Abstract
Probiotics have been documented to promote growth, immunity, and disease resistance in farmed fish. In the present study, we aimed to evaluate single or conjoint effects of some lactic acid bacteria (LAB), namely, Lactococcus lactis, Lactobacillus rhamnosus, and Enterococcus faecalis on the growth performance, immune response, and disease resistance of Carassius auratus. Fish were fed a basal diet supplemented with L. lactis (group B), L. rhamnosus (group C), E. faecalis (group D), L. lactis + L. rhamnosus (group E), L. rhamnosus + E. faecalis (group F), L. lactis + E. faecalis (group G), and L. lactis + L. rhamnosus + E. faecalis (group H) at 5.0 × 108 CFU/g diet for 34 days. After feeding, the final body weight (FBW), weight gain (WG), specific growth rate (SGR), and survival had significantly increased (p < 0.05) for group E. The results indicated that single or conjoint administration of LAB induced high levels of IgM, LZM, AKP, and SOD activity in serum, which may effectively induce humoral immunity, and group E induced even higher levels. At the same time, when compared to the basal diet (group A), the results of qPCR showed that probiotic administration significantly upregulated (p < 0.05) the expression of IL-10, IL-1β, TNF-α, and IFN-γ in the spleen, kidney, liver, and intestine of C. auratus. After challenge with the Aeromonas hydrophila, the survival rates in all probiotic-fed groups were significantly higher (p < 0.05) than those of group A, and the relative protection rates of groups B, C, D, E, F, G, and H were 27%, 30.7%, 40.94%, 42.32%, 39.38%, 33.61%, and 37.5%, respectively. Our data indicated that probiotics could promote growth, enhance immune indicators, and enhance immune capacity. In summary, combined administration of probiotics had a better effect on C. auratus. This experiment could provide a reference for the optimal combination of probiotic additives for aquatic animals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, an increasing number of fishery disease outbreaks have caused major economic losses in the aquaculture industry (Mahmoud et al. 2016; Anjan et al. 2019; Camilo et al. ; Zhang et al. 2017). The improper use of antibiotics is one of the important factors that cause diseases (Kong et al. 2019). Not only can improper use of antibiotics or abuse of antibiotics increase drug residues in water and animal bodies, decrease animal immunity, and increase resistance to pathogenic bacteria, but it can also increase the cost of aquaculture and cause serious environmental problems, which ultimately affects human health (Giri et al. 2014). There is an urgent need to explore safe and high-quality feed additives and potential alternatives to antibiotics. At present, lactic acid bacteria (LAB) have been increasingly widely used as a substitute for veterinary antibiotics (Wang et al. 2019; Zuo et al. 2018; Bo et al. 2015; Marjan et al. 2016). As a feed additive, LAB can colonize in the intestine or reproductive system of animals, produce excellent effects on the animal’s body and improve the animal’s microecological balance (Wang et al. 2019; Mahmoud et al. 2016). As a probiotic that can replace antibiotics in fish farming, it has the characteristics of no pollution, residue, toxic side effects, or drug resistance (Gao et al. 2018; Batista et al. 2016; Yang et al. 2019). The addition of LAB in the feed not only produces excellent effects on the body of fish but also improves the water environment, prevents the growth of pathogenic bacteria, and improves the growth and immune function of the fish (Gregor et al. 2006; Qi et al. 2009). Therefore, LAB is used more and more widely. In our previous study, strains of three probiotics originally isolated from the intestine of C. auratus were noticed to be antagonistic against aquatic pathogens, which might have the better potential to inhibit pathogens and enhance immunity systems.
In recent years, many studies have shown that a certain amount of LAB intake may be beneficial to the health of aquatic animals, such as Scylla serrate (Talib et al. 2017), Cromileptes altivelis (Sun et al. 2018), Oreochromisspp (Yu et al. 2017), Channa argus (Kong et al. 2020), etc. Probiotics can be added to feed as a single strain or a mixture of two or more strains. Some previous studies demonstrated that a combined use of probiotics was better than single use. The combined application of L. lactis and L. plantarum strains can improve the growth, immune response, and disease resistance of Paralichthys olivaceus compared to a single probiotic preparation (Bo et al. 2015, 2016). In addition, dietary supplementation with the mixture of L. rhamnosus and L. lactis could improve growth, immunity, antioxidant, and lipid-lowering effects for Pagrus major compared to supplementation with a single strain (Xia et al. 2018). However, some scholars have shown that a single probiotic is more advantageous in some aspects. A previous study demonstrated that feeding L. lactis and/or E. faecalis improved the growth rate, immune status, and disease resistance of A. veronii infected with Channa argus (Kong et al. 2020). Single L. lactis showed higher survival than did a combination. Different types of LAB and their matching ratios with different types of fish will have different effects. Therefore, the purpose of our research is to explore reasonable ways of adding probiotics. Consequently, the present investigation made an attempt to evaluate the effects of a single or conjoint application of LAB as potential probiotics on growth, blood-immune parameters, immune response, and disease resistance of C. auratus.
Materials and methods
Probiotic bacteria and diets preparation
The three probiotics, including L. lactis, L. rhamnosus, E. faecalis, and the pathogen Aeromonas hydrophila TPS strain (LD50 = 1 × 106 CFU/mL), were all isolated from healthy Carassius auratus intestines and provided by our lab. The three probiotics were cultured in Man-Rogosa-Sharpe (MRS) medium (Solarbio, China) for 48 h at 37 °C in an anaerobic environment. The cultures were centrifuged (Beckman Coulter, AK, USA) at 5000 g for 10 min. The pellets were washed twice with phosphate-buffered saline (PBS) (137 mM NaCl, 10.1 mM NaH2P4, 2.7 mM KCl, 1.8 mM KH2PO4, and pH 7.4) and suspended in PBS. The number of bacterial cells in the suspensions was determined by turbidimetry and administered to C. auratus in the experimental diets at a concentration of 5.0 × 108 CFU/g with slight modifications. Every 50 mL of LAB is coated with 20 g of feed, mixed with 1.5% sodium alginate solution, and dried in a drying oven at 37 °C for later use. The formulation and chemical composition of the basal diet are indicated in Table 1. The basal diet was used as a control. The addition ratio of LAB and the content of live bacteria in each group of fish food are shown in Table 2, which makes the addition ratio of different lactic acid bacteria in the same group the same, and the number of viable lactic acid bacteria should be the same between different groups.
