Abstract
This study aimed to compare the susceptibility of tomato pinworm, Tuta absoluta, from four Brazilian regions to insect growth disruptor (IGD) insecticides by tomato leaf-dip bioassays. Larval mortality was assessed seven days after exposure to insecticide-treated leaves. The control failure likelihood (CFL) was estimated after larval bioassays with IGDs recommended label rate. Mortality data were subjected to Probit and variance analysis. All populations of tomato pinworm showed significant resistance to one or more insecticides. The LC50 values ranged from 0.34 to 0.63 g L−1 (chlorfluazuron), 0.17 to 0.92 g L−1 (teflubenzuron), 0.13 to 1.28 g L−1 (novaluron), 0.29 to 0.46 g L−1 (lufenuron), and 0.71 to 4.60 g L−1 (methoxyfenozide). The resistance ratios varied from 1.2 to 1.8-fold (chlorfluazuron), 1.4 to 5.5-fold (teflubenzuron), 1.2 to 9.9-fold (novaluron), 1.0 to 1.6-fold (lufenuron), and 1.5 to 6.5-fold (methoxyfenozide). Despite the low resistance ratios (< 10-fold), all populations showed low mortality and CFL > 54%, indicating likely control failure at the IGD label rates. We detected significant variations among populations of T. absoluta for activity of enzymes glutathione S-transferases (GST), cytochrome P450-dependent monooxygenases (MFO) and α-esterase (EST), however there was no significant difference between the populations for activity of β-esterase (EST). The evolution of resistance in T. absoluta populations to IGDs observed in this study is likely due to high selection pressure, demanding insecticide resistance management practices and environmentally sustainable tactics to obtain a more effective control of this pest.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The tomato pinworm, Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae), is one of the most damaging pests of tomato crops in the world. It was first reported in Brazil in the beginning of the 1980s, but at the end of this decade its presence was already reported in many tomato production fields throughout the country. In the American continent, it is predominantly present in the southern hemisphere, but has also been reported in few countries of Central America, and it is a quarantine pest in North America (NAPPO 2013). It was first detected in 2006 in Spain, and to date it is spread to the Mediterranean region, and European, African and Asian countries (Desneux et al. 2011). It has recently reached China, the world biggest tomato producer (Zhang et al. 2020). The most susceptible phenological stage of the tomato crop to this pest is during the vegetative growth when larvae of T. absoluta heavily damage leaves, moving later to buds, branches, flowers, and fruits. In Brazil, this pest usually causes great losses (often reaching 100%) as previously shown in other tomato-producing areas around the world (Souza and Reis 1992), demanding a great number of insecticide treatments.
Chemical control is the method of control most adopted by growers to minimize losses inflicted by the tomato pinworm, often requiring 36 sprays during the cropping season (Picanço et al. 2000). These successive insecticide sprays in tomato crops have certainly led to intoxication of producers and increased environmental pollution and detection of pesticide residues in food (Benvenga et al. 2007). Moreover, overuse of insecticides altered the pattern of use due to the emergence of populations resistant to available insecticides (Siqueira et al. 2000; Guedes and Siqueira 2012). Organophosphates and pyrethroids were the first chemical groups used against T. absoluta in the country, followed by alternate use or rotations with cartap hydrochloride. These products were succeeded by abamectin in the early 1990’s. Later, first cases of resistance were reported in Chile (Salazar and Araya 1997), Brazil (Siqueira et al. 2000; Siqueira et al. 2001), and Argentina (Lietti et al. 2005). These insecticides were then succeeded in the mid 1990’s by benzoylphenylureas (BPUs) (insect growth disruptors - IGDs) and indoxacarb (an oxadiazine insecticide), to which moderate to high resistance was reported (Silva et al. 2011). In an attempt to reduce the damage of T. absoluta to the crop, the use of methoxyfenozide (a diacylhydrazine insecticide) was then intensified.
Methoxyfenozide acts as a molt-accelerating compound. It is rapidly absorbed into the insect’s circulatory system after exposure and binds with high affinity to the ecdysone receptor complex (EcR:USP). Feeding stops within hours of exposure and a premature, lethal molt is initiated in immature stages by mimicking the action of 20-hydroxyecdysone (20E) (Carlson et al. 2001). This disruption of the normal molt cycle prevents the larvae from completely shedding its old cuticle resulting in starvation, dehydration, and death within a few days. The mode of action for methoxyfenozide is uniquely different from chitin biosynthesis inhibitors (Dhadialla et al. 1998).
The chitin biosynthesis inhibitor insecticides comprise most of the IGDs recommended to control T. absoluta in Brazil (MAPA 2014). These insecticides disrupt the chitin synthesis process (Ishaaya and Casida 1974; Post et al. 1974), impairing the normal insect ecdysis. Diflubenzuron, the first commercially available active substance of this group, causes the cuticle composition alteration, acting mainly in the chitin formation process, thus affecting the elasticity and firmness of the endocuticle (Grosscurt 1978; Grosscurt and Anderson 1980). The search for more potent benzoylureas resulted in the development of structural formulas such as chlorfluazuron, teflubenzuron, lufenuron, and novaluron among others (Ishaaya 1990; Ishaaya and Horowitz 1998).
These insecticides present residual activities that range between 10 and 30 days, depending on the environmental conditions in the field (Ishaaya et al. 2002), besides the reasonable selectivity towards populations of natural enemies such as parasitoids and phytoseiid mites (Ishaaya et al. 2001; Ishaaya et al. 2002). Therefore, IGDs may be used as an important component in integrated pest management. However, despite their effectiveness against populations of insect pests (Miranda and Bettini 2006), control failures were reported in tomato pinworm populations (Silva et al. 2011) associated with resistance evolution.
