7–8, respectively. Chemical factors in water were measured as follows: temperature by mercury thermometer (Zomorodazma Company, Iran), dissolved oxygen by Cyberscan Eutech instruments (DO 110, Singapore), and pH by Hanna instrument (8314, USA). The level of total ammonia-nitrogen was measured every 2 weeks which was lower than 0.05 mg/L [43].
Feed and Feeding
The basal diet was formulated with 50% fishmeal as the protein source (Table 1). The feeding trial was conducted in four experimental groups with three replicas each. The treatments included control (C), capsule without probiotic (E), sprayed probiotic to the diet (L.r), and encapsulated probiotic supplemented diet (E-L.r). Free probiotic with a concentration of 109 CFU/mL was sprayed with canola oil to the 100-g diet, mixed manually, and air dried on a clean bench for 24 h (to reach approximately 108 CFU/100 g). The encapsulated probiotic was added to the basal diet in the same way as for the unencapsulated. To establish the same conditions, the control diet was sprayed with distilled water and canola oil. During the 60 days of experimental period, the diets including different forms of probiotics were prepared every 2 weeks. For protecting the diets from spoilage, all diets were stored at 4 °C. To assess the viability of the sprayed bacterium, 1-g L.r diet was dissolved, and its bacterial count was performed on MRS agar at different time intervals (0, 5, 10, and 15 days).
Growth Performance and Sampling
At the end of the experiment, the fish were left fasted for 24 h and then anesthetized with clove oil stock solution (50 ppm). The body weight and length of each fish were measured. Growth parameters―weight gain (WG), specific growth rate (SGR), and feed conversion ratio (FCR)―were calculated using the common equations as follows:
Biochemical Analysis
Crude protein, crude lipid, moisture, and ash contents in the diets and whole body of rainbow trout were determined according to the standard methods of AOAC (2005) [48]. For more details about the standard methods and instruments utilized, please check our previous papers [41,42,43]. Liver superoxide dismutase (SOD) and catalase (CAT) activity were assayed using commercial kits (ZEIIBio, GmbH, Germany), according to the mentioned methods in kits.
Immunological Analysis
Collection of Blood and Blood Biochemistry
Heparinized syringes were used to collect blood from the caudal vein. The plasma was obtained through centrifugation at 1500 g for 5 min at 4 °C and kept in a freezer at − 80 °C until subsequent analysis. Plasma samples were thawed and the biochemical indices including total proteins (g/dL), albumin (g/dL), cholesterol (mg/dL), triglycerides (mg/dL), and glucose (mg/dL) were measured using commercial spectrophotometric kits (Pars Azmun Co. Ltd., Iran) by a chemistry analyzer (model AE 600, ERMA, Tokyo, Japan). Three fish from each tank were randomly taken for analyzing blood biochemistry and immune parameters.
Alternative Complement Pathway Hemolytic Activity (ACH50)
The ACH50 was assayed by hemolysis of rabbit red cells (RaRBC) [49]. Briefly, a series of volumes of diluted plasma ranging from 0.1 to 0.25 mL was dispensed to test tubes where the total volume was brought up to 0.25 mL with a barbitone buffer in the presence of ethylene glycol-bis (2-aminoethoxy)-tetraacetic acid (EGTA), and Mg+2. Then, 0.1 mL of RaRBC was added to each tube. After incubation for 2 h at 22 °C, 3.15 mL of 0.9% NaCl was added. In the next step, the sample was centrifuged at 836 g for 5 min at 4 °C to eliminate unlysed RaRBC. The absorbance of the supernatant was measured at 414 nm. Finally, the volume yielding 50% hemolysis was used for determining the complement activity of the sample as follows:
In the above relation, K represents the volume of serum in mL, which causes 50% hemolysis, 0.5 is constant, and finally the dilution factor in this test is 0.01.
Lysozyme Activity Assay
Lysozyme activity was determined in plasma through measuring (530 nm after 1 and 5 min at 22 °C) the reduction of the turbidity of a Micrococcus lysodeikticus suspension, as previously described [50]. Lysozyme activity was determined using as standard lyophilized hen egg-white lysozyme (Sigma-Aldrich, St. Louis, USA) serially diluted in a sodium phosphate buffer (0.05 M; pH 6.2). A unit of enzyme activity was defined as a reduction in absorbance by 0.001/min.
Plasma Immunoglobulin M Assay
Total immunoglobulin (IgM) levels were measured by using an enzyme-linked immunosorbent assay (ELISA) mouse kit purchased from Eastbiopharm Company (Hangzhou Eastbiopharm Co., Ltd. China). Briefly, wells of flat-bottomed 96-well plates were coated with trout plasma samples and washed with wash buffer (20× (PBS with 1% Tween™ 20)) to eliminate the excess of Ag. Then, a biotinylated detection antibody specific for mouse Ig M and avidin-Horseradish Peroxidase (HRP) conjugate were successively added to each well and incubated at 37 °C for 1 h. Free components were washed away. The HRP substrate (tetramethylbenzidine) solution was added to each well and the plate was incubated at 37 °C for 1 h. The oxidation of tetramethylbenzidine by peroxidase develops a blue color in wells. The enzyme-substrate reaction was terminated by the addition of stop solution and the color turned yellow. The optical density (OD) was measured spectrophotometrically at a wavelength of 450 nm in a plate reader (model Epoch 2, Biotek, USA). Blank wells were chromogen solutions and stop solution. The mean absorbance of negative controls for each plate was subtracted from the optical density (OD) at 450 nm. The OD value is proportional to the concentration of mouse Ig M and we calculated the concentration of Ig M in the samples by comparing the OD of the samples to the standard curve.