Experimental design and rearing trial
Healthy C. auratus weighing 25.28 ± 0.5 g were obtained from a commercial fish farm (Changchun, China), acclimatized in 300-L tanks with aeration filtered dechlorinated water at 25 ± 1 °C for 2 weeks and fed the basal diet three times daily. After acclimation, C. auratus were randomly divided into four treatment groups. The glass tanks were kept under experimental conditions (temperature: 25 ± 1 °C; pH: 7.2 ± 0.1; ammonia nitrogen: < 0.5 mg/L; nitrites: < 0.05 mg/L; dissolved oxygen: 7.0 mg/L) and a specific cycle (12 h light/12 h dark photoperiod). Water pH, nitrite, ammonia, temperature, and dissolved oxygen were monitored weekly and maintained at optimal levels. The fish were fed two times (08:00 and 16:00) a day for 34 days at a rate of 3% of their body weight. Approximately 1/3 to 1/2 of the water in each plastic tank was changed every 2 days.
Growth performance and sampling
At 0, 7, 16, 25, and 34 days, fish were fasted for 24 h, anesthetized using 300 mg/L methane-sulfonate-222 (MS-222), counted, and weighed. Three fish per tank were randomly collected. The blood was sampled by caudal venipuncture, centrifuged at 4000 rpm for 15 min to acquire serum, and stored at − 20 °C until analysis. In addition, the spleen, kidney, gill, liver, and intestine were immediately sampled. All tissues were flash-frozen in liquid nitrogen and stored at − 80 °C until analysis. The final body weight (FBW), weight gain (WG), feed efficiency ratio (FER), specific growth rate (SGR), and protein efficiency ratio (PER) were calculated as described previously, using the following formulas:
Moisture was determined by the constant-temperature drying weight loss method at 105 °C (GB 5009.3—2010). Crude protein was determined by Kjeldahl’s method (GB 5009.5—2010). Crude lipids were determined by the Soxhlet ether extraction method (GB 5009.6—2010). Ash was determined by the muffle furnace burning method (GB 5009.4—2010).
Humoral immunity parameters
Immunoglobulin M (IgM), lysozyme (LZM) activity, alkaline phosphatase (AKP), and superoxide dismutase (SOD) content in the serum were measured with commercial assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China), as described by Liu et al. (2019) and Gou et al. (2018).
RNA extraction and cDNA synthesis
Total RNA from the spleen, kidney, gill, liver, and intestine was extracted using a High Pure RNA Tissue Kit (Takara, Japan). RNA quality and quantity were analyzed by 1.0% agarose gel electrophoresis and NanoDrop 2000 spectrophotometer (Thermo Scientific USA). Subsequently, cDNA was synthesized using a PrimeScriptTM RT reagent kit (Perfect Real Time, Takara, Japan) according to the manufacturer’s instructions.
Immune-related gene expression
The selected immune-related genes were IL-10, IL-1β, IFN-γ, and TNF-α. qPCR was performed to determine gene expression levels with an SYBR Premix ExTaq™ П kit (Takara, Japan) and subjected to the Stratagene MxPro system (Stratagene Mx3005 P, USA) in 96-well reaction plates. The GenBank accession numbers for the selected genes and primer sequences are indicated in Table 3. The fluorescent quantitative PCR solution consisted of 12.5 μL SYBR® Premix Ex Taq™ (2 ×), 1.0 μL forward primer, 1.0 μL reverse primer (10 mM), 1.0 μL cDNA, and 9.5 μL ddH2O. The reaction conditions were as follows 94 °C for 30 s, followed by 40 cycles of 94 °C for 5 s, annealing for 30 s, and 60 °C for 30 s. Data were quantified by the 2−ΔΔCT method.
Challenge test
After 34 days, 20 fish from each tank were injected intraperitoneally with 100 μL of A. hydrophila TPS strain (LD50 = 1 × 106 CFU/g), which was provided by our lab. Injected fish were monitored for clinical signs, postmortem lesions, and daily mortalities for 14 days to calculate the relative protection rates (RPS).
Statistical analysis
Statistical analysis was performed using SPSS 20.0 for Windows (SPSS Inc.). All results data are presented as the mean ± SD (standard deviation), using one-way analysis of variance (ANOVA), followed by Duncan’s multiple range test. p < 0.05 was considered to be significant.
Results
Growth performance
The growth efficiency results of C. auratus fed the LAB are depicted in Table 4. The results indicated that FBW, BWG, SGR, and other indicators of the probiotics group were higher than those of the control group. Compared with the control group, each index of the compound probiotics groups was significantly improved (p < 0.05). The indicators of Group E were the highest and were significantly higher than those of the other probiotic groups. There was no significant difference among the other probiotic groups. The probiotic groups had no significant difference in C. auratus WG, BWG, or survival rate (p > 0.05).
As shown in Table 5, the content of crude protein in the muscle of the probiotic groups was higher than that of the control group. In addition, group E was significantly higher than the control group (p < 0.05). However, there was no significant difference between group E and the other probiotic groups (p > 0.05) and the rest of the probiotic groups were not significantly different than the control group (p > 0.05). Additionally, no significant difference in crude fat, crude ash, or moisture was observed between the control and treatment groups (p > 0.05).