The control failure of an insecticide is based on the significant decrease in efficacy of a (commercial) product (e.g., an insecticide formulation) used at its recommended label rate but not reaching an expected control level (Guedes 2017). Thus, after years of using IGDs against tomato pinworm populations, there is no specific information about the susceptibility levels of this pest to these insecticides, except for reports of control failures. The determination of the risk of control failure (or control failure likelihood, CFL), requires use of realistic methods simulating field exposure (Gontijo et al. 2013; Guedes 2017). In addition, there is no information on the role of detoxifying enzymes associated with potential resistance of T. absoluta to IGDs. Therefore, this work aimed to assess the susceptibility status of tomato pinworm populations to benzoylureas and diacylhydrazines, the likelihood of control failure and evaluate possible involvement of detoxifying enzymes in the resistance.
Material and methods
Insect growth regulators
Concentration-response curves were estimated for the larval stage of each population of T. absoluta using the following insecticides: chlorfluazuron (Atabron 50 EC, ISK Biosciences do Brasil Defensivos Agrícolas Ltda, Yokkaichi-City, Mie, Japan), teflubenzuron (Nomolt 150 SC, BASF S.A., Leverkusen, Germany), novaluron (Rimon 100 EC, Milenia Agrociências S.A., Beer-Sheva, Israel), lufenuron (Match 50 EC, Syngenta Proteção de Cultivos Ltda, Monthey, Switzerland) (benzoylphenylureas) and methoxyfenozide (Intrepid 240 SC, Dow Agrosciences Industrial Ltda, Bergamo, Italy) (diacylhydrazine). The control failure likelihood for each insecticide against T. absoluta was also assessed using the recommended label rate.
Insects
Eight different populations of tomato pinworm from commercial crops in the Northeast, Midwest, Southeast and South of Brazil were collected in the period between 2010 and 2011 and used to study the resistance and control failure of IGDs in these regions (Table 1). Stems, leaves and fruits infested with larvae of T. absoluta were harvested from different spots in tomato crops. The populations were established and reared individually in cages with leaves of tomato cultivar “Santa Clara”, free of any treatment to prevent interference with the results. Bioassays were always carried out between one and two generations after field collected. Second instar larvae were used in the bioassays.
Concentration-response bioassays
Preliminary tests were performed on each population for establishing an “all or nothing” response, i.e., the range of concentrations where there is a concentration-response relationship. At least seven concentrations were used for each insecticide to obtain insect mortality between 0 and 100%. The concentrations used in the bioassays for all insecticides ranged from 0.16 to 42.00 g L−1. Tomato leaves of the cultivar “Santa Clara” were used in bioassays modified from Galdino et al. (2011). Tomato leaves, containing at least five leaflets and without any insecticide treatment, were used. The leaves were wrapped in hydrophobic cotton by the petioles and inserted into 100 mL amber colored vials containing 2% sodium hypochlorite solution as preservative. The leaves were dipped into insecticide treatments for 5 s and left to dry at room temperature. After drying, leaves were transferred to each experimental unit (consisting of a 2.5 L PET bottle). All treatments used Triton X-100 at 0.01% (v/v) as adhesive spreader, except for teflubenzuron that used Assist® 1.0% (v/v) as spreader. Two leaves were used per concentration and bioassay, to where 20 T. absoluta second-instar larvae were transferred per leaf, totaling 40 larvae per concentration. All bioassays were repeated one more time. Bioassays were conducted under controlled environmental conditions (25 ± 1 °C temperature; 65 ± 5% relative humidity; 12 h:12 h L:D photoperiod). The mortality assessment was performed seven days after transferring larvae to treated leaves. Insects that showed no motility after stimuli with soft brush were considered dead (Silva et al. 2011).
Bioassays to estimate control failure likelihood (CFL)
These bioassays were similar to the concentration-response bioassays, but they used only label rates recommended by manufacturers. Leaves were immersed in the following application rates: chlorfluazuron (50 mg a. i. L−1), teflubenzuron (37.5 mg a. i. L−1), novaluron (20 mg a. i. L−1), lufenuron (40 mg a. i. L−1), and methoxyfenozide (120 mg a. i. L−1) for 5 s, left to dry at room temperature, and finally transferred to 2.5 L PET bottles. Following, 20 T. absoluta second instar larvae were transferred to each experimental unit (consisting of a leaf packed in a 2.5 L PET bottle). Five repetitions were performed in this experiment. Bioassays were carried out in the same controlled conditions mentioned earlier. The mortality assessment was performed seven days after transferring larvae to treated leaves. Insects that showed no motility after stimuli with soft brush were considered dead (Silva et al. 2011).
Enzyme sample extraction
For assessing the detoxification enzyme activities of T. absoluta populations, 30 larvae of each population were collected and transferred to three microfuge tubes totaling 10 larvae per tube (3 replicates). For esterase and glutathione S–transferase assays, each sample was homogenized in 200 μL of sodium phosphate buffer (0.02 M, pH 7.2) and sodium phosphate buffer (0.1 M, pH 7.5), respectively using a Potter-Elvehjem homogenizer (Iwaki Glass Co., Ltd., Tokyo, Japan). Homogenates were centrifuged (Eppendorf 5810R) at 15,000 g and 4 °C for 15 min. The supernatant was harvested and stored at −80 °C. The protein concentration was titrated following the bicinchoninic acid method (Smith et al. 1985) using bovine serum albumin as standard (BSA). For cytochrome P450-dependent monooxygenase assays, the samples contained 30 third instar larvae per tube (with 3 replicates), homogenized in 1.0 mL of sodium phosphate buffer (0.1 M; pH 7.5) containing 10% glycerol [vol:vol], 1 mM ethylenediaminetetraacetic acid [EDTA], 0.1 mM dithiothreitol [DTT], 1 mM 1-phenyl-2-thiourea [PTU] and 1 mM phenylmethylsulfonyl fluoride [PMSF]. The homogenate was centrifuged at 10,000 x g for 15 min. The supernatant was centrifuged at 100,000 x g for 1 h in an ultracentrifuge (Optima L-80 XP, Beckman Coulter, USA). The microsomal pellets were then resuspended in resuspension buffer (0.1 M sodium phosphate buffer, pH 7.5, containing 10% [vol:vol] glycerol, 1 mM EDTA, 0.1 mM DTT, and 1 mM PMSF).