Real-Time RT-PCR
At the end of the experimental period, 50 to 100 mg of kidney tissue was collected and immediately stored in liquid nitrogen. Then, theses samples were transferred to − 80 °C until RNA extraction. Total RNA extraction was performed according to Biozol Reagent company protocol (Bio flux; China). The quality and quantity of total RNA were measured by 1% agarose gel electrophoresis and NanoDrop spectrophotometer at 280 and 260 nm (Thermo Fisher Scientific, USA). The first-strand cDNA was synthesized using SuPrime Script RT Premix (2×) cDNA Synthesis kit (GeNet BIO Inc.; Daejeon, South Korea) by following the manufacturer’s protocol. Primers for target genes (TNF-1α and IL-1) and reference gene (β-Actin) in rainbow trout had already been designed (Table 2). The expression of target genes was examined using SYBR Green Kit (Bio Pars Cyber, Iran) on iQ5 System (Bio-Rad, USA). The complete method was described by Miandare et al. (2016) [51].
Bacterial Challenge by Y. ruckeri
At the end of the experimental trial, Y. ruckeri bacterial challenge was done. For this purpose, bacteria were grown in tryptic soy broth (TSB; QUELAB) for 24 h at 22 °C, centrifuged at 6000 g for 10 min, and suspended in sterile PBS. In this way, 18 fish per treatment (6 fish per tank) were intraperitoneally (IP) challenged with 0.1 mL per fish of bacterial suspension (1 × 107 CFU/mL). The cumulative mortality (%) was recorded for 14 days once the challenge and any moribund fish were removed and bacteriologically examined to confirm the presence of the pathogen in their skin, liver, and kidney.
Statistical Analysis
The results are presented as the mean ± standard deviation (S.D). Statistical analysis was performed using SPSS (version 19). Normality of the data was assessed using the Kolmogorov-Smirnov test and the significant differences were determined using one-way analysis of variance (ANOVA). Duncan’s test was applied for multiple comparisons. Differences were considered statistically significance when P < 0.05.
Result
Growth Performance
The growth performance of fish fed the experimental diets, supplemented or not with Lact. rhamnosus ATCC 7469, is presented in Table 3. According to these results, fish fed dietary E-L.r had a significantly higher value of WG (84.98 g) and SGR (2.78) compared to the other groups. On the other hand, rainbow trout fed E-L.r diet had a significantly lower value of FCR (0.95) as compared with those fed the control diet (1.34) (P < 0.05).
Body Composition
Table 4 reports the results of whole-body composition in rainbow trout fed diets supplemented or not with Lact. rhamnosus ATCC 7469. As observed, the fish fed dietary E-L.r had a significantly higher (17.51%) content of protein and a lower (4.82%) lipid content compared to the rainbow trout fed the control diet (15.75% and 6.75%) (P < 0.05). Also, there was no significant difference in ash and moisture contents in whole body of those fed the experimental diets.
Biochemical Blood Parameters
The biochemical parameters of blood in the fish fed experimental diets are presented in Table 5. According to the results, the fish fed E-L.r diet had a higher content of total proteins (4.98 g/dL) compared with those fed the control diet. Albumin, glucose, and triglyceride levels showed no significant difference between the treatments. Total cholesterol levels in the fish fed E-L.r (160.67 mg/dL) and L.r (156.5 mg/dL) diets were significantly lower than in those fed the control (185.00 mg/dL) and E (185.50 mg/dL) diets (P < 0.05).
Immune System Parameters and Gene Expression
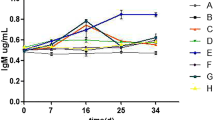
Figure 1 demonstrates the results of immune system factors such as lysozyme, ACH50, and IgM of the fish fed experimental diets. Accordingly, diet supplementation with probiotic, in any way used, significantly improved the lysozyme and ACH50 activities (P < 0.05). Precisely, lysosome contents in the rainbow trout fed dietary E-L.r (30.66 U/mL) were significantly higher than in the control (22.33 U/mL) and E (17.00 U/mL) fed diets (P < 0.05). Regarding ACH50, the rainbow trout fed L.r (132 U mL−1) and E-L.r (134 U mL−1) diets had significantly higher values of the ACH50 as compared with those fed dietary E (119.00 U/mL) and control (110.67 U/mL). The plasma IgM changes revealed no significant differences between the treatments.
Immune system parameters of rainbow trouts fed different diets for 60 days. Control basal diet (C), diet with empty microcapsules added (E), diet sprayed with Lactobacillus rhamnosus ATCC 7469 (L.r.), and diet with encapsulated Lact. rhamnosus added (E-L. r.). Values are means ± SE (n = 9). Values with different letters are significantly different (P < 0.05).
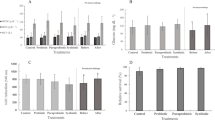
The gene expression of IL-1 and TNF-1α in the kidney was affected by both encapsulated and unencapsulated Lact. rhamnosus ATCC 7469 administrations as well as by spraying Lact. rhamnosus ATCC 7469 to the diet (Fig. 2). In this regard, rainbow trout fed E-L.r diet induced the highest expression of the IL-1 gene in the kidney tissue (0.25) compared to those fed the dietary control (0.06) and E (0.07) (P < 0.05). The expression of TNF-1α gene was significantly higher in fish fed L.r diet (0.73) as compared to those fed the control (0.35) and E (0.33) diets (P < 0.05).
Expression of immune system related genes in rainbow trouts fed different diets for 60 days. Control basal diet (C), diet with empty microcapsules added (E), diet sprayed with Lactobacillus rhamnosus ATCC 7469 (L.r.), and diet with encapsulated Lact. rhamnosus added (E-L. r.). Values are means ± SE (n = 9). Values with different letters are significantly different (P < 0.05)
Antioxidant Activities
Figure 3 reveals the results of antioxidant activities such as SOD and CAT of fish fed experimental diets. According to this figure, diet supplementation with probiotic, in any way used, significantly improved the SOD and CAT activities (P < 0.05). Thus, the fish fed dietary E-L.r had a significantly higher activity of SOD (203 U/mg protein) and CAT (528.33 U/mg protein) as compared to the rainbow trout fed the control diet (P < 0.05).