Humoral immunity analysis
Serum AKP, SOD, and LZM activities and IgM concentrations of Carassius auratus fed LAB are presented in Fig. 1. As shown in Fig. 1, there was no significant change in IgM levels in group A during the trial period. However, in the treatment groups, serum IgM antibody levels were all increased. In addition, the IgM levels of groups C and G started to rise from day 0, reached a maximum at day 16, and subsequently began to fall, which were significantly different from groups A, B, D, F, and H (p < 0.05). The IgM level in group E showed an upward trend from 0 to 34 days and reached a maximum at 34 days, which was significantly higher than that of groups A, B, C, D, F, G, and H (p < 0.05).
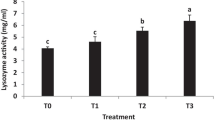
As shown in Fig. 2, there was no significant change in the LZM level in group A during the trial period. However, the level of serum LZM in the treatment groups increased. The LZM level of group G increased from day 0 and reached a maximum at day 16, which was significantly higher than that of group A (p < 0.05) and then decreased slightly. The levels of LZM in groups B, C, D, E, F, and H reached a maximum at 34 days, which was significantly higher than that of group A (p < 0.05). Group E reached the highest level at 34 days compared with the other groups. There was no significant difference between group E and groups D and H (p > 0.05). However, there was a significant difference from group G (p < 0.05).
In Fig. 3, the AKP level in group A did not change significantly during the experiment. However, the serum AKP level of C. auratus in the test groups was increased. The serum AKP level in groups B, C, D, E, F, G, and H reached its maximum value at 34 days, which was significantly higher than that in group A (p < 0.05). Group E reached the highest value among the groups at 34 days, but there was no significant difference from groups B, C, D, F, G, and H (p > 0.05).
The SOD level is shown in Fig. 4. In group A, there was no significant change in SOD level, but the SOD level of C. auratus in the test group first increased and then decreased. Groups B, C, and D increased from 0 to 16 days, reached the maximum value, which was significantly higher than that in group A (p < 0.05), and then decreased. In addition, SOD levels in groups E, G, and H reached their maximum value at 25 days, which was significantly higher than those in group A (p < 0.05). There was no significant difference in SOD content among the test groups at 34 days (p > 0.05).
Cytokine expression analysis
The cytokine expression levels of immune-related genes (IFN-γ, TNF-α, IL-1β, and IL-10) in the spleen, kidney, liver, and intestine are shown in Fig. 5.
As shown in Fig. 6, except for group A, the other groups had increased expression levels of IFN-γ in various tissues, and the expression level of IFN-γ in various tissues continued to rise, reaching the highest value at 34 days, which was significantly higher than that of group A (p < 0.05). The expression levels of IFN- in the spleen, kidney, and intestine were relatively high. When the experiment was carried out for 34 days, the expression level of IFN-γ in the spleen of the E group was the highest, and it was significantly higher than that of the A, C, D, F, and H groups (p < 0.05). At 34 days, the expression level in the kidney of group F was the highest and significantly higher than that of groups A, B, C, D, G, and H (p < 0.05) and the expression level in the liver of groups C, E, F, and H was the highest, which was significantly higher than that of groups A, B, D, and G (p < 0.05). Group H had the highest expression level in the intestinal tract, which was significantly higher than that of groups A, B, C, D, and F (p < 0.05).
Relative expression levels of IFN-γ in various tissues. Note: Different superscript letters in the same indicator indicate significant differences (p < 0.05); the same or unlabeled letters indicate no significant differences (p > 0.05). qPCR analysis of the expression of IFN-γ in spleen (A), kidney (B), liver (C) and gut (D) of C. auratus
As shown in Fig. 7, except for group A, the expression level of TNF-α in each tissue was increased in each group, and the expression level of TNF-α in various tissues showed a continuous upward trend, reaching the highest value at 34 days, which was significantly higher than that in group A (p < 0.05). In addition, the expression in the spleen, kidney, and liver was higher. At 34 days, the expression level of TNF-α in the spleen in group E was the highest and was significantly higher than that in groups A, B, C, D, and H (p < 0.05). The expression level in the kidney of group E was the highest and was significantly higher than that of groups A, B, C, and F (p < 0.05), and the expression level in the liver of group E was the highest and was significantly higher than that of groups A, B, D, F, G, and H (p < 0.05). The highest expression level was found in group E, which was significantly higher than that in groups A, B, C, D, F, and H (p < 0.05).
Relative expression levels of TNF-α in various tissues. Different superscript letters in the same indicator indicate significant differences (p < 0.05); the same or unlabeled letters indicate no significant differences (p > 0.05). qPCR analysis of the expression of TNF-α in spleen (A), kidney (B), liver (C) and gut (D) of C. auratus
As shown in Fig. 8, the expression level of IL-1β in the treatment groups was increased. When the experiment was carried out for 34 days, the expression level of IL-1β in the spleen of groups E and F was the highest and was significantly higher than that of groups A, B, D, and H (p < 0.05). At 34 days, the expression level of group D in the kidney was the highest, which was significantly higher than groups A, B, C, E, F, G, and H (p < 0.05). However, in the liver, group H was the highest and significantly higher than that of groups A, B, D, E, F, and G (p < 0.05). In addition, groups D, E, and G had the highest expression in the intestine, which was significantly higher than that of groups A, B, C, F, and H (p < 0.05).
