Abstract
Pectolinaringenin was isolated from chloroform extract of Clerodendrum phlomidis L. and was evaluated at 12.5 to 100 ppm concentrations for its effects on total protein, esterase and glutathione S-transferase (GST) activities of Earias vittella and Helicoverpa armigera. At 100 ppm, the compound reduced total protein content by 55.75% and 53.01% over control with IC50 values of 74.37 and 212.31 ppm in E. vittella and H. armigera, respectively. At 100 ppm it also reduced GST and esterase enzyme activities in E. vittella by 37.53% and 43.09% over control, with IC50 values of 133.00 and 111.76 ppm, respectively. It also reduced GST and esterase activities in H. armigera by 43.14% and 47.421% over control with IC50 values of 114.38 and 98.78 ppm, respectively. Data were analyzed for their normality using the Shapiro-Wilk test and Levene’s statistics to determine the significant deviation. Pectolinaringenin could be used for the management of agricultural pests.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plant-derived substances like tannins, phenolics, gossypol, some saponins, and proteinase inhibitors have the potential to interfere directly with an animal’s ability to digest dietary protein, and thus reduce the efficiency of utilization of the dietary protein (Birk & Peri 1980; Singleton 1981; Whitaker 1981; Zucker 1983). Flavonoids isolated from Circium and Cardus species exhibited a broad spectrum of biological activities such as enzyme inhibitors (α-glucosidase and α-amylase), larval growth inhibitor, etc., against insect pests; these have been reported by many researchers (Elliger et al. 1980; Hedin & Waage 1986; Kim et al. 2000).
Researchers around the world have reported the activity of plant-derived substances on protein content and enzyme activities of different insects. Khalaf (1998) reported that volatile oils of Cymbopogon citratus and Rosmarinus officinalis induced biochemical disturbances and decreased protein content in Musca stabulans. Melia azedarach methanol extract was studied for its effect on protein content of Spodoptera littoralis and Agrotis ypsilon; it was observed that at 100 ppm, the hemolymph concentration of both the insects was reduced after 6 days of treatment (Schmidt et al. 1998). Boreddy et al. (2000) evaluated the effect of Annona squamosa seed extracts on S. litura for its total quantitative and qualitative protein contents; they observed that it reduced the protein content in all stages of the insects. Azadirachtin-rich commercial insecticides were evaluated against 4th instar larvae of H. armigera and it was found that all the treatments reduced the protein level (Neoliya et al. 2007). Rharrabe et al. (2007) investigated the effects of 20-hydroxyecdysone on protein content of Plodia interpunctella; the protein content was reduced when the larvae consumed treated diet compared with control. Glutathione S-transferases (GSTs) are the multifunctional group of active enzymes with detoxification functions (Wood et al. 1986, 1990). Esterases constitute a widely distributed family of enzymes that involve hydrolysis of carboxylester, amide, and thioester bonds in a variety of compounds. These enzymes have the ability to detoxify insecticides (Conyers et al. 1998). GST and esterases are important enzymes involved in the metabolism of a broad range of foreign and endogenous compounds in insects (Francis et al. 2001). The important function of GST is detoxification, conjugating reduced glutathione with a large number of electrophilic metabolites derived from a variety of xenobiotics, including carcinogens, toxins and drugs (Zanden et al. 2004). Cho et al. (1995) suggested that esterase and mixed function oxidase play an important role in the detoxification of organophosphorus and pyrethroid insecticides in S. litura.
The bhendi fruit borer, Earias vittella Fab. (Lepidoptera: Noctuidae), is a serious pest causing more than 80% damage to okra (Radake & Undirwade 1981; Srinivasan & Gowder 1960). It reduced the seeds by 16.47% per fruit (Sinha et al. 1978). Helicoverpa armigera Hübner (Lepidoptera: Noctuidae) is a major pest of many crops worldwide (Fakrudin et al. 2004; Jallow et al. 2004) and it is also a polyphagous pest causing severe loss to economically important crops (Subramanian & Mohankumar 2006). H. armigera alone damages more than 80 species of crop plants. In India, it caused a yield loss of 158 million US\$ during 1996–1997 and about 54% of the total insecticides was used for the protection of the cotton crop for this serious pest (Jalali et al. 2004; Jayaraj 2003). Hence, an attempt has been made to study the effect of pectolinaringenin (Fig. 1), a flavonoid isolated from chloroform extract of Clerodendrum phlomidis L. leaves, on total protein and esterase and GST enzymes activities in two lepidopteran pests, E. vittella and H. armigera.