Antioxidant system parameters of rainbow trouts fed different diets for 60 days. Control basal diet (C), diet with empty microcapsules added (E), diet sprayed with Lactobacillus rhamnosus ATCC 7469 (L.r.), and diet with encapsulated Lact. rhamnosus added (E-L. r.). Values are means ± SE (n = 9). Values with different letters are significantly different (P < 0.05).
Challenge with Y. ruckeri
Microencapsulation of Lact. rhamnosus ATCC 7469 enhanced the resistance of rainbow trout against Y. ruckeri infection (Fig. 4). The highest post-challenge survival rate (70.36%) was observed in fish fed the E-Lr diet. The fish group fed the diet supplemented with free Lact. rhamnosus ATCC 7469 indicated 59.25% survival rate. Overall, the results indicated that the fish fed dietary L.r and E-L.r had a significantly higher survival ratio as compared with those fed the control and E diets.
Survival of rainbow trouts challenged with Yersinia ruckeri. Fish fed different diets for 60 days were intraperitoneally challenged with 107 CFU/mL of Yersinia and, after 14 days, the cumulative mortalities were recorded. Control basal diet (C), diet with empty microcapsules added (E), diet sprayed with Lactobacillus rhamnosus ATCC 7469 (L.r.), and diet with encapsulated Lact. rhamnosus added (E-L. r.). Values are means ± SE (n = 18). Values with different letters are significantly different (P < 0.05)
Discussion
Growth Performance
Probiotics are the most effective additives which provide a wide range of benefits such as growth promotion and immune system stimulation in the aquaculture industry. However, their efficiency can be negatively affected by poor bioavailability of viable microorganisms in the gastrointestinal tract. The microencapsulation of probiotic cells can increase the viability of probiotic bacteria during processing and delivery to the gastrointestinal tract [28, 29, 32]. Earlier studies have indicated the efficacy of administration of different species of Lactobacillus as a potential growth promoter in tilapia [21, 52], orange-spotted grouper (Epinephelus coioides), [53], roho (Lobeo rohito) [54], rainbow trout [35], and Caspian brown trout (Salmo trutta Caspius) [40]. Also, some studies have shown the role of different strains of Lact. rhamnosus in improving the growth in fish species [16,17,18,19,20,21,22]. However, there is no study on the administration of ATCC 7469 in aquaculture research. Although the administration of probiotics in aquatic animals has been broadly investigated, few works have focused on encapsulating probiotics [29]. In the present study, we showed that chitosan-alginate encapsulated Lact. rhamnosus ATCC 7469 improves the WG, SGR, and FCR of rainbow trout. Fish fed L.r diet had also significantly higher values of these parameters as compared with the control fish. We can conclude that probiotic Lact. rhamnosus ATCC 7469 could improve the growth performance in both sprayed and encapsulated forms. Interestingly, probiotic encapsulation had the highest growth performance effect. The best FCR value was observed in the fish fed encapsulated Lact. rhamnosus ATCC 7469 supplemented diet. Several in vivo works showed some significant positive effects of the encapsulation method to enhance the viability and/or the functional properties of the probiotic cells. Probiotics can improve nutrients’ digestibility by increasing the activity of digestive enzymes, maintaining normal intestinal microbiota, and stimulating the synthesis of vitamins [6, 7, 38]. These improvements finally enhance growth performance and FCR [6, 7, 38]. Definitely, investigating digestibility, amino acids, and fatty acids profile of fish fed encapsulated probiotic supplemented diet can help us better understand the mechanisms. In our future work, we will investigate these factors to illustrate the possible reasons behind the enhanced growth performance and feed efficiency of rainbow trout fed the encapsulated probiotic supplemented diet.
Body Composition
Body compositions in the present study were affected by encapsulated probiotic where the fish fed dietary E-L.r had significantly higher and lower protein and lipid contents respectively. Although the chemical composition of aquatic species depends on internal factors and external factors (age, gender, size) and external factors (water quality, season, and geographical area), the main reason is usually the diet [55]. Our previous works also indicated changes in the body composition arising from the diet [56,57,58,59]. Parallel with the present study, some works indicated that inclusion of probiotics to the diet can increase the protein and reduce the lipid contents in the body [60,61,62,63]. We observed similar improvement of flesh quality (increased protein and decreased lipid) in the fish fed herbal medicine [64,65,66] and microalgae [67]. On the other hand, some studies have reported elevated lipid in the body upon probiotic feeding [68, 69]. It was not clear how encapsulated Lact. rhamnosus ATCC 7469 could improve the flesh quality of rainbow trout in our study. Although we hypothesize that improving nutrient retention, digestibility, and absorption can explain this, more research is required.
Blood Biochemistry
Levels of plasma components in the blood are valuable tools for assessing the fish health [18]. In our study, biochemical blood parameters indicated that the diet supplemented with encapsulated Lact. rhamnosus ATCC 7469 increased the total protein in the blood. Some researchers have reported that the rise in total proteins is an indicator of healthy condition as regards immunity in fish [7, 42, 70]. Other researchers reported a significant increase in the protein levels in the rainbow trout plasma fed diets including Lact. rhamnosus [18, 22] which were compatible with our study. Also, it was reported that the capsule including Lact. rhamnosus could activate the anabolic capacity of the hepatocytes to produce blood proteins [71]. Although plasma glucose levels remained unaffected, plasma cholesterol was significantly lower in the group fed free and encapsulated Lact. rhamnosus ATCC 7469 diets. Some studies previously showed that lipid components in the blood can be influenced by probiotic supplementation [8, 19, 70].