Relative expression levels of IL-1β in various tissues. Different superscript letters in the same indicator indicate significant differences (p < 0.05); the same or unlabeled letters indicate no significant differences (p > 0.05). qPCR analysis of the expression of IL-1β in spleen (A), kidney (B), liver (C) and gut (D) of C. auratus
As shown in Fig. 9, the expression level of IL-10 in all tissues was improved in the other groups except the control group. At 25 days, the expression level of IL-10 in the spleen of group F decreased slightly and then increased, but the expression level of IL-10 in the other tissues continued to increase, reaching the highest value at 34 days, which was significantly higher than that in all tissues of group A (p < 0.05). In comparison, the expression level of IL-10 was higher in the kidney and liver. At 34 days, the expression level in the spleen in groups E, F, and H was the highest and was significantly higher than that in groups A, B, C, D, and G (p < 0.05). Groups C, E, and H showed levels in the kidney that was the highest and were significantly higher than those of the A and G groups (p < 0.05). However, groups C and E had the highest expression in the liver, which was significantly higher than that of groups A and B (p < 0.05). Group E had the highest intestinal expression and was significantly higher than that of groups A, B, C, D, F, G, and H (p < 0.05).
Relative expression levels of IL-10 in various tissues. Different superscript letters in the same indicator indicate significant differences (p < 0.05); the same or unlabeled letters indicate no significant differences (p > 0.05). qPCR analysis of the expression of IL-10 in spleen (A), kidney (B), liver (C) and gut (D) of C. auratus
Resistance against A. hydrophila TPS strain
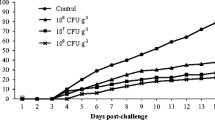
C. auratus was injected with the A. hydrophila TPS strain in each group. As shown in Fig. 10, all fish died within 7 days after the challenge with the A. hydrophila TPS strain. The relative immune protection rate of the probiotic group was higher than that of group A. Group E had the highest relative immune protection rate. The relative protection rates of groups B, C, D, E, F, G, and H were 27%, 30.7%, 40.94%, 42.32%, 39.38%, 33.61%, and 37.5%, respectively.
Discussion
As a safe and high-quality feed additive and potential alternative to antibiotics, the main benefits of LAB as a probiotic used in aquaculture are to improve growth performance, immunity, antioxidant capacity, and disease resistance (Hoseinifar et al. 2019; Doan et al. 2016). In addition, there are a large number of microbial flora in fish intestines, which have varying degrees of influence on the growth and health of fish. The composition and structure of fish intestinal microbes are easily affected by nutrients in food and the environment in the intestine. Therefore, a good source of food is essential for the balance of fish intestinal microbes (Llewellyn et al. 2014). In the present study, C. auratus fed three different LAB strains singly or conjointly, group E significantly increased in FBW, WG, and SGR than the other probiotic groups, and the control group did at the end of the work (34 days). However, compared with the control group, the other probiotic groups showed a certain improvement, but the difference was not significant. Similarly, preceding studies reported that supplementation with probiotics (LAB) significantly increased the WG and SGR in Lutjanus peru (Reyes-Becerril et al. 2014), Pangasius bocourti (Doan et al. 2014), Oreochromis niloticus (Mariya et al. 2019), Rutilus frisii kutum (Mirghaed et al. 2018; Tarkhani et al. 2020), etc. A similar observation was shown by Hoseinifar et al. who reported a significant increase in the SGR of Xiphophorus helleri–fed Lactobacillus acidophilus (L. acidophilus) as a probiotic. The results demonstrate the beneficial effects of dietary L. acidophilus on mucosal immune parameters, intestinal microbiota, stress resistance, and growth parameters of Xiphophorus helleri, and the appropriate inclusion is 6 × 108 CFU/g (Hoseinifar et al. 2015). Therefore, the results indicated that different concentrations of compound probiotics would have different effects on fish growth performance. Combined with this experiment, we found that under the same concentration, different combinations of probiotics had different effects on the growth performance of C. auratus. However, compared with single probiotics, the production performance of composite probiotics was improved to a certain extent. There was no significant difference between the compound probiotic groups and the single probiotic groups except Group E, which may be caused by the symbiotic, antagonistic, or competitive coexistence between different strains in the intestinal tract at the same concentration. Moreover, the physiological characteristics of different strains were different, and their metabolites had different effects on fish tissues and organs. Environmental factors, individual differences, and differences in the optimal concentration of strains were also possible reasons for the results of this experiment. Similarly, the diet supplemented with 1.0 × 108 CFU/g L. plantarum, alone or in combination for 28 days, showed a significant increase in SGR compared with that of the control group. Analysis of the possible reason for the results of this experiment is that the growth of the fish body is mainly achieved through the synthesis of proteins in the body, and fish can be used for tissue repair and renewal through the absorption and utilization of proteins and amino acids in food to promote growth. LAB can secrete bacterial toxins, produce organic acids, occupy nutrient-receiving sites, and inhibit the proliferation and growth of harmful bacteria in the intestinal tract to promote the health of the intestinal tract of fish and enhance the effective absorption and utilization of nutrients in the intestinal tract.