Materials and methods
Collection, extraction, fractionation and isolation of pectolinaringenin
Collection of plant materials, extraction of crude extracts, identification of active crude extract, fractionation and isolation of the compound pectolinaringenin have been described in our earlier reports (Muthu et al. 2012a,b).
Rearing of insects
Earias vittella larvae were collected from Thandalam village near Thirupporur, Kancheepuram district, Tamil Nadu. They were reared until pupation in glass jars (21 cm x 15 cm) and fed with bhendi fruits (Abelmoschus esculentus L.) under laboratory conditions (27 ± 2°C and 75 ± 5% r.h.). After pupation, the pupae (cocoon) were collected and kept in different glass jars covered with white muslin cloth. After emergence of the adults (8–10 days), they were fed with 10% honey solution absorbed in cotton swabs inside glass jars. Muslin cloth was provided as an oviposition substrate. The eggs laid were kept in a glass jar covered with muslin cloth for hatching. After hatching, and in the neonate stage, the larvae were fed with tender leaves of bhendi; after that they were fed with bhendi fruit. A laboratory-reared culture was used for the experiment.
Helicoverpa armigera
Larvae were collected from a farmer’s field in Melakondayar village in the Tiruvallur district of Tamil Nadu and the collected larvae were reared individually in a plastic container (vials) and fed regularly with bhendi fruit until the larvae became pupae under the laboratory conditions. Sterilized soil was provided for pupation. After pupation, the pupae were collected from soil and placed inside a cage for emergence of adults. Cotton soaked with 10% honey solution mixed with a few drops of multivitamins was provided for adult feeding to increase fecundity. A potted cotton plant was kept inside the adult emergence cage for egg laying (Baskar & Ignacimuthu 2012). After hatching, the larvae were collected from the cage and fed with a standard artificial diet as recommended by Koul et al. (1997) and were used for the experiment.
Treatments
Toxic effects of pectolinaringenin on protein content and detoxifying enzymes in the midgut and hemolymph of 3rd instar larvae of E. vittella and H. armigera were studied after 24 h of oral treatment using a fruit disc of A. esculentus for E. vittella and a cotton leaf disc for H. armigera under no-choice conditions. Fresh bhendi fruit discs (10 mm thickness) for E. vittella and fresh cotton leaves (4 cm diam) punched with a cork borer for H. armigera were used. The tested materials – bhendi fruit discs and cotton leaves – were dipped individually in pectolinaringenin at 12.5, 25, 50 and 100 ppm concentrations. Leaf discs and fruit discs dipped in acetone + Tween 80, used to dissolve the compound, were used as negative control. For comparative analysis, a reference control – azadirachtin (purity 40.86%), was used. Ten replicates were maintained for each concentration of the tested compound, reference compound and control.
Protein
The total protein content in the hemolymph and midgut of E. vittella and H. armigera was estimated according to Bradford’s method using bovine serum albumin as the standard (Bradford 1976).
Glutathione S-transferase
GST activity was studied according to the method of Oppenoorth (1979). Enzyme activity was determined using chlorodinitrobenzene (CDNB) as a substrate. The reaction mixture contained 10 μl of diluted enzyme solution (the stock solution was diluted tenfold with 0.1 M, pH 7.6, sodium phosphate buffer), 90 μl of 0.1 M sodium phosphate buffer, 100 μl of 1.2 mM CDNB and 100 μl of 6 mM GSH. Optical density at 340 nm was recorded at intervals of 10 s for 5 min. Controls without the enzyme always accompanied each assay. Enzyme activity was expressed as mOD/sec/mg protein at 27°C, and the specific activity was calculated (Huang & Han 2007).
Esterase
General esterase activity was determined using α-naphthyl acetate as the substrate following the procedure of Han et al. (1998). Fifty microliters of 0.1 M sodium phosphate buffer (pH 7.6) and 200 μl mixture solution containing 0.3 mM eserine, 10 mM α-naphthyl acetate solution and 4 mM fast blue RR salt were added into each sample. The reaction was initiated by addition of 20 μl of diluted enzyme solution (the stock solution was diluted tenfold with 0.1 M, pH 7.6, sodium phosphate buffer). Optical density at 450 nm was recorded at intervals of 30 s for 10 min. Controls without the enzyme always accompanied each assay. Enzyme activity was expressed as mOD/sec/mg protein at 27°C, and the specific activity was calculated (Huang & Han 2007).