Immune Parameters and Immune-Related Genes
Immune response parameters such as lysozyme, ACH50, and IgM are considered as some appropriate indicators of the health status of fish under various nutritional and environmental conditions [72]. The results of the present study revealed that lysozyme and ACH50 were enhanced in the probiotic-treated fish groups, especially those receiving encapsulated Lact. rhamnosus ATCC 7469. Similarly, many studies indicated the positive effect of Lact. rhamnosus in improving the immune response [16,17,18,19,20,21,22]. We found no significant difference in the total IgM levels between different groups. Probiotics including Lactobacillus spp. had increased lysozyme and ACH50 in fish [18, 19, 73, 74]. Lysozyme, one of the important bactericidal enzymes of innate immunity, is an important tool for fighting against infectious agents in the fish body [71]. One of the most important factors in innate immunity is ACH50 [16]. In the same way, some studies found that ACH50 was significantly higher in the fish fed different strains of Lact. rhamnosus diets [17, 53, 75]..
The expressions of two immune-related genes were evaluated in the fish fed encapsulated and sprayed Lact. rhamnosus ATCC 7469 supplemented diets. In the present work, IL-1 was significantly upregulated in the kidney of the fish fed dietary encapsulated Lact. rhamnosus ATCC 7469 and TNF-1α in the free probiotic group, when compared to the control. There is an agreement between our finding and previous studies, showing that the supplementation of probiotic bacteria in tilapia increased the expression of pro-inflammatory cytokines, including IL-1 and TNF-α [17]. Similarly, higher levels of IL10 and TNF-α induction have been reported in RAW264.7 murine macrophages treated with Lactobacillus salivarus microencapsulated into alginate/chitosan [76]. Furthermore, encapsulation of Lactobacillus plantarum into alginate/chitosan/alginate microcapsules induced secretion of TNF-1α and IL6 from macrophages and dendritic cells, proving the immunomodulatory effect of such a probiotic [77]. Higher plasma ACH50 corresponded with the TNF-α and IL-1 expression in the fish fed supplemented diet with the probiotic [17]. Overall, in the present study, encapsulated and sprayed Lact. rhamnosus ATCC 7469 diets could improve the immune response in both ACH50 and lysozyme, as well as gene expression level. Another point is that we can match the improvement of immune response and growth performance in rainbow trout fed E-L.r diets. Boosting immune response could indirectly enhance the growth performance of fish.
Antioxidant Activity
Superoxide anions, along with hydroxyl radicals and nitric oxides, are induced reactive oxygen species, supporting macrophages to kill microbial communities. Probiotics, as an immunostimulant, can enhance the phagocytic activity and the production of reactive oxygen metabolites by macrophages [78]. In fish species, SOD and CAT detoxify reactive oxygen species and they are thus considered as an indicator of antioxidant activity. SOD and CAT were significantly higher in the liver of fish fed E-L.r diet when compared with those fed the control. Similarly, in different species such as Japanese eel (Anguilla japonica) [78], red sea bream [79], common carp Cyprinus carpio [80], turbot (Scophthalmus maximus) [81], and tilapia [82] lactobacillus spp. could increase SOD and CAT activities. Based on the results, there was no significant difference in antioxidant activities of fish fed the encapsulated and sprayed probiotic to the diets. It means that antioxidant enzymes in rainbow trout are stable where an increase in the loading of probiotic could not enhance these enzymes. In other words, a specific level of probiotic was enough to enhance these enzymes beyond which this value did not change SOD and CAT activities. On the other hand, some authors found diminished SOD and CAT activities when the fish were fed a diet supplemented with probiotics [53]. However, most studies have reported enhanced antioxidant activities such as SOD and CAT in fish upon administration of probiotics to the diets rather than a decline.
Challenge with Y. ruckeri
Probiotics not only can promote the growth and boost immune response, they can also control bacterial infectious disease. The challenge test showed that Lact. rhamnosus ATCC 7469 increased the survival rates of rainbow trout against Y. ruckeri. The highest survival rate was obtained in free and encapsulated Lact. rhamnosus ATCC 7469 groups. In line with our findings, some studies found that probiotics increased disease resistance in tilapia [14, 83] and rainbow trout [38]. In aquaculture studies, many works have reported potential of different strains of Lact. rhamnosus against A. salmonicida, V. anguillarum, and F. psychrophilum in rainbow trout [13], E. tarda [14], and S. agalactiae [15] in tilapia. Also, probiotics lactobacillus spp. in either free or bio-encapsulated form can improve the natural resistance and survival rate of larvae and postlarvae of fish [84]. Encapsulation causes the entrance of more bacteria into the intestines. Thus, encapsulated Lact. rhamnosus ATCC 7469 could enhance the immune response and eventually, caused a higher survival ratio in this group. However, the effect of encapsulation on resistance against bacteria and disease have rarely been investigated. Some studies indicated that the encapsulation of different probiotic bacteria and applying resistant starch as prebiotics and chitosan as a coating material significantly enhanced the survival rate of microorganisms in vitro [33, 85].
Conclusion
The current study showed how designing and developing a diet contains encapsulated probiotic in the aquaculture industry can improve growth and immune response. In general, encapsulated Lact. rhamnosus ATCC 7469 supplemented diets positively influenced the growth performance, body composition, blood biochemistry, antioxidant activity, and the immune system of rainbow trout. Furthermore, encapsulated Lact. rhamnosus ATCC 7469 more effectively enhanced the survival rate of rainbow trout after the challenge test with Y. ruckeri. As little works have been done to understand how encapsulated probiotics can affect the fish physiology, we would focus in the future research on the effect of encapsulation on digestibility, digestive enzymes, fatty acids, and amino acids profiles of fish.