Serum immune parameters are valuable tools for monitoring fish health. SOD, AKP and LZM in fish serum are important components in evaluating the immune performance of fish (Magnadottir et al. 2010; Lee et al. 2017). Superoxide dismutase (SOD) is a metal enzyme that widely exists in animals, plants, and microorganisms. Studies have shown that SOD can remove ROS and improve the antioxidant capacity in fish. AKP is an important nonspecific immune marker enzyme for fish (Magnadottir et al. 2006). It is widely present in the tissues and organs of fish. It plays a role in immune defense and digestion and can regulate metabolism and the phosphorus ratio. APK is also a marker enzyme of cell lysosomes in blood and plays an important role in the digestion and degradation of foreign bodies in lysosomes (Boshra et al. 2006). LZM exists in the skin mucus and blood of fish and can decompose bacterial cell walls and peptidoglycans to achieve immune defense (Saurabh et al. 2008). In this experiment, SOD, AKP, and LZM in C. auratus serum of the probiotics group in the test group were all increased, which was significantly higher than that of the control group (p < 0.05), while the levels of single probiotics and composite probiotics peaked at different time stages. Similarly, the health-promoting effects of dietary combined probiotics have been reported to stimulate innate immune responses in gilthead Sparus aurata (Salinas et al. 2005, 2008), Epinephelus coioides (Sun et al. 2012) and Labeo rohita (Giri et al. 2014). The results showed that some indices of the composite probiotics were higher than those of the single probiotics group at different time stages. For instance, the serum SOD level of groups E and G was higher than that of the other groups at 25 days. However, the compound probiotics did not show an absolute advantage during the overall test, which might be related to the different experimental animals and the different types and contents of strains. In addition, the immune system of fish contains a complex physiological response, and the role of probiotics in the intestinal tract is often influenced by the body itself, the internal environment, and other factors. The specific immunity of fish is also an important part of the fish immune system, and it is mainly involved in IgM, which can directly act on pathogenic microorganisms (Bilal et al. 2016; Vervarcke et al. 2005; Van-Muiswinkel et al. 1991). Bahi et al. studied the addition of single or compound probiotics to the diet of Sparus aurata and found that the probiotic group could upregulate the level of IgM after three weeks, which was similar to the results of this test (Bahi et al. 2017). The result may be that probiotics can stimulate the secretion of IgM antibodies by B lymphocytes in C. auratus and increase the level of antibodies in the body, thereby obtaining a higher level of resistance to foreign pathogens. The composite probiotic groups all had peak levels of IgM at different time periods, but the IgM levels of the remaining probiotic groups had relatively slow growth because there are many factors that can affect differences in antibody levels in fish blood, e.g., the level of IL-4 and IL-13 (Yamaguchi et al. 2011), fish body length, age, and weight (Davis et al. 1999), environmental factors, individual differences, and et al.
Cytokines are essential mediators secreted from immune cells that regulate immune responses, repair damaged tissue, and defend against infection. IL-1β and TNF-α are two important pro-inflammatory genes that act as bacterial and viral invasion and are important markers for evaluating fish health. Il-1β is a cytokine produced when an immune response occurs that has pro-inflammatory effects and a wide range of biological activities and is involved in the regulation of a variety of immune active cells (Kono et al. 2013). TNF-α has a proinflammatory effect and is a cytokine that can directly kill tumor cells. The effector cells of nonspecific immunity mainly include monocytes-macrophages, neutrophils, and natural killer cells (NK). Mononuclear macrophages can be activated by IFN-γ and can secrete IFN-γ and other cytokines (Hasan et al. 2018). IL-10 is mainly synthesized by mononuclear macrophages and T helper cells, which are multicellular pluripotent cytokines that regulate cell growth and differentiation, participate in inflammation and immune responses, and are inflammatory and immunosuppressive factors. The spleen, kidney, liver, and intestine are the main immune organs of bony fish. In this experiment, supplementation of single or conjoin administration of probiotics exhibited significant inductions of IFN-γ, TNF-α, Il-1β, and IL-10 in the spleen, kidney, liver, and intestine, showing that dietary supplementation of single or combined LAB might induce nonspecific immunity, thereby boosting the immune system of fish against invading pathogens. A previous study showed that Biswas et al. also found that L. paracei and L. plantarum could stimulate the expression of TNF-α and Il-1β to different degrees in Takifugu rubripes (Biswas et al. 2013), which is similar to the results of this experiment. Similarly, Panigrahi et al. (2011) indicated that L. rhamnosus could induce the upregulation of TNF-α cytokines in the kidney and spleen of rainbow trout, and the above results were like those in this study. Likewise, LAB can significantly increase the levels of the proinflammatory cytokines TNF-α and IL-1β and upregulate the anti-inflammatory cytokine IL-10 by adding 5 × 108 CFU/g three different LAB to the diet (Feng et al. 2019). The results may be due to supplementation of single or conjoin administration of probiotics changed the microbiome in the gut and the interaction between probiotics, which stimulated other signaling pathways. Probiotics live in the fish intestinal tract and secrete related immune-promoting factors, metabolites, and enzymes from cells to stimulate the secretion of IL-10 by mononuclear macrophages or other immune cells in the body and promote and enhance the immune performance of the body. Relevant mechanisms should be further studied and discussed.
The immune-protective effect of probiotics was evaluated through an immune protection test. The results showed that all the patients in the control group died within 7 days after the challenge. The relative protection rates of probiotic groups B, C, D, E, F, G, and H were 27%, 30.7%, 40.94%, 42.32%, 39.38%, 33.61%, and 37.5%, respectively, which showed that the probiotic groups had a certain protective effect on C. auratus infection with A. hydrophila. The reason may be that the probiotic groups promoted the upregulation of immune factors in the body, enhanced the barrier function of the first immune defense system of fish, such as skin, scales, and mucous membranes, and effectively prevented the invasion of pathogenic microorganisms. The colonization ability of different probiotics in the intestine and the adaptability to the environment in the intestine are different, resulting in different immune protection effects.
In this study, different combinations of probiotics were fed to C. auratus to explore the effects of different combinations on the immune performance of C. auratus, providing a reference for the development of high-quality probiotic additives in feed. The induction mechanism of probiotics on the immune performance of C. auratus is being studied and explored. In addition, adequate theories are still needed for reference. The mechanism of compound probiotics and single probiotics on the immune performance of fish still needs to be further explored. However, the mutual influence between different strains under environmental conditions in the body is also an area that deserves further exploration. At present, the evaluation standards for probiotics are not unified, and the functions of probiotics are mostly different. Although numerous studies have shown that probiotics can improve the immune performance of fish, it is impossible to uniformly evaluate the pros and cons of a certain type of probiotic in aquaculture due to the wide variety of probiotics and different test subjects. Therefore, the establishment of a probiotic evaluation system is of great significance for the application of probiotics in aquaculture.