Esterase assays
Esterase activity was measured with method adapted from van Asperen (1962). Stock solutions (250 mM) of α–naphthyl acetate and β–naphthyl acetate substrates were prepared in acetone. For reaction, a 2 μL α–naphthyl acetate substrate was used, 10 μL of sample that was diluted to 1:100 and 188 μL of sodium phosphate buffer (0,02 M, pH 7,2) per well of the microtiter plate. The same procedure was done to esterase activity quantification using β–naphthyl acetate substrate; however, the samples were diluted to 1:10. Samples were incubated at 30 °C for 15 min. Reaction was stopped using 33.2 μL of 0.3% FAST Blue B. Absorbance was read at 595 nm on a microtiter plate reader (Elx800, BioTek®, Winooski, VT, USA). Each biological sample (total of 3) was analyzed in triplicates, comprising a total of nine technical replicates (measures). Alpha–naphthol and β–naphthol standard curves were used to calculate these products in the samples. Esterase activity is expressed as mmol naphthol x min−1 x mg protein−1.
Glutathione S-transferase assays
Conjugation activity of reduced glutathione was determined using CDNB (1–chloro–2,4–dinitrobenzene) substrate in the presence of glutathione S–transferase forming 2,4–dinitrophenyl–S–glutathione (Habig et al. 1974). CDNB solution (150 mM) was prepared in ethanol and reduced glutathione (10 mM) was dissolved in sodium phosphate buffer (0.1 M, pH 7.5). For reaction, 138 μL of sodium phosphate buffer (0.1 M, pH 7.5), 10 μL of sample containing 1 μg of protein, 150 μL of reduced glutathione (10 mM) were mixed. Premix was incubated in water bath at 30 °C for 5 min. Following, 2 μL of CDNB (150 mM) were added to the reaction. Immediately, formation of 2,4–dinitrophenyl–S–glutathione was measured using a biophotometer (Eppendorf) at 340 nm. Reaction was analyzed for 5 min with read intervals of 30 s. Each biological sample (total of 3) was analyzed in triplicates, and measures comprised a total of nine replicates. Absorbance data were analyzed as function of reaction time after addition of CDNB. The slope of the line (absorbance min−1) was transformed into concentration unit using the extinction coefficient of CDNB (9.6 mM−1.cm−1).
Cytochrome P450 monooxygenase (O-demethylase) assays
Activity of cytochrome P450 was determined through method O–demethylation using substrate p–nitroanisole (O2N–C6H4–O–CH3) to p–nitrophenol. The procedure of O–demethylation is lightly alkaline and absorbs light in wavelength of 420 nm, not interfering in the microsomal protein or substrate (Netter and Seidel 1964). Therefore, in the presence of p–nitroanisole, cytochrome P450-dependent O–demethylase activity can be measured (Rose and Brindley 1985). Reaction mix comprised (in this order) 178.8 μL of sodium phosphate resuspension buffer (0.1 M, pH 7.5), 56.2 μL of sample, 2.5 μL p–nitroanisole (150 mM in ethanol), 12.5 μL of reduced NADPH (9.6 mM) in each microfuge tube. The reaction mix was incubated for 30 min at 30 °C, and then stopped by adding 50 μL of HCl (1 M). After centrifugation at 3000 g for 15 min, 200 μL of reaction mix supernatant was transferred to microplate wells and read at 405 nm (ELx800 reader, Biotek, USA). Each biological sample (total of 3) was analyzed in three replicates, and measures comprised a total of nine replicates. Activity of cytochrome P450-dependent monooxygenases per sample was determined based on a standard curve of p–nitrophenol in nmol p–nitrophenol x min−1 x mg of protein−1.
Cytochrome P450 monooxygenase (N-dealkylase) assays
Assays were performed according to Silva et al. (2015) with slight modification. The substrate 4-chloro-N-methylaniline was used to determine N-demethylation activity. Reactions were run with 50 μl of sodium phosphate buffer (PBS) with 2% Tween-20 (0.1 M, pH 7.5), 25 μl of enzyme extract (sample), 25 μl of 4-chloro-N-methylaniline (4-CNMA) 7.5 mM diluted in 20% v/v ethanol, and 25 μl of reduced NADPH (9.6 mM). The reaction was processed for 15 min at 37 °C then stopped by the addition of 187.5 μl p-dimethylaminobenzaldehyde to 233.33 mM prepared in 3.0 N sulphuric acid. Samples were then centrifuged for 15 min at 10,000 g at room temperature and 200 μl of the supernatant read at 450 nm on a microtiter plate reader. Activity of N-demethylase (alkylase) per sample was determined based on a standard curve of 4-chloroaniline and expressed as nmol 4-chloroaniline × min−1 × mg protein−1. Assays used three different protein preparations (biological samples) and each one had three technical replicates.