References
FAO (2018) The state of world fisheries and aquaculture 2018. Food and Agriculture Organization of the United Nations, Rome http://www.fao.org/state-of-fisheries-aquaculture
Long L, Zhang H, Ni Q, Liu H, Wu F, Wang X (2019) Effects of stocking density on growth, stress, and immune responses of juvenile Chinese sturgeon (Acipenser sinensis) in a recirculating aquaculture system. Comp Biochem Physiol C Toxicol 219:25–34
Mirghaed AT, Hoseini SM, Ghelichpour M (2018) Effects of dietary 1, 8-cineole supplementation on physiological, immunological and antioxidant responses to crowding stress in rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol 81:182–188
Santos L, Ramos F (2018) Antimicrobial resistance in aquaculture: current knowledge and alternatives to tackle the problem. Int J Antimicrob Agents 52(2):135–143
Xiong W, Sun Y, Zhang T, Ding X, Li Y, Wang M, Zeng Z (2015) Antibiotics, antibiotic resistance genes, and bacterial community composition in fresh water aquaculture environment in China. Microb Ecol 70(2):425–432
Hai N (2015) The use of probiotics in aquaculture. J Appl Microbiol 119(4):917–935
Dawood MA, Koshio S, Abdel-Daim MM, Van Doan H (2018) Probiotic application for sustainable aquaculture. Rev Aquacult 11(3):907–924
Dawood MA, Koshio S, Esteban MÁ (2018) Beneficial roles of feed additives as immunostimulants in aquaculture: a review. Rev Aquacult 10(4):950–974
Verschuere L, Rombaut G, Sorgeloos P, Verstraete W (2000) Probiotic bacteria as biological control agents in aquaculture. Microbiol Mol Biol Rev 64(4):655–671
Cordero H, Esteban MÁ, Cuesta A (2014) Use of probiotic bacteria against bacterial and viral infections in shellfish and fish aquaculture. Sustainable Aquaculture Techniques. IntechOpen. https://www.intechopen.com/books/sustainable-aquaculture-techniques/use-of-probiotic-bacteria-against-bacterial-and-viral-infections-in-shellfish-and-fish-aquaculture, In. https://doi.org/10.5772/57198
Land MH, Rouster-Stevens K, Woods CR, Cannon ML, Cnota J, Shetty AK (2005) Lactobacillus sepsis associated with probiotic therapy. Pediatrics 115(1):178–181
Sanders ME, Guarner F, Guerrant R, Holt PR, Quigley EM, Sartor RB, Sherman PM, Mayer EA (2013) An update on the use and investigation of probiotics in health and disease. Gut 62(5):787–796
Nikoskelainen S, Salminen S, Bylund G, Ouwehand AC (2001) Characterization of the properties of human-and dairy-derived probiotics for prevention of infectious diseases in fish. Appl Environ Microbiol 67(6):2430–2435
Pirarat N, Kobayashi T, Katagiri T, Maita M, Endo M (2006) Protective effects and mechanisms of a probiotic bacterium Lactobacillus rhamnosus against experimental Edwardsiella tarda infection in tilapia (Oreochromis niloticus). Vet Immunol Immunopathol 113(3–4):339–347
Pirarat N, Pinpimai K, Rodkhum C, Chansue N, Ooi EL, Katagiri T, Maita M (2015) Viability and morphological evaluation of alginate-encapsulated Lactobacillus rhamnosus GG under simulated tilapia gastrointestinal conditions and its effect on growth performance, intestinal morphology and protection against Streptococcus agalactiae. Anim Feed Sci Tech 207:93–103
Nikoskelainen S, Ouwehand AC, Bylund G, Salminen S, Lilius E-M (2003) Immune enhancement in rainbow trout (Oncorhynchus mykiss) by potential probiotic bacteria (Lactobacillus rhamnosus). Fish Shellfish Immunol 15(5):443–452
Pirarat N, Pinpimai K, Endo M, Katagiri T, Ponpornpisit A, Chansue N, Maita M (2011) Modulation of intestinal morphology and immunity in nile tilapia (Oreochromis niloticus) by Lactobacillus rhamnosus GG. Res Vet Sci 91(3):e92–e97
Panigrahi A, Kiron V, Satoh S, Watanabe T (2010) Probiotic bacteria Lactobacillus rhamnosus influences the blood profile in rainbow trout Oncorhynchus mykiss (Walbaum). Fish Physiol Biochem 36(4):969–977
Dawood MA, Koshio S, Ishikawa M, Yokoyama S, El Basuini MF, Hossain MS, Nhu TH, Dossou S, Moss AS (2016) Effects of dietary supplementation of Lactobacillus rhamnosus or/and Lactococcus lactis on the growth, gut microbiota and immune responses of red sea bream, Pagrus major. Fish Shellfish Immunol 49:275–285
Xia Y, Cao J, Wang M, Lu M, Chen G, Gao F, Liu Z, Zhang D, Ke X, Yi M (2019) Effects of Lactococcus lactis subsp. lactis JCM5805 on colonization dynamics of gut microbiota and regulation of immunity in early ontogenetic stages of tilapia. Fish Shellfish Immunol 86:53–63
Xia Y, Lu M, Chen G, Cao J, Gao F, Wang M, Liu Z, Zhang D, Zhu H, Yi M (2018) Effects of dietary Lactobacillus rhamnosus JCM1136 and Lactococcus lactis subsp. lactis JCM5805 on the growth, intestinal microbiota, morphology, immune response and disease resistance of juvenile Nile tilapia, Oreochromis niloticus. Fish Shellfish Immunol 76:368–379
Topic Popovic N, Strunjak-Perovic I, Sauerborn-Klobucar R, Barisic J, Jadan M, Kazazic S, Kesner-Koren I, Prevendar Crnic A, Suran J, Beer Ljubic B (2017) The effects of diet supplemented with Lactobacillus rhamnosus on tissue parameters of rainbow trout, (Oncorhynchus mykiss Walbaum). Aquac Res 48(5):2388–2401
Reid G, Sanders M, Gaskins HR, Gibson GR, Mercenier A, Rastall R, Roberfroid M, Rowland I, Cherbut C, Klaenhammer TR (2003) New scientific paradigms for probiotics and prebiotics. J Clin Gastroenterol 37(2):105–118