Statistical analysis
One-way ANOVA was used to analyze all the data. Significant differences between treatments were determined using Tukey’s HST multiple range tests (P ≤ 0.05). Homogeneity of Variances (Levene Statistic) was used to determine significant deviation. Analyses were performed with the original data. Distribution of the data exhibited significant deviations from normality (Shapiro-Wilk test). IC50 and IC90 values were calculated using probit analysis (Finney 1971). Statistical package SPSS version 11.5 was used for statistical analysis.
Results
Protein
The effects of pectolinaringenin at 100, 50, 25 and 12.5 ppm concentrations on total protein content of E. vittella and H. armigera are presented in Table 1. Pectolinaringenin reduced the protein content of E. vittella and H. armigera in a dose-dependent manner. Maximum reductions of 174.98 mg ml-1 (55.75%) and 210.01 mg ml-1 (53.01%) of total protein content over control were recorded against E. vittella and H. armigera, at 100 ppm concentration. Total protein reduction was statistically significant at all the concentrations. Effective IC50 and IC90 values for total protein content were 74.37, 83.33 and 212.31, 186.15 ppm for E. vittella and H. armigera, respectively (Table 2). The chi-square values were significant for both the insects.
Enzyme activity
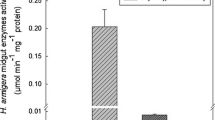
The effect of different concentrations of pectolinaringenin on GST and esterase enzyme activities was studied against the larvae of E. vittella (Table 3). Compared with control, the isolated compound, pectolinaringenin, reduced GST and esterase enzyme activities; the activities were 0.146 and 0.143 OD/5 min/larva, respectively, at 100 ppm concentration; the percentages of reduction over control were 37.53 and 43.09, respectively. The compound reduced the enzyme activity in a dose-dependent manner. A statistically significant reduction was observed at all the concentrations for both the enzymes. The effective concentration of pectolinaringenin to reduce GST and esterase activities is presented in Table 4. The IC50 and IC90 values for GST and esterase activity of E. vittella were 133.00, 111.76 ppm and 318.81, 262.96 ppm, respectively. The chi-square value for GST showed significant activity.
The compound reduced GST and esterase activities in H. armigera; the activities were 0.184 and 0.122 OD/5 min/larva, respectively, at 100 ppm concentration; the percentages of reduction over control were 43.14 and 47.42, respectively. The reduction of the enzyme activity by the compound was in a concentration-dependent manner. Statistically significant reduction over control was observed at all the concentrations (Table 5). Effective inhibitory concentrations of pectolinaringenin for GST and esterase activities of H. armigera are presented in Table 6. The IC50 and IC90 values for GST and esterase activities of H. armigera were 114.38, 98.78 and 272.06, 272.42 ppm, respectively.
Discussion
The present study revealed that the larvae treated with pectolinaringenin showed reduced total protein content in a dose-dependent manner against both the pests. It may be due to the interaction of the compound with the hormones which are related to protein synthesis (Sieber & Rembold 1983). Senthilkumar & Murugan (1999) reported that azadirachtin at 2 ppm concentration reduced the total protein to 54.26 μg g-1 in H. armigera. Pectolinaringenin reduced the feeding and utilization of digested food. Diet containing pectolinaringenin reduced 55.75% of the total protein content against E. vittella and 53.2% in H. armigera at 100 ppm concentration and the reduction was dose-dependent. The present finding is in agreement with the earlier results of Rharrabe et al. (2007), who studied the effects of 20-hydroxyecdysone isolated from Plypodium vulgare on protein of Plodia interpunctella larvae at 50, 100, 200 and 500 ppm concentrations; they found that 20-hydroxyecdysone reduced the protein content (in a dose-dependent manner) when the larvae consumed the treated diet. Neoliya et al. (2007) evaluated the effect of azadirachtin from A. indica against the larvae of H. armigera and observed a significant reduction in protein. Limonoids, deacetylnimbin, 17-hydroxyazadiradione, gedunin, salannin and deacetyl-gedunin from A. indica were studied against ovary protein of H. armigera (Jayabalan & Murugan 1997). They found that all the compounds reduced the protein concentration
Enzyme activity