Data analysis
The mortality data were corrected by the mortality observed in the control (Abbott 1925) and subjected to Probit analysis (Finney 1971) using the POLO-Plus program (LeOra-Software 2005) with P > 0.05 to estimate concentration-response curves parameters for each population and insecticide. The resistance ratios (RR) based on the LC50 value and confidence intervals were calculated by the method of Robertson et al. (2007), using as reference the most susceptible population to each insecticide. The confidence interval (CI) at 95% on resistance ration (RR50) not include 0. The recommended label rate mortality data were corrected as mentioned above (Abbott 1925) and mean mortality and standard error calculated. These results were used to assess the control failure likelihood (CFL) by an insecticide as in Guedes (2017), where CFL = 100 - [observed mortality (%) × 100] / expected mortality (considered as 80% following the minimum requirement from the Brazilian Ministry of Agriculture for minimum efficacy threshold; Gontijo et al. 2013). CFL values <0% indicate negligible risk of control failure (or control failure likelihood).
Means of esterase, glutathione S-transferase and cytochrome P450-dependent monooxygenase activities were subjected to analysis of variance (PROC ANOVA) and Tukey’s test (HSD) at P < 0.05 for scoring differences after checking ANOVA assumptions were tested using SAS (PROC UNIVARIATE; SAS Institute 2016). Canonical correlation analysis was performed as in Fragoso et al. (2013) between the IGDs LC50 estimates and the enzyme activities using the procedure CANCORR from SAS (SAS Institute 2016). The insecticide and enzymes with highest canonical loadings on the significant canonical pairs were further subjected to single (Pearson’s) correlation (PROC CORR; SAS Institute 2016) was eventually conducted to test that possible relationship. Multivariate normality assumptions were tested using Mardia’s test through the macro “%MULTNORM” (SAS Institute 2016) and no data transformation was necessary. Besides the standardized canonical function coefficients (or canonical weights) and the structure coefficients (loadings), we also used the squared canonical structure coefficient (rs2), which indicates the percentage of shared variance between the observed variable and the synthetic variable generated from the observed variable’s set as well as the canonical communality coefficient (h2), which is the proportion of variance in each observable variable that is reproducible across the function (indicates the usefulness of a variable in the model) for interpretation as in Sherry and Henson (2005).
Results
Concentration-response bioassays
Mortality data from concentration-response assays fitted the Probit model (χ2 not significant, P > 0.05). The Guaraciaba population presented lower LC50 for the insecticide chlorfluazuron and methoxyfenozide, while Paulínia population presented lower LC50 for the insecticide novaluron and the Anápolis population presented lower LC50 for the insecticides teflubenzuron and lufenuron (Table 2). Therefore, these populations were used as reference susceptible strains for respective insecticides (populations maintained in the laboratory without exposure to IGDs).
The LC50 values for chlorfluazuron (Table 2) were higher than the recommended label rate (Table 3) in all the populations of T. absoluta, varying from 0.34 to 0.63 g L−1 (Table 2). Five populations of T. absoluta showed significant chlorfluazuron resistance ratios since the confidence intervals did not include the value 1.0. The values of resistance ratio for chlorfluazuron varied from 1.20 to 1.82 fold (Table 2).
The LC50 values for teflubenzuron were high in all the tomato pinworm populations, varying from 0.17 to 0.92 g L−1 (Table 2). The resistance ratio values for teflubenzuron ranged between 1.44 and 5.51 fold.
Among all the insecticides, methoxyfenozide showed the highest LC50 values for T. absoluta populations (Table 2). The highest values of resistance ratio were observed for novaluron followed by methoxyfenozide varying from 1.22 to 9.87 and 1.53 to 6.51, respectively (Table 2). However, there were more populations showing resistance in the case of methoxyfenozide. Among the insecticides used, lufenuron showed a higher homogeneity of response among the populations with resistance ratio values varying from 1.04 to 1.60 times (Table 2). The slopes of the Probit regression curves for all insecticides varied from 0.73 to 3.86 (Table 2).
Control failure likelihood
The diagnosis for control failure indicated significant departure from the expected target mortality of 80%, which is the minimum efficacy threshold for the context under investigation. Results of the assays conducted at the label recommended rates for chlorfluazuron, teflubenzuron, novaluron, lufenuron and methoxyfenozide for the control of T. absoluta suggested that field control failures would occur in all field populations, since mortalities were significantly lower than 80% presenting high CFL values (Table 3).
Enzyme activity assays
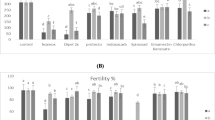
Esterase activity differed significantly among T. absoluta populations using α-naphthyl acetate (Fig. 1a). Activity of α-esterase varied from 1.13 ± 0.10 μmol/min/mg protein (PLT) to 2.32 ± 0.10 μmol/min/mg protein (IRQ), a variation of 2.05-times in α-esterase activity. Activity of β-esterase varied from 1.02 ± 0.06 μmol/min/mg protein (ANP) to 1.30 ± 0.06 μmol/min/mg protein (TNG), a variation range of 1.27-times in β-esterase activity.
Regarding oxidative metabolism, significant differences were observed in cytochrome P450-dependent monooxygenases among T. absoluta populations. Activity of N-dealkylation varied from 1.03 ± 0.13 nmol/min/mg protein (PLN) to 5.86 ± 0.17 nmol/min/mg protein (ANP), with a variation of 5.69-times among T. absoluta populations (Fig. 1b). Activity of O-dealkylation varied from 4.23 ± 0.72 nmol/min/mg protein (PLN) to 18.13 ± 1.31 nmol/min/mg protein (IRA), with a variation of 4.29-times among T. absoluta populations (Fig. 1b).