Vaughan EE, Heilig HG, Ben-Amor K, De Vos WM (2005) Diversity, vitality and activities of intestinal lactic acid bacteria and bifidobacteria assessed by molecular approaches. FEMS Microbiol Rev 29(3):477–490
Ramos PE, Cerqueira MA, Teixeira JA, Vicente AA (2018) Physiological protection of probiotic microcapsules by coatings. Crit Rev Food Sci Nutr 58(11):1864–1877
Kim J, Muhammad N, Jhun BH, Yoo J-W (2016) Probiotic delivery systems: a brief overview. J Pharm Investig 46(4):377–386
Corona-Hernandez RI, Álvarez-Parrilla E, Lizardi-Mendoza J, Islas-Rubio AR, de la Rosa LA, Wall-Medrano A (2013) Structural stability and viability of microencapsulated probiotic bacteria: a review. Compr Rev Food Sci F 12(6):614–628
Chávarri M, Marañón I, Villarán MC (2012) Encapsulation technology to protect probiotic bacteria. Probiotics. IntechOpen. https://www.intechopen.com/books/probiotics/encapsulation-technology-to-protect-probiotic-bacteria, In. https://doi.org/10.5772/50046
Masoomi Dezfooli S, Gutierrez-Maddox N, Alfaro A, Seyfoddin A (2018) Encapsulation for delivering bioactives in aquaculture. Rev Aquacult 11(3):631–660
Dong QY, Chen MY, Xin Y, Qin XY, Cheng Z, Shi LE, Tang ZX (2013) Alginate-based and protein-based materials for probiotics encapsulation: a review. IJFST 48(7):1339–1351
Đorđević V, Paraskevopoulou A, Mantzouridou F, Lalou S, Pantić M, Bugarski B, Nedović V (2016) Encapsulation technologies for food industry. Emerging and traditional technologies for safe, healthy and quality food. Springer, In, pp 329–382
Shori AB (2017) Microencapsulation improved probiotics survival during gastric transit. HAYATI J Biosci 24(1):1–5
Călinoiu L-F, Ştefănescu BE, Pop ID, Muntean L, Vodnar DC (2019) Chitosan coating applications in probiotic microencapsulation. Coatings 9 (3):194, doi:/https://doi.org/10.3390/coatings9030194
Burgain J, Gaiani C, Linder M, Scher J (2011) Encapsulation of probiotic living cells: from laboratory scale to industrial applications. J Food Eng 104(4):467–483
Mohammadian T, Dezfuly ZT, Motlagh RG, Jangaran-Nejad A, Hosseini SS, Khaj H, Alijani N (2019) Effect of encapsulated lactobacillus bulgaricus on innate immune system and hematological parameters in rainbow trout (Oncorhynchus mykiss), post-administration of Pb. Probiotics Antimicro:1–14
Rosas-Ledesma P, León-Rubio JM, Alarcón FJ, Moriñigo MA, Balebona MC (2012) Calcium alginate capsules for oral administration of fish probiotic bacteria: assessment of optimal conditions for encapsulation. Aquac Res 43(1):106–116
Cordero H, Guardiola FA, Tapia-Paniagua ST, Cuesta A, Meseguer J, Balebona MC, Moriñigo MÁ, Esteban MÁ (2015) Modulation of immunity and gut microbiota after dietary administration of alginate encapsulated Shewanella putrefaciens Pdp11 to gilthead seabream (Sparus aurata L.). Fish Shellfish Immunol 45(2):608–618
Ghosh B, Cain K, Nowak B, Bridle A (2016) Microencapsulation of a putative probiotic Enterobacter species, C6-6, to protect rainbow trout, Oncorhynchus mykiss (Walbaum), against bacterial Coldwater disease. J Fish Dis 39(1):1–11
Rodrigues JB, Leitão NJ, Chaves KS, Gigante ML, Portella MC, Grosso CR (2014) High protein microparticles produced by ionic gelation containing Lactobacillus acidophilus for feeding pacu larvae. Food Res Int 63:25–32
Jami MJ, Kenari AA, Paknejad H, Mohseni M (2019) Effects of dietary b-glucan, mannan oligosaccharide, Lactobacillus plantarum and their combinations on growth performance, immunity and immune related gene expression of Caspian trout, Salmo trutta caspius (Kessler, 1877). Fish Shellfish Immunol 91:202–208
Esmaeili M, Abedian Kenari A, Rombenso A (2017a) Effects of fish meal replacement with meat and bone meal using garlic (Allium sativum) powder on growth, feeding, digestive enzymes and apparent digestibility of nutrients and fatty acids in juvenile rainbow trout (Oncorhynchus mykiss Walbaum, 1792). Aquac Nutr 23(6):1225–1234
Esmaeili M, Kenari AA, Rombenso A (2017b) Immunohematological status under acute ammonia stress of juvenile rainbow trout (Oncorhynchus mykiss Walbaum, 1792) fed garlic (Allium sativum) powder-supplemented meat and bone meal-based feeds. Comp Clin Path 26(4):853–866
Hosseinpour Aghaei R, Abedian Kenari A, Yazdani Sadati MA, Esmaeili M (2018) The effect of time-dependent protein restriction on growth factors, nonspecific immunity, body composition, fatty acids and amino acids in the Siberian sturgeon (Acipenser baerii). Aquac Res 49(9):3033–3044
Ghosi Mobaraki MR, Abedian Kenari A, Bahrami Gorji S, Esmaeili M (2020) Effect of different levels of fish and vegetable oil on the growth performance, body composition, fatty acid profiles, reproductive performance, and larval resistance in pearl gourami (Trichogaster leeri). Aquac, Nutr In press
Sultana K, Godward G, Reynolds N, Arumugaswamy R, Peiris P, Kailasapathy K (2000) Encapsulation of probiotic bacteria with alginate–starch and evaluation of survival in simulated gastrointestinal conditions and in yoghurt. Int J Food Microbiol 62(1–2):47–55