In the present investigation, pectolinaringenin-treated larvae showed a reduction in GST and esterase activities. The compound reduced GST activity by 37.61% and esterase activity by 43.25% over control against E. vittella. In the case of H. armigera, the same pectolinaringenin reduced the GST and esterase activities by 43.21% and 47.41%, respectively, over control. The reduction was in a dose-dependent manner in both the pests. The present findings corroborated the findings of Mukanganyama et al. (2003), who investigated the effect of DIMBOA (2,4-dihydroxy-7-methoxy-1,4-benzoxazin-3-one) isolated from Triticum aestivum on GST and esterase of Rhopalosiphum padi at 0.5, 1, 2 and 4 mM concentrations. The insects fed with treated diet exhibited decreased GST activity in vitro and in vivo by 33% and 30%, respectively,, and also reduced esterase activity by 50% and 75%, respectively. The effect of cyclodepsipeptidic mycotoxin, destruxin from Metarhizium anisopliae on glutathione and oxidized glutathione in larvae of S. litura was studied by Sree & Padmaja (2008); they observed that after 24 and 48 h of treatment, the levels of glutathione and oxidized glutathione were decreased in direct proportion to concentration. The larvae treated with Celangulin V isolated from the Chinese plant Celastrus augulatus reduced the esterase activity of M. separata by 7% (Lu et al. 2008). Azadirachtin from A. indica inhibited the enzyme activity of acetylcholinesterase in Nilaparvata lugens at concentrations of 0.10, 0.25 and 0.50 ppm, in a dose-dependent manner (Senthil Nathan et al. 2008).
Sosa et al. (2000) investigated 20 flavonoids isolated from Argentina native plants and some others purchased commercially against T. molitor larval growth. Quercetin was the most effective growth inhibitor for T. molitor larvae and it decreased the GST activity of nymphs of Triatoma infestans. Sintim et al. (2009) observed that Sesamum indicum extract reduced the GST activity of 1st and 2nd instar larvae of S. litura.
Thiboldeaux et al. (1998) reported the effects of 1,4-naphthoquinones on mid gut glutathione levels of Actias luna and Callosamia larvae at 0.05% and 0.5% concentrations. After 3 days mid gut total glutathione decreased in A. luna. Bullangpoti et al. (2007) studied the effect of magnostin from the fruit extract of Garcinia mangostana against the brown planthopper Nilaparvata lugens. They observed that magnostin reduced the detoxifying enzyme level of GST by 1.01–3.34-fold. Rambutan’s seed extracts from Nephilium lappaceum and mangosteen’s peel extracts from Garcinia mangostana reduced esterase and GST activities on rice weevil (Bullangpoti et al. 2004).
Pectolinaringenin significantly reduced the total protein content, esterase and GST enzyme activities of E. vittella and H. armigera in this study. Renuga & Sahayaraj (2009) reported that plant-derived substances reduced the head protein content of Spodoptera litura. Many investigations demonstrated the effect of botanicals on different parameters such as feeding of insects, digestibility of consumed food, conversion efficiency of the ingested food, conversion efficiency of digested food and consumption index (Shekari et al. 2008). Inhibitory effects of botanical insecticides on digestive enzymes have been illustrated by Zibaee & Bandani (2010a). Plant-derived substances induced the expression of esterase activity in the early stages of the insects and reduced it in the later stages due to their toxicity (Zibaee & Bandani 2010b).
Conclusion
In the present investigation, pectolinaringenin isolated from C. phlomidis, reduced the total protein content, esterase and GST enzyme activities. Reductions in protein and enzyme activities affect the growth and survival of E. vittella and H. armigera larvae. It is concluded that pectolinaringenin could be used to develop an effective botanical insecticidal formulation for the management of important agricultural pests.
References
Baskar, K., & Ignacimuthu, S. (2012). Antifeedant, larvicidal and growth inhibitory effect of ononitol monohydrate isolated from Cassia tora L. against Helicoverpa armigera (Hub.) and Spodoptera litura (Fab.) (Lepidoptera: Noctuidae). Chemosphere, 88, 384–388.
Birk, Y., & Peri, I. (1980). Saponins. In I. E. Liener (Ed.), Toxic constituents of plant foodstuff (2nd ed., pp. 161–182). New York, NY: Academic Press.
Boreddy, Y., Chitra, K. C., & Reddy, E. (2000). Studies on sublethal concentration (LC30) of Annona seed extract on total proteins of Spodoptera litura (Fab.). Entomon, 25, 351–355.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilising the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254.