Significant differences were also observed for conjugation activity by glutathione S-transferase among T. absoluta populations. Activity varied from 0.29 ± 0.08 μmol/min/mg protein (GCB) to 2.93 ± 0.43 μmol/min/mg protein (ANP), a variation of 10.10-times in glutathione S-transferase activity (Fig. 1c).
Canonical correlation analysis
Canonical correlation was performed to test for independence (null hypothesis) of both sets of variables (IGDs and enzymes). The null hypothesis was rejected, and the two sets of variables were dependent (Wilks’ lambda = 0.02644; F = 15.38; DF = 25,231.82; P < 0.0001). Five canonical correlations were obtained but only three of them were significant (P < 0.05) (Table 4). However, the focus was on the first pair because the second and third canonical pairs, although significant, showed little contribution in explaining variances of the variables set (Rd < 0.13). Also, although the three significant canonical pairs accounted for 99.3% of the data, the first alone accounted for 82.8%. Said that, according to canonical correlation and structure coefficients (loadings), susceptibility to novaluron and teflubenzuron prevailed together with metabolism by N-dealkylation and α-esterase as predictors of the first canonical pair, followed by β-esterase and O-dealkylase as secondary contributors. The communality coefficients supported them as predictors as well. Despite that, the metabolism was a moderate predictor of the susceptibility to IGDs. The proportion of variance (by canonical redundancy analysis) in LC50 values explained by enzymes was 0.25 and the opposite relationship was 0.42. Based on that, correlation analysis complementary to the canonical correlation showed significant positive single correlation with novaluron for N-dealkylase (rp = 0.85; P < 0.0001; N = 72), α-esterase (rp = 0.50; P < 0.0001; N = 72), glutathione S-transferase (rp = 0.44; P < 0.0001; N = 72), β-esterase (rp = 0.33; P = 0.0043; N = 72), and O-dealkylase (rp = 0.32; P = 0.0061; N = 72). Besides that, methoxyfenozide significantly correlated with N-dealkylase activity (rp = 0.37; P = 0.0012; N = 72).
Discussion
A set of previous reported resistance cases in T. absoluta populations to virtually all classes of insecticides registered for its control was recently compiled (Guedes et al. 2019), and only a few alternatives are left to reduce infestations by this pest. Because of the high destructive potential of T. absoluta besides the market demand for high quality crops, growers have heavily relied on insecticides in Brazil (Picanço and Marquini 1999; Picanço et al. 2000). Moreover, despite the long-term use of IGDs in Brazil (since late 1980’s), grower concerns about control failures, and the suspicion of resistance evolution, very few cases of this phenomenon to benzoylureas in T. absoluta have been reported (Silva et al. 2011). This work showed that populations were highly resistant to four chitin synthesis disruptors though the resistance ratios values were low (< 10-fold), resulting from lack of a standard susceptible population as reference. However, the LC50 values for all of these insecticides were higher than their respective recommended label rates, and all assessed IGDs virtually killed less than 20% of the populations tested with their label rates.
Resistance may be defined as a heritable change in the sensitivity of a pest population that is reflected in the repeated failure of a product to achieve the expected level of control when used according to the label recommendation for that pest species (IRAC 2020). These results demonstrated a reduction in the effectiveness of benzoylureas for T. absoluta control after a long period of use in Brazil, evidencing the evolution of resistance. Silva et al. (2011) reported some cases of resistance to these insecticides in T. absoluta and associated this evolution with control failures. That was very clear in this survey with IGD label rates, denoting a high selection pressure that has been posed in the field against these populations for long time. Resistance to IGDs is still widespread across all tomato producing regions of Brazil as observed previously for triflumuron and teflubenzuron (Silva et al. 2011). Many of these resistance cases could be derived from the early nationwide use of triflumuron, the first benzoylurea to be registered in Brazil, which possibly impacted the efficacy of other insecticides in the IGD class insecticides.
Most populations presented very high LC50 values for methoxyfenozide compared to benzoylureas, well above the label rate. These are the first cases of resistance to diacylhydrazine insecticides reported in T. absoluta, and to know when or how fast this resistance evolved to high levels in the field is an incognita, as no previous data existed to infer on that. Conversely to benzoylureas, methoxyfenozide was registered later (early 2000’s) in Brazil, and despite this, the reduced susceptibility in different populations may be associated with resistance through higher activity of N-dealkylase.
The faster evolution of methoxyfenozide resistance may be a consequence of potential cross resistance with benzoylureas and other insecticides used against T. absoluta via higher expression of this enzyme complex. Furthermore, both benzoylureas and diacylhydrazines have amide linkages, and there are reports of insect carboxylesterases with low level amidase activity (Farnsworth et al. 2010), and esterases showed a high percentage of association in the enzyme overall model (canonical communality). We would expect a high activity of O-demethylation of methoxyfenozide with further increase in GST activity (phase II metabolism) because O-demethylation is the first metabolic step that methoxyfenozide undergoes (Andrew et al. 2004) in general. The presence of the methoxy moiety in the molecule would possibly trigger an increase of O-demethylase group upon insecticide selection, though no correlation was observed for cytochrome P450-dependent monooxygenases. Perhaps that was a random result of variation usually seen with detoxification enzymes, and in this case, it is recalled that indeed all populations were resistant to the assessed insecticides, and in fact O-dealkylases presented the lowest contribution in the overall canonical model.