Shu X, Zhu K (2002) The release behavior of brilliant blue from calcium–alginate gel beads coated by chitosan: the preparation method effect. Eur J Pharm Biopharm 53(2):193–201
Krasaekoopt W, Bhandari B, Deeth H (2004) The influence of coating materials on some properties of alginate beads and survivability of microencapsulated probiotic bacteria. Int Dairy J 14(8):737–743
AOAC (1995) AOAC (Association of Official Analytical Chemists), official methods of analysis (16th ed.), P. Cunniff (Ed.), Airlington, VA
Amar EC, Kiron V, Satoh S, Okamoto N, Watanabe T (2000) Effects of dietary β-carotene on the immune response of rainbow trout Oncorhynchus mykiss. Fisheries Sci 66(6):1068–1075
Demers NE, Bayne CJ (1997) The immediate effects of stress on hormones and plasma lysozyme in rainbow trout. Dev Comp Immunol 21(4):363–373
Miandare HK, Niknejad M, Shabani A, Safari R (2016) Exposure of Persian sturgeon (Acipenser persicus) to cadmium results in biochemical, histological and transcriptional alterations. Comp Biochem Physiol C Toxicol Pharmacol 181:1–8
Van Nguyen N, Onoda S, Van Khanh T, Hai PD, Trung NT, Hoang L, Koshio S (2019) Evaluation of dietary heat-killed Lactobacillus plantarum strain L-137 supplementation on growth performance, immunity and stress resistance of Nile tilapia (Oreochromis niloticus). Aquaculture 498:371–379
Son VM, Chang C-C, Wu M-C, Guu Y-K, Chiu C-H, Cheng W (2009) Dietary administration of the probiotic, Lactobacillus plantarum, enhanced the growth, innate immune responses, and disease resistance of the grouper Epinephelus coioides. Fish Shellfish Immunol 26(5):691–698
Giri SS, Sukumaran V, Oviya M (2013) Potential probiotic Lactobacillus plantarum VSG3 improves the growth, immunity, and disease resistance of tropical freshwater fish, Labeo rohita. Fish Shellfish Immunol 34(2):660–666
Shearer KD (1994) Factors affecting the proximate composition of cultured fishes with emphasis on salmonids. Aquaculture 119(1):63–88
Matani Bour H, Esmaeili M, Abedian Kenari A (2018) Growth performance, muscle and liver composition, blood traits, digestibility and gut bacteria of beluga (Huso huso) juvenile fed different levels of soybean meal and lactic acid. Aquac Nutr 24(4):1361–1368
Roohani AM, Abedian Kenari A, Fallahi Kapoorchali M, Borani MS, Zoriezahra SJ, Smiley AH, Esmaeili M, Rombenso AN (2019) Effect of spirulina Spirulina platensis as a complementary ingredient to reduce dietary fish meal on the growth performance, whole-body composition, fatty acid and amino acid profiles, and pigmentation of Caspian brown trout (Salmo trutta caspius) juveniles. Aquac Nutr 25(3):633–645
Abtahi B, Yousefi M, Kenari AA (2013) Influence of dietary nucleotides supplementation on growth, body composition and fatty acid profile of Beluga sturgeon juveniles (Huso huso). Aquac Res 44(2):254–260
Tazikeh T, Abedian Kenari A, Esmaeili M (2019) Effects of fish meal replacement by meat and bone meal supplemented with garlic (Allium sativum) powder on biological indices, feeding, muscle composition, fatty acids and amino acids profile of whiteleg shrimp (litopenaeus vannamei). Aquac Res, ahead of print. https://doi.org/10.1111/are.14416
Ebrahimi G, Ouraji H, Khalesi M, Sudagar M, Barari A, Zarei Dangesaraki M, Jani Khalili K (2012) Effects of a prebiotic, Immunogen®, on feed utilization, body composition, immunity and resistance to Aeromonas hydrophila infection in the common carp Cyprinus carpio (Linnaeus) fingerlings. J Anim Physiol Anim Nutr 96(4):591–599
Yilmaz E, Genc MA, Genc E (2007) Effects of dietary mannan oligosaccharides on growth, body composition, and intestine and liver histology of rainbow trout, Oncorhynchus mykiss. Isr J Aquac 59(3):182–158
Ghosh S, Sinha A, Sahu C (2008) Dietary probiotic supplementation in growth and health of live-bearing ornamental fishes. Aquac Nutr 14(4):289–299
Allameh S, Yusoff F, Ringø E, Daud H, Saad C, Ideris A (2016) Effects of dietary mono-and multiprobiotic strains on growth performance, gut bacteria and body composition of Javanese carp (Puntius gonionotus, B leeker 1850). Aquac Nutr 22(2):367–373
Ramezanzadeh S, Abedian Kenari A, Esmaeili M (2019a) Immunohematological parameters of rainbow trout (Oncorhynchus mykiss) fed supplemented diet with different forms of barberry root (Berberis vulgaris). Comp Clin Path, ahead of print 29:177–187. https://doi.org/10.1007/s00580-019-03032-8
Ramezanzadeh S, Abedian Kenari A, Esmaeili M, Rombenso A (2020) Effects of different forms of barberry root (Berberis vulgaris) on growth performance, muscle fatty acids profile, whole body composition and digestive enzymes of rainbow trout (Oncorhynchus mykiss). J World Aquacult Soc, In press
Zeilab Sendijani R, Abedian Kenari A, Smiley AH, Esmaeili M (2019) The effect of extract from dill (Anethum graveolens) on the growth performance, body composition, immune system and antioxidant system of rainbow trout (Oncorhynchus mykiss). N Am J Aquacult, ahead of print. https://doi.org/10.1002/naaq.10123
Safavi SV, Abedian Kenari A, Tabarsa M, Esmaeili M (2019) Effect of sulfated polysaccharides extracted from marine macroalgae (Ulva intestinalis and Gracilariopsis persica) on growth performance, fatty acid profile, and immune response of rainbow trout (Oncorhynchus mykiss). J Appl Phycol, In press