Conclusion
In conclusion, the present study demonstrates that a single or combined LAB-supplemented diet improves growth performance and humoral immunity, as well as regulates immune-related gene expression and disease resistance against A. hydrophilia. Moreover, the mixture of L. lactis + L. rhamnosus was more effective than those of the other groups for promoting growth performance and enhancing the immune response and disease resistance of C. auratus.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Anjan M, Goutam C, Koushik G (2019) Single or conjoint application of autochthonous Bacillus strains as potential probiotics: effects on growth, feed utilization, immunity and disease resistance in Rohu, Labeo rohita(Hamilton). Aquaculture 512:1–15
Bahi A, Guardiola FA, Messina C, Mandhi A, Cerezuela R, Santulli A, Bakhrouf A, Esteban MA (2017) Effects of dietary administration of fenugreek seeds, alone or incombination with probiotics, on growth performance parameters, humoral immune response and gene expression of gilthead seabream(Sparus aurata). Fish Shellfish Immunol 60:50–58
Batista S, Medina A, Pires MA, Morinigo MA, Sansuwan K, Fernandes JMO, Valente LMP, Ozorio ROA (2016) Innate immune response, intestinal morphology and microbiota changes in Senegalese sole fed plant protein diets with probiotics or autolysed yeast. Appl Microbiol Biotechnol 100:7223–7238
Bilal S, Lie K, Karisen OA, Hordvik I (2016) Characterization of IgM in Norwegian cleaner fish (lumpfish and wrasses). Fish Shellfish Immunol 53:9–17
Biswas G, Korenaga H, Nagamine R, Takayama H, Kawahara S, Takeda S, Kikuchi Y, Dashnyam B, Kono T, Sakai M (2013) Cytokine responses in the Japanese pufferfish (Takifugurubripes) head kidney cells induced with heat-killed probiotics isolated from the Mongolian dairy products. Fish Shellfish Immunol 34:1170–1177
Bo RB, Daniel K, Jongsu J, Sun ML, Hui KK, Oi-Jin K, Jae IIL, Byung SS, Hyung KD, Kwan HL, Wilhelm HH, Jee YH, Mun GK, Seong KS (2015) The effects of combined dietary probiotics Lactococcus lactis BFE920 and Lactobacillus plantarum FGL0001 on innate immunity and disease resistance in olive flounder (Paralichthys olivaceus). Fish Shellfish Immunol 42:177–183
Bo RB, Joon HS, Byung SP, Daniel K, Jin-Hwan K, Hyung KD, Ah-Ram K, Woo-Jin K, Seong KS (2016) Distinct immune tones are established by Lactococcus lactis BFE920 and Lactobacillus plantarum FGL0001 in the gut of olive flounder (Paralichthys olivaceus). Fish Shellfish Immunol 55:434–443
Boshra H, Li J, Sunyer JO (2006) Recent advances on the complement system of teleost fish. Fish Shellfish Immunol 20:239–262
Camilo P, Delbert MGIII (2014) Interrelationships between fish nutrition and health. Aquaculture 431:111–117
Davis CR, Marty GD, Adkison MA, Freiberg EF, Hedrick RP (1999) Association of plasma IgM with body size, histopathologic changes, and plasma chemistries in adult Pacific herring Clupea pallas. Dis Aquat Org 38:125–133
Dawood MAO, Koshio S (2016) Recent advances in the role of probiotics and prebiotics in carp aquaculture: a review. Aquaculture 454:243–251
Doan HV, Doolgindachbaporn S, Suksri A (2014) Effects of low molecular weight agar and Lactobacillus plantarum on growth performance, immunity, and disease resistance of basa fish (Pangasius bocourti, Sauvage 1880). Fish Shellfish Immunol 41:340–345
Doan HV, Hoseinifar SH, Tapingkae W, Tongsiri S, Khamtavee P (2016) Combined administration of low molecular weight sodium alginate boosted immunomodulatory, disease resistance and growth enhancing effects of lactobacillus plantarum in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol 58:678–685
Feng JC, Chang XL, Zhang YR, Yan X, Zhang JX, Nie GX (2019) Effects of Lactococcus lactis from Cyprinus carpio L.as probiotics on growth performance, innate immune response and disease resistance against Aeromonas hydrophila. Fish Shellfish Immunol 93:73–81
Gao XL, Zhang M, Li X, Han Y, Wu FC, Liu Y (2018) The effects of feeding Lactobacillus pentosus on growth, immunity, and disease resistance in Haliotis discus hannai Ino. Fish Shellfish Immunol 78:42–51
Giri SS, Sukumaran V, Sen SS, Jena PK (2014) Effects of dietary supplementation of potential probiotic Bacillus subtilis VSG1 singularly or in combination with Lactobacillus plantarum VSG3 or/and Pseudomonas aeruginosa VSG2 on the growth, immunity and disease resistance of Labeo rohita. Aquac Nutr 20:163–171
Gou CL, Wang JZ, Wang YQ, Dong WL, Shan XF, Lou YJ, Gao YH (2018) Hericium caput-medusae pers. polysaccharide enhance innate immune response, immune-related genes expression and disease resistance against Aeromonas hydrophila in grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol 72:604–613
Gregor R (2006) Safe and efficacious probiotics: what are they? Trends Microbiol 14:348–352
Hasan MT, Jang WJ, Tak JY, Lee BJ, Kim KW, Hur SW, Han HS, Kim BS, Min DH, Kim SK, Kong IS (2018) Effects of Lactococcus >lactis subsp. lactis I2 with β-glucooligosaccharides on growth, innate immunity and Streptococcosis resistance in olive flounder (Paralichthys olivaceus). J Microbiol Biotechnol 28:1433–1442