The high LC50 and LC80 values and the low percentage of mortality observed from assessed recommended label rates can be related to direct or indirect exposure of T. absoluta to these insecticides. In Brazil, tomato growers usually apply insecticide without any resistance management concerns, and the lack of a systematic management and monitoring plan for insecticide susceptibility may have contributed significantly to the development of resistance. The evolution of resistance in insect pests to an active ingredient can also lead to resistance to other insecticides that act in a similar way or even between different chemical groups (Metcalf 1989). Benzoylureas have been available in the Brazilian market for nearly 50 years, and during that time new benzoylureas have introduced in the market in some regions without the scientifically backed supervision. Nevertheless, cross resistance between insecticides of the same chemical group has already been observed in studies with T. absoluta (diamides) (Silva et al. 2016; Roditakis et al. 2015), and this work reinforces such perception.
High levels of insect resistance to insecticides are generally associated with alteration of their target sites. Douris et al. (2016) in their studies showed that the resistance of insects and mites to IGDs was related to a point mutation (I1042M, I1017F) present in the chitin synthase 1 (CHS1) gene, respectively observed in resistant Plutella xylostella (L.) (Lepidoptera: Plutellidae) and mite individuals, interfering with chitin biosynthesis. Therefore, it is possible that the high values of LC50 associated with high percentages of survival in the populations of T. absoluta might be associated with an alteration of CHS1 as well, requiring further studies to confirm this hypothesis. In support of that is the fact that resistance to different IGDs did not show a significant correlation and it did not contribute positively to enzyme activities based on the canonical functions as was the case for chlorfluazuron, suggesting that target site alteration might be a factor involved in the resistance.
IGDs are important tools for integrated pest management because they have low acute toxicity for mammals, birds, and fish (Fulton et al. 2013). Methoxyfenozide for example presents low impact on most beneficial insects including bees, predators, and parasitoids (Fulton et al. 2013). However, the evolution of resistance may compromise the environmental performance that these insecticides possibly possess because of the increased rates being applied in the fields. Although this survey was performed in the years 2011 and 2012, these results provide information that call the attention for managing the resistance of T. absoluta to IGDs in different tomato cropping regions. Resistance levels have certainly changed since then, but they are not likely to have been reversed because resistance to these products appears to be stable (laboratory observation) over time and growers still spray insecticides without using scouting techniques and economic injury levels (EILs). Insecticides must be applied rationally and at the first instance should be used associated within an integrated pest management program, involving the monitoring of T. absoluta, as well as its natural enemies (Silva et al. 2016). Areas where BPUs are used not only have to focus on the rotation of active ingredients with different modes of action associated with refuge areas, but also to use other tactics of control such as pheromone-based mass trapping and the elimination of cropping remains, which together will make the control of T. absoluta more efficient and sustainable.
Conclusions
Our results demonstrate that despite the relative low levels of resistance found among populations of T. absoluta to growth disrupting insecticides, the recommended label rates established for their control were not effective and caused control failures. In addition, evidence of selected metabolic mechanisms of resistance (detoxification enzymes) was observed using canonical correlation analysis, but not to all IGDs, indicating that other factors such as target-site alteration may be involved, contributing to the high levels of resistance observed and consequently to control failures.
References
Abbott, W. S. (1925). A method of computing the effectiveness of an insecticide. Journal of Economic Entomology, 18(2), 265–267.
Andrew, D., Shillaker, R., & Dewhurst, I. (2004). Methoxyfenozide. In: joint meeting of the FAO panel of experts on pesticide residues in food and environment and the who core assessment group. Pesticides residues in food – 2003: Toxicological Evaluations. Geneva: World Health Organization.
Benvenga, S. R., Fernandes, O. A., & Gravena, S. (2007). Tomada de decisão de controle da traça-do-tomateiro através de armadilhas com feromônio sexual. Horticultura Brasileira, 25, 164–169.
Carlson, G. R., Dhadialla, T. S., Hunter, R., Jansson, R. K., Jany, C. S., Lidert, Z., & Slawecki, R. A. (2001). The chemical and biological properties of methoxyfenozide, a new insecticidal ecdysteroid agonist. Pest Management Science, 57(2), 115–119. https://doi.org/10.1002/1526-4998(200102)57:2<115::Aid-ps245>3.0.Co;2-a.
Desneux, N., Luna, M. G., Guillemaud, T., & Urbaneja, A. (2011). The invasive south American tomato pinworm, Tuta absoluta, continues to spread in afro-Eurasia and beyond: The new threat to tomato world production. Journal of Pest Science, 84(4), 403–408. https://doi.org/10.1007/s10340-011-0398-6.
Dhadialla, T. S., Carlson, G. R., & Le, D. P. (1998). New insecticides with ecdysteroidal and juvenile hormone activity. Annual Review of Entomology, 43(1), 545–569. https://doi.org/10.1146/annurev.ento.43.1.545.
Douris, V., Steinbach, D., Panteleri, R., Livadaras, I., Pickett, J. A., Van Leeuwen, T., et al. (2016). Resistance mutation conserved between insects and mites unravels the benzoylurea insecticide mode of action on chitin biosynthesis. [article]. Proceedings of the National Academy of Sciences of the United States of America, 113(51), 14692–14697. https://doi.org/10.1073/pnas.1618258113.
Farnsworth, C. A., Teese, M. G., Yuan, G., Li, Y., Scott, C., Zhang, X., Wu, Y., Russell, R. J., & Oakeshott, J. G. (2010). Esterase-based metabolic resistance to insecticides in heliothine and spodopteran pests. Journal of Pesticide Science, 35(3), 275–289. https://doi.org/10.1584/jpestics.R10-13.
Finney, D. J. (1971). Probit Analysis. London: Cambridge University Press.
Fragoso, D. B., Guedes, R. N. C., & Ladeira, J. A. (2013). Seleção na evolução de resistência a organofosforados em Leucoptera coffeella (Guérin-Mèneville) (Lepidoptera: Lyonetiidae). Neotropical Entomology. 32(2), 329–334. https://doi.org/10.1590/S1519-566X2003000200020.