Sáenz de Rodrigáñez M, Díaz-Rosales P, Chabrillón M, Smidt H, Arijo S, León-Rubio J, Alarcón F, Balebona M, Moriñigo M, Cara J (2009) Effect of dietary administration of probiotics on growth and intestine functionality of juvenile Senegalese sole (Solea senegalensis, Kaup 1858). Aquac Nutr 15(2):177–185
Mazurkiewicz J, Przybyl A, Sip A, Grajek W (2007) Effect of Carnobacterium divergens and Enterococcus hirae as probiotic bacteria in feed for common carp, Cyprinus carpio L. Arch Pol Fisheries 15(2):93–102
Dawood MA, Koshio S, Ishikawa M, El-Sabagh M, Yokoyama S, Wang W-L, Yukun Z, Olivier A (2017) Physiological response, blood chemistry profile and mucus secretion of red sea bream (Pagrus major) fed diets supplemented with Lactobacillus rhamnosus under low salinity stress. Fish Physiol Biochem 43(1):179–192
Nayak S (2010) Probiotics and immunity: a fish perspective. Fish Shellfish Immunol 29(1):2–14
Zwollo P (2018) The humoral immune system of anadromous fish. Dev Comp Immunol 80:24–33
Jinendiran S, Nathan AA, Ramesh D, Vaseeharan B, Sivakumar N (2019) Modulation of innate immunity, expression of cytokine genes and disease resistance against Aeromonas hydrophila infection in goldfish (Carassius auratus) by dietary supplementation with Exiguobacterium acetylicum S01. Fish Shellfish Immunol 84:458–469
Hoseinifar SH, Van Doan H, Dadar M, Ringø E, Harikrishnan R (2019) Feed additives, gut microbiota, and health in finfish aquaculture. Microbial Communities in Aquaculture Ecosystems. Springer, In, pp 121–142
Panigrahi A, Kiron V, Kobayashi T, Puangkaew J, Satoh S, Sugita H (2004) Immune responses in rainbow trout Oncorhynchus mykiss induced by a potential probiotic bacteria Lactobacillus rhamnosus JCM 1136. Vet Immunol Immunopathol 102(4):379–388
Bajracharya P, Islam MA, Jiang T, Kang S-K, Choi Y-J, Cho C-S (2012) Effect of microencapsulation of Lactobacillus salivarus 29 into alginate/chitosan/alginate microcapsules on viability and cytokine induction. J Microencapsul 29(5):429–436
Jiang T, Kim Y-K, Singh B, Kang S-K, Choi Y-J, Cho C-S (2013) Effect of microencapsulation of Lactobacillus plantarum 25 into alginate/chitosan/alginate microcapsules on viability and cytokine induction. J Nanosci Nanotechnol 13(8):5291–5295
Lee J-S, Cheng H, Damte D, Lee S-J, Kim J-C, Rhee M-H, Suh J-W, Park S-C (2013) Effects of dietary supplementation of Lactobacillus pentosus PL11 on the growth performance, immune and antioxidant systems of Japanese eel Anguilla japonica challenged with Edwardsiella tarda. Fish Shellfish Immunol 34(3):756–761
Dawood MA, Koshio S, Ishikawa M, Yokoyama S (2015) Effects of heat killed Lactobacillus plantarum (LP20) supplemental diets on growth performance, stress resistance and immune response of red sea bream, Pagrus major. Aquaculture 442:29–36
Zhang C-N, Zhang J-L, Guan W-C, Zhang X-F, Guan S-H, Zeng Q-H, Cheng G-F, Cui W (2017) Effects of Lactobacillus delbrueckii on immune response, disease resistance against Aeromonas hydrophila, antioxidant capability and growth performance of Cyprinus carpio Huanghe var. Fish Shellfish Immunol 68:84–91
Li Z, Bao N, Ren T, Han Y, Jiang Z, Bai Z, Hu Y, Ding J (2019) The effect of a multi-strain probiotic on growth performance, non-specific immune response, and intestinal health of juvenile turbot. Scophthalmus maximus L Fish Physiol Biochem:1–15
Dawood MA, Magouz FI, Salem MF, Abdel-Daim HA (2019) Modulation of digestive enzyme activity, blood health, oxidative responses and growth-related gene expression in GIFT by heat-killed Lactobacillus plantarum (L-137). Aquaculture 505:127–136
Ngamkala S, Futami K, Endo M, Maita M, Katagiri T (2010) Immunological effects of glucan and Lactobacillus rhamnosus GG, a probiotic bacterium, on Nile tilapia Oreochromis niloticus intestine with oral Aeromonas challenges. Fisheries Sci 76(5):833–840
Abraham TJ, Babu S, Mondal S, Banerjee T (2007) Effects of dietary supplementation of commercial human probiotic and antibiotic on the growth rate and content of intestinal microflora in ornamental fishes. Bangladesh J Fish Res 11(1):57–63
Abbaszadeh S, Gandomi H, Misaghi A, Bokaei S, Noori N (2014) The effect of alginate and chitosan concentrations on some properties of chitosan-coated alginate beads and survivability of encapsulated Lactobacillus rhamnosus in simulated gastrointestinal conditions and during heat processing. J Sci Food Agric 94(11):2210–2216
NRC (2011) National Research Council, nutrient requirements of fish and shrimp, the National Academies Press, Washington DC. National academies press
Acknowledgments
The authors gratefully acknowledge of the Tarbiat Modares University for the financial supported this research as a Ph.D thesis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hooshyar, Y., Abedian Kenari, A., Paknejad, H. et al. Effects of Lactobacillus rhamnosus ATCC 7469 on Different Parameters Related to Health Status of Rainbow Trout (Oncorhynchus mykiss) and the Protection Against Yersinia ruckeri. Probiotics & Antimicro. Prot. 12, 1370–1384 (2020). https://doi.org/10.1007/s12602-020-09645-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-020-09645-8