Hoseinifar SH, Roosta Z, Hajimoradloo A, Vakili F (2015) The effects of Lactobacillus acidophilus as feed supplement on skin mucosal immune parameters, intestinal microbiota, stress resistance and growth performance of black swordtail (Xiphophorus helleri). Fish Shellfish Immunol 42:533–538
Hoseinifar SH, Hosseini M, Paknejad H, Safari R, Jafar A, Yousefi M, Doan HV, Mozanzadeh MT (2019) Enhanced mucosal immune responses, immune related genes and growth performance in common carp (Cyprinus carpio) juveniles fed dietary Pediococcus acidilactici MA18/5M and raffinose. Dev Comp Immunol 94:59–65
Kenneth JL, Thomas DS (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 25(4):402–408
Kong YD, Kang YH, Tian JX, Zhang DX, Zhang L, Tao LT, Wu TL, Li Y, Wang GQ, Shan XF (2019) Oral immunization with recombinant Lactobacillus casei expressing flaB confers protection against Aeromonas veronii challenge in common carp, Cyprinus carpio. Fish Shellfish Immunol 87:627–637
Kong YD, Gao CS, Du XY, Zhao J, Li M, Shan XF, Wang GQ (2020) Effects of single or conjoint administration of lactic acid bacteria as potential probiotics on growth, immune response and disease resistance of snakehead fish (Channa argus). Fish Shellfish Immunol 102:412–421
Kono T, Takayama H, Nagamine R, Korenaga H, Sakai M (2013) Establishment of amultiplex RT-PCR assay for the rapid detection of fish cytokines. Vet Immunol Immunopathol 151:90–101
Lee S, Katya K, Park Y, Won S, Seong M, Hamidoghli A, Bai SC (2017) Comparative evaluation of dietary probiotics Bacillus subtilis WB60 and Lactobacillus plantarum KCTC3928 on the growth performance, immunological parameters, gut morphology and disease resistance in Japanese eel, Anguilla japonica. Fish Shellfish Immunol 61:201–210
Liu F, Qu YK, Wang AM, Yu YB, Yang WP, Lv F, Nie Q (2019) Effects of carotenoids on the growth performance, biochemical parameters, immune responses and disease resistance of yellow catfish (Pelteobagrus fulvidraco) under high-temperature stress. Aquaculture 503:293–303
Llewellyn MS, Boutin S, Hoseinifar SH, Derome N (2014) Teleost microbiomes: the state of the art in their characterization, manipulation and importance in aquaculture and fisheries. Front Microbiol 5:207
Magnadottir B (2006) Innate immunity of fish (overview). Fish Shellfish Immunol 20:137–151
Magnadottir B (2010) Immunological control of fish diseases. Mar Biotechnol 12:361–379
Mahmoud AOD, Shunsuke K, Manabu I, Saichiro Y, Mohammed FEB, Md SH, Truong HN, Serge D, Amina SM (2016) Effects of dietary supplementation of Lactobacillus rhamnosus or/and Lactococcus lactis on the growth, gut microbiota and immune responses of red sea bream, Pagrus major. Fish Shellfish Immunol 49:275–285
Mariya S, Trullas C, Chotiko A, Rodkhum C, Chansue N, Boonanuntanasarn S, Pirarat N (2019) Efficacy of synbiotic Jerusalem artichoke and Lactobacillus rhamnosus GG- supplemented diets on growth performance, serum biochemical parameters, intestinal morphology, immune parameters and protection against Aeromonas veronii in juvenile red tilapia (Oreochromis spp.). Fish Shellfish Immunol 86:260–268
Marjan H, Hamed KM, Seyed HH, Ali S, Peyman Y (2016) Dietary Lactobacillus acidophilus modulated skin mucus proteinprofile, immune and appetite genes expression in gold fish(Carassius auratus gibelio). Fish Shellfish Immunol 59:149–154
Mirghaed AT, Yarahmadi P, Hosseinifar SH, Tahmasebi D, Gheisvandi N, Ghaedi A (2018) The effects singular or combined administration of fermentable fiber and probiotic on mucosal immune parameters, digestive enzyme activity, gut microbiota and growth performance of caspian white fish (Rutilus frisii kutum) fingerlings. Fish Shellfish Immunol 77:194–199
Panigrahi A, Viswanath K, Satoh S (2011) Real-time quantification of the immune gene expression in rainbow trout fed different forms of probiotic bacteria Lactobacillus rhamnosus. Aquac Res 42:906–917
Qi ZZ, Zhang XH, Boon N, Bossier P (2009) Probiotics in aquaculture of China-currents state, problems and prospect. Aquaculture 290:15–21
Reyes-Becerril M, Angulo C, Estrada N, Murillo Y, Ascencio-Valle F (2014) Dietary administration of microalgae alone or supplemented with Lactobacillus sakei affects immune response and intestinal morphology of Pacific red snapper (Lutjanus peru). Fish Shellfish Immunol 40:208–216
Salinas I, Cuesta A, Esteban MA, Meseguer J (2005) Dietary administration of Lactobacillus delbrüeckii and Bacillus subtilis, single or combined, on gilthead seabream cellular innate immune responses. Fish Shellfish Immunol 19:67–77
Salinas I, Abelli L, Bertoni F, Picchietti S, Roque A, Furones D, Cuesta A, Meseguer J, Esteban MA (2008) Monospecies and multispecies probiotic formula-tions produce different systemic and local immunostimulatory effects in the gilthead seabream (Sparus aurata L.). Fish Shellfish Immunol 25:114–123
Saurabh S, Sahoo PK (2008) Lysozyme: an important defence molecule of fish innate immune system. Aquac Res 39:223–239