Bullangpoti, V., Visetson, S., Milne, J., Milne, M., Sudthongkong, C., & Pornbanlualap, S. (2007). Effects of alpha-mangostin from mangosteen pericarp extract and imidacloprid on Nilaparvata lugens (Stål.) and non-target organisms: toxicity and detoxification mechanisms. Communications in Agricultural and Applied Biological Sciences, 72, 361–712.
Bullangpoti, V., Visetson, S., Milne, J., & Pornbanlualap, S. (2004). Effects of mangosteen’s peels and rambutan’s seeds on toxicity, esterase and glutathione-S-transferase in rice weevil (Sitophilus oryzae L.). Kasetsart Journal: Natural Science, 38, 84–89.
Cho, J. R., Kim, H. S., Song, H. S., & Lee, J. D. (1995). Synergism and detoxification mechanism in the larvae of Helicoverpa assulta and Spodoptera litura (pp. 413–431). Crops: USDA Research Reports of the Rural Development Administration.
Conyers, C. M., MacNicoll, A. D., & Price, N. R. (1998). Purification and characterisation of an esterase involved in resistance to organophosphorus insecticides in the saw toothed grain beetle, Oryzaephilus surinamensis, (Coleoptera: Silvernidae). Insect Biochemistry and Molecular Biology, 28, 435–448.
Elliger, C. A., Chan, B. C., & Waiss, A. C., Jr. (1980). Flavonoids as larval growth inhibitors. Naturwissenschaften, 67, 358–360.
Fakrudin, B., Kumar, V., Krishnareddy, K. B., Patil, B. V., & Kuruvinashetti, M. S. (2004). Insecticide resistance vis-à-vis Cry1Ac delta endotoxin resistance in South Indian cotton ecosystem. Resistant Pest Management Newsletter, 13, 15–17.
Finney, D. J. (1971). Probit analysis (3rd ed.). London, UK: Cambridge University Press.
Francis, F., Haubruge, E., Gaspar, C., & Dierickx, P. J. (2001). Glutathione S-transferases of Aulacorthum solani and Acyrthosiphon pisum: Partial purification and characterisation. Comparative Biochemistry and Physiology Part B, 129, 165–171.
Han, Z., Moores, G., Devonshire, A., & Denholm, I. (1998). Association between biochemical markers and insecticide resistance in the cotton aphid, Aphis gossypii. Pesticide Biochemistry and Physiology, 62, 164–171.
Hedin, P. A., & Waage, S. K. (1986). Roles of flavonoids in plants resistant to insects. In V. Cody, E. Middleton, & J. B. Harborne (Eds.), Plant flavonoids in biology and medicine: Biochemical, pharmacological and structure activity relationships (pp. 87–100). New York, NY: Alan R. Liss Inc.
Huang, S., & Han, Z. (2007). Mechanisms for multiple resistances in field populations of common cutworm, Spodoptera litura (Fabricius) in China. Pesticide Biochemistry and Physiology, 87, 14–22.
Jalali, S. K., Mohan, K. S., Singh, S. P., Manjunath, T. M., & Lalitha, Y. (2004). Baseline susceptibility of the old-world bollworm, Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) population from India to Bacillus thuringiensis Cryl Ac insecticides protein. Crop Protection, 23, 53–59.
Jallow, M. F. A., Cunningham, J. P., & Zalucki, M. P. (2004). Intraspecific variation for host plant use in Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae): Implications for management. Crop Protection, 23, 955–964.
Jayabalan, D., & Murugan, K. (1997). Effect of neem limonoids on feeding and reproduction of Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae). Entomon, 22, 15–20.
Jayaraj, S. (2003). Insect biodiversity: Conservation and enhancement of entomophage diversity for biological pest suppression in rice ecosystem. In S. Ignacimuthu & S. Jayaraj (Eds.), Biological control of insect pests (pp. 232–249). New Delhi, India: Phoenix Publishing House.
Khalaf, A. A. (1998). Biochemical and physiological impacts of two volatile plants oils on Muscina stabulans (Diptera: Muscidae). Journal of the Egyptian-German Society of Zoology, 27, 315–329.
Kim, J. S., Kwon, C. S., & Son, K. H. (2000). Inhibition of ά-glucosidase and ά-amylase by luteolin, a flavonoid. Bioscience, Biotechnology, and Biochemistry, 64, 2458–2461.