Fulton, M. H., Key, P. B. & DeLorenzo, M. E. (2013). 6 – Insecticide Toxicity in Fish. Fish Physiology, 33, 309–368. https://doi.org/10.1016/B978-0-12-398254-4.00006-6.
Galdino, T. V. d. S., Picanço, M. C., Morais, E. G. F. d., Silva, N. R., Silva, G. A. R. d., & Lopes, M. C. (2011). Bioassay method for toxicity studies of insecticide formulations to Tuta absoluta (meyrick, 1917). Ciência e Agrotecnologia, 35, 869–877.
Gontijo, P. C., Picanço, M. C., Pereira, E. J. G., Martins, J. C., Chediak, M., & Guedes, R. N. C. (2013). Spatial and temporal variation in the control failure likelihood of the tomato leaf miner,Tuta absoluta. Annals of Applied Biology, 162(1), 50–59. https://doi.org/10.1111/aab.12000.
Grosscurt, A. C. (1978). Effects of diflubenzuron on mechanical penetrability, chitin formation, and structure of the elytra of Leptinotarsa decemlineata. Journal of Insect Physiology, 24(12), 827–831. https://doi.org/10.1016/0022-1910(78)90103-8.
Grosscurt, A., & Anderson, S. O. (1980). Effect of diflubenzurom on some chemical and mechanical properties of the elytra of Leptinotarsa decemlineata. Proceedings of the Koniklijke Nederlandse Akademie van Wetenschappen, 83(2), 143–150.
Guedes, R. N. C. (2017). Insecticide resistance, control failure likelihood and the first law of geography. Pest Management Science, 73(3), 479–484. https://doi.org/10.1002/ps.4452.
Guedes, R. N. C., & Siqueira, H. A. A. (2012). The tomato borer Tuta absoluta: Insecticide resistance and control failure. CAB Reviews: Perspectives in Agriculture, Veterinary Science, Nutrition and Natural Resources, 7(055), 1–7. https://doi.org/10.1079/pavsnnr20127055.
Guedes, R. N. C., Roditakis, E., Campos, M. R., Haddi, K., Bielza, P., Siqueira, H. A. A., Tsagkarakou, A., Vontas, J., & Nauen, R. (2019). Insecticide resistance in the tomato pinworm Tuta absoluta: Patterns, spread, mechanisms, management and outlook. Journal of Pest Science, 92, 1329–1342. https://doi.org/10.1007/s10340-019-01086-9.
Habig, W. H., Pabst, M. J., & Jakoby, W. B. (1974). Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. The Journal of Biological Chemistry, 249(22), 7130–7139.
IRAC. (2020). IRAC mode of action classification scheme. IRAC International Workgroup. http://irac-online.org. Acessed 25 Oct 2020.
Ishaaya, I. (1990). Benzoylphenyl ureas and other selective control agents: Mechanism and application. In J. E. Casida (Ed.), Pesticides and alternatives (pp. 365–376). Amsterdan: Elsevier Science Publisher BV.
Ishaaya, I., & Casida, J. E. (1974). Dietary TH 6040 alters composition and enzyme activity of housefly larval cuticle. Pesticide Biochemistry and Physiology, 4(4), 484–490. https://doi.org/10.1016/0048-3575(74)90073-X.
Ishaaya, I., & Horowitz, A. R. (1998). Insecticides with novel modes of action: An overview. In I. Ishaaya & D. Degheele (Eds.), Insecticides with novel modes of action, mechanism and application (pp. 1–24). New York: Springer-Verlag.
Ishaaya, I., Kontsedalov, S., Mazirov, D., & Horowitz, A. R. (2001). Biorational agents--mechanism and importance in IPM and IRM programs for controlling agricultural pests. Mededelingen (Rijksuniversiteit te Gent. Fakulteit van de Landbouwkundige en Toegepaste Biologische Wetenschappen), 66(2a), 363–374.
Ishaaya, I., Horowitz, A. R., Tirry, L., & Barazani, A. (2002). Novaluron (Rimon), a novel IGR--mechanism, selectivity and importance in IPM programs. Mededelingen (Rijksuniversiteit te Gent. Fakulteit van de Landbouwkundige en Toegepaste Biologische Wetenschappen), 67(3), 617–626.
LeOra-Software. (2005). POLO-Plus, POLO for Windows computer program, version 2.0. Petaluma: LeOra-Software.
Lietti, M. M. M., Botto, E., & Alzogaray, R. A. (2005). Insecticide resistance in argentine populations of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Neotropical Entomology, 34, 113–119.
MAPA. (2014). Ministério da agricultura, pecuária e abastecimento. AGROFIT: sistema de agrotóxicos fitossanitários. http://extranet.agricultura.gov.br/agrofit_cons/principal_agrofit_cons. Accessed 15 December 2014.
Metcalf, R. L. (1989). Insect resistance to insecticides. Pesticide Science, 26, 333–358. https://doi.org/10.1002/ps.2780260403.
Miranda, J. E., & Bettini, P. C. (2006). Resistência ou não. Revista Cultivar, 84, 18–22.
NAPPO – North American Plant Protection Organization. (2013). Surveillance protocol for the tomato leaf miner, Tuta absoluta, for NAPPO member countries. USDA. https://www.aphis.usda.gov/import_export/plants/plant_exports/downloads/Tuta_absoluta_surveillanceprotocol. Acessed 20 Dec 2016.