Sun YZ, Yang HL, Ma RL, Song K, Li JS (2012) Effect of Lactococcus lactis and Enterococcus faecium on growth performance, digestive enzymes and immuneresponse of grouper Epinephelus coioides. Aquac Nutr 18:281–289
Sun Y, He MW, Cao ZJ, Xie ZY, Liu CS, Wang SF, Guo WL, Zhang X, Zhou YC (2018) Effects of dietary administration of Lactococcus lactis HNL12 on growth, innate immune response, and disease resistance of humpback grouper (Cromileptes altivelis). Fish Shellfish Immunol 82:296–303
Talib A, Onn KK, Chowdury MA, Din WMW, Yahya K (2017) Bacillus as probiotics in enhancing culture performance for mud crab Scylla paramamosain larval culture. Aquacult Int 25:849–866
Tarkhani R, Imani A, Hoseinifar SH, Ashayerizadeh O, Moghanlou KS, Manaffar R, Doan HV, Reverter M (2020) Comparative study of host-associated and commercial probiotic effects on serum and mucosal immune parameters, intestinal microbiota, digestive enzymes activity and growth performance of roach (Rutilus rutilus caspicus) fingerlings. Fish Shellfish Immunol 98:661–669
Van-Muiswinkel WB, Lamers CH, Rombout JH (1991) Structural and functional aspects of the spleen in bony fish. Res Immunol 142:362–366
Wang CA, Liu HB, Mu GQ, Lu SX, Wang D, Jiang HB, Sun X, Han SC, YLiu Y. (2019) Effects of traditional Chinese medicines on immunity and culturable gut microflora to Oncorhynchus masou. Fish Shellfish Immunol 93:322–327
Xia Y, Lu MX, Chen G, Cao JM, Gao FY, Wang M, Liu ZG, Zhang DF, ZhuH P, Yi MM (2018) Effects of dietary Lactobacillus rhamnosus JCM1136 and Lactococcus lactis subsp. lactis JCM5805 on the growth, intestinal microbiota, morphology, immune response and disease resistance of juvenile Nile tilapia Oreochromis Niloticus. Fish & Shellfish Immunol 76:368–379
Yang QH, Lv YL, Zhang M, Gong Y, Li ZZ, Tran NT, He YY, Zhu CH, Lu YS, Zhang YL, Li SK (2019) Lactic acid bacteria, Enterococcus faecalis Y17 and Pediococcus pentosaceus G11, improved growth performance, and immunity of mud crab (Scylla paramamosain). Fish Shellfish Immunol 93:135–143
Yu LL, Zhai QX, Zhu JM, Zhang CC, Li TQ, Liu XM, Zhao JX, Zhang H, Tian FW, Chen W (2017) Dietary Lactobacillus plantarum supplementation enhances growth performance and alleviates aluminum toxicity in tilapia. Ecotoxicol Environ Saf 143:307–314
Zhang CN, Zhang JL, Guan WC, Zhang XF, Guan SH, Zeng QH, Cheng GF, Cui W (2017) Effects of Lactobacillus delbrueckii on immune response, disease resistance against Aeromonas hydrophila, antioxidant capability and growth performance of Cyprinus carpio, Huanghe var. Fish Shellfish Immunol 68:84–91
Zuo ZH, Shang BJ, Shao YC, Li WY, Sun JS (2018) Screening of intestinal probiotics and the effects of feeding probiotics on the growth, immune, digestive enzyme activity and intestinal flora of Litopenaeusvannamei. Fish Shellfish Immunol 86:160–168
Vervarcke S, Ollevier F, Kinget R, Michoel A (2005). Mucosal response in African catfish after administration of Vibrio anguillarum O2 antigens via different routes. Fish & Shellfish Immunology 18, 0–133.
Yamaguchi T, Katakura F, Shitanda S, Niida Y, Toda H, Ohtani M, Yabu T, Suetake H, Moritomo T, Nakanishi T (2005). Clonal growth of carp (Cyprinus carpio) T cells in vitro. Developmental and Comparative Immunology 35, 0–202.
Acknowledgements
This work was supported by the earmarked fund for the Project of Jilin Provincial Education Department (project no. JJKH20210367KJ).
Funding
This study was funded the Project of Jilin Provincial Education Department (project no. JJKH20210367KJ).
Author information
Authors and Affiliations
Contributions
Ruoming Li completed the main experimental work and wrote the paper. Teng Chi and Rui Zhou analyzed the data. Qing Xu contributed significantly to the analysis and manuscript preparation. Juntong Liu helped perform the analysis with constructive discussions. Xiaofeng Shan and Jiayun Yao contributed analysis tools and materials. Wuwen Sun designed the experiments. Guiqin Wang contributed to the conception of the study.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All the experimental fish handling procedures were used in this study were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Ethics Committee of Jilin Agricultural University.
Human and animal ethics
Not applicable.
Consent for publication
The author agrees to publication in the journal indicated below and also to publication of the article in English by Springer in Springer’s corresponding English-language journal.
Competing interests
The authors declare no competing interests.
Additional information
Handling Editor: Brian Austin
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, R., Chi, T., Xu, Q. et al. Effects of single or conjoint administration of lactic acid bacteria as potential probiotics on the growth, immune responses, and disease resistance of Carassius auratus. Aquacult Int 31, 157–177 (2023). https://doi.org/10.1007/s10499-022-00969-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-022-00969-6