Koul, O., Shankar, J. S., Mehta, N., Taneja, S. C., Tripathi, A. K., & Dhar, K. L. (1997). Bioefficacy of crude extracts of Aglaia species (Meliaceae) and some active fractions against lepidopteran larvae. Journal of Applied Entomology, 121, 245–248.
Lu, M., Wu, W., & Liu, H. (2008). Effect of celangulin V on detoxification enzymes in Mythimna separata and Agrotis ypsilon. Pesticide Biochemistry and Physiology, 90, 114–118.
Mukanganyama, S., Figueroa, C. C., Hasler, J. A., & Niemeyer, H. M. (2003). The effect of DIMBOA on detoxification enzymes of the aphid Rhopalosiphum padi (Homoptera: Aphididae). Journal of Insect Physiology, 49, 223–229.
Muthu, C., Baskar, K., Kingsley, S., & Ignacimuthu, S. (2012a). Bioefficacy of Clerodendrum phlomidis Linn. F. and Flueggea leucopyrus (Koen.) Willd. against Earias vittella Fab. Journal of Entomology, 9, 332–342.
Muthu, C., Reegan, A. D., Kingsley, S., & Ignacimuthu, S. (2012b). Larvicidal activity of pectolinaringenin from Clerodendrum phlomidis L. against Culex quinquefasciatus Say and Aedes aegypti L. (Diptera: Culicidae). Parasitology Research, 111, 1059–1065.
Neoliya, N. K., Singh, D., & Sangwan, R. S. (2007). Azadirachtin-based insecticides induce alteration in Helicoverpa armigera Hub. head polypeptides. Current Science, 92, 94–99.
Oppenoorth, F. J. (1979). Glutathione-S-transferase and hydrolytic activity in a tetrachlorvinphos-resistant strain of house fly and their influence on resistance. Pesticide Biochemistry and Physiology, 11, 176–178.
Radake, S. G., & Undirwade, R. S. (1981). Seasonal abundance and insecticidal control of shoot and fruit borer, Earias spp. on okra, Abelmoschus esculentus (L.). Indian Journal of Entomology, 43, 283–287.
Renuga, F. B., & Sahayaraj, K. (2009). Influence of botanicals in total head protein of Spodoptera litura (Fab.). Journal of Biopesticides, 2, 52–55.
Rharrabe, K., Bakrim, A., Ghailani, N., & Sayah, F. (2007). Bioinsecticidal effect of harmaline on Plodia interpunctella development (Lepidoptera: Pyralidae). Pesticide Biochemistry and Physiology, 89, 137–145.
Schmidt, G. H., Rembold, H., Ahmed, A. A. I., & Breuer, M. (1998). Effect of Melia azedarach fruit extract on juvenile hormone titer and protein content in the haemolymph of two species of noctuid lepidopteran larvae (Insecta: Lepidoptera: Noctuidae). Phytoparasitica, 26, 283–292.
Senthil Nathan, S., Choi, M. Y., Paik, C. H., Seo, H. Y., Kalaivani, K., & Kim, J. D. (2008). Effect of azadirachtin on acetylcholinesterase (AChE) activity and histology of the brown planthopper Nilaparvata lugens (Stål). Ecotoxicology and Environmental Safety, 70, 244–250.
Senthilkumar, N., & Murugan, K. (1999). Impact of NPV and azadirachtin on the digestive enzyme activity and biochemical composition of the gut of Helicoverpa armigera Hübner (Insecta: Lepidoptera: Noctuidae). Tropical Agricultural Research, 11, 393–407.
Shekari, M., Sendi, J. J., Etebari, K., Zibaee, A., & Shadparvar, A. (2008). Effects of Artemisia annua L. (Asteracea) on nutritional physiology and enzyme activities of elm leaf beetle, Xanthogaleruca luteola Mull (Coleoptera: Chrysomellidae). Pesticide Biochemistry and Physiology, 91, 66–74.
Sieber, K. P., & Rambold, H. (1983). The effects of azadirachtin on the endocrine control of moulting in Locusta migratoria. Journal of Insect Physiology, 29, 523–527.
Singleton, V. L. (1981). Naturally occurring food toxicants: Phenolic substances of plant origin common in foods. Advances in Food Research, 27, 149–242.
Sinha, S. N., Sharma, S. P., & Chakrabarti, A. K. (1978). Effect of spotted bollworm infestation on seed quality