Netter, K. J., & Seidel, G. (1964). An adaptively stimulated O-demethylating system in rat liver microssomes and its kinetic properties. Journal of Pharmacology and Experimental Therapeutics, 146(1), 61–65.
Picanço, M. C., & Marquini, F. (1999). Manejo integrado de pragas de hortaliças em ambiente protegido. Informe Agropecuário, 20, 126–133.
Picanço, M. C., Gusmão, M. R., & Galvan, T. L. (2000). Manejo integrado de pragas de hortaliças. In L. Zambolim (Ed.), Manejo integrado de doenças, pragas e ervas daninhas (pp. 275–324). Viçosa: Universidade Federal de Viçosa.
Post, L. C., de Jong, B. J., & Vincent, W. R. (1974). 1-(2,6-disubstituted benzoyl)-3-phenylurea insecticides: Inhibitors of chitin synthesis. Pesticide Biochemistry and Physiology, 4(4), 473–483. https://doi.org/10.1016/0048-3575(74)90072-8.
Robertson, J. L., Russell, R. M., Preisler, H. K., & Savin, N. E. (2007). Bioassays with arthropods (2nd ed.). Boca Raton: CRC Press Taylor & Francis Group.
Roditakis, E., Vasakis, E., Grispou, M., Stavrakaki, M., Nauen, R., Gravouil, M., & Bassi, A. (2015). First report of Tuta absoluta resistance to diamide insecticides. Journal of Pest Science, 88, 9–16.
Rose, R. L., & Brindley, W. A. (1985). An evaluation of the role of oxidative enzymes in Colorado potato beetle resistance to carbamate insecticides. Pesticide Biochemistry and Physiology, 23(1), 74–84. https://doi.org/10.1016/0048-3575(85)90080-X.
Salazar, E. S., & Araya, J. E. (1997). Detección de resistência a inseticidas em la polilla del tomate. Simiente, 67, 8–22.
SAS Institute. (2016). SAS user’s guide: statistics, version 9.4. Cary: SAS Institute.
Sherry, A., & Henson, R. K. (2005). Conducting and interpreting canonical correlation analysis in personality research: A user-friendly primer. Journal of Personality Assessment, 84(1), 37–48. https://doi.org/10.1207/s15327752jpa8401_09.
Silva, G. A., Picanço, M. C., Bacci, L., Crespo, A. L. B., Rosado, J. F., & Guedes, R. N. C. (2011). Control failure likelihood and spatial dependence of insecticide resistance in the tomato pinworm, Tuta absoluta. Pest Management Science, 67(8), 913–920. https://doi.org/10.1002/ps.2131.
Silva, W. M., Berger, M., Bass, C., Balbino, V. Q., Amaral, M. H. P., Campos, M. R., & Siqueira, H. A. A. (2015). Status of pyrethroid resistance and mechanisms in Brazilian populations of Tuta absoluta. Pesticide Biochemistry and Physiology, 122, 8–14. https://doi.org/10.1016/j.pestbp.2015.01.011.
Silva, J. E., Assis, C. P. O., Ribeiro, L. M. S., & Siqueira, H. A. A. (2016). Field-evolved resistance and cross-resistance of Brazilian Tuta absoluta (Lepidoptera: Gelechiidae) populations to diamide insecticides. Journal of Economic Entomology, 109, 2190–2195. https://doi.org/10.1093/jee/tow161.
Siqueira, H. A. A., Guedes, R. N. C., & Picanço, M. C. (2000). Insecticide resistance in populations of Tuta absoluta (Lepidoptera: Gelechiidae). Agricultural and Forest Entomology, 2(2), 147–153. https://doi.org/10.1046/j.1461-9563.2000.00062.x.
Siqueira, H. A. A., Guedes, R. N. C., Fragoso, D. B., & Magalhaes, L. C. (2001). Abamectin resistance and synergism in Brazilian populations of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). International Journal of Pest Management, 47(4), 247–251. https://doi.org/10.1080/09670870110044634.
Smith, P. K., Krohn, R. I., Hermanson, G. T., Mallia, A. K., Gartner, F. H., Provenzano, M. D., Fujimoto, E. K., Goeke, N. M., Olson, B. J., & Klenk, D. C. (1985). Measurement of protein using bicinchoninic acid. Analytical Biochemistry, 150(1), 76–85. https://doi.org/10.1016/0003-2697(85)90442-7.
Souza, J. C., & Reis, P. R. (1992). Traça-do-tomateiro: histórico, reconhecimento, biologia, prejuízos e controle ((EPAMIG, Boletin Técnico, 2).). Belo Horizonte: EPAMIG.
van Asperen, K. (1962). A study of housefly esterases by means of a sensitive colorimetric method. Journal of Insect Physiology, 8(4), 401–416. https://doi.org/10.1016/0022-1910(62)90074-4.
Zhang, G.-F., Ma, D.-Y., Wang, Y.-S., Gao, Y.-H., Liu, W.-X., Zhang, R., et al. (2020). First report of the south American tomato leafminer, Tuta absoluta (Meyrick), in China. Journal of Integrative Agriculture, 19(7), 1912–1917. https://doi.org/10.1016/S2095-3119(20)63165-3.
Acknowledgments
Thanks to the Universidade Federal Rural de Pernambuco and the IRAC-Brazil which made possible this work and the CAPES for the assistantship granted to the first author. We greatly appreciate the anonymous reviewers that patiently help us to improve this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical standards
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Silva, J.E., Silva, W.M., Silva, T.B.M. et al. High resistance to insect growth disruptors and control failure likelihood in Brazilian populations of the tomato pinworm Tuta absoluta. Phytoparasitica 49, 689–701 (2021). https://doi.org/10.1007/s12600-021-00895-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12600-021-00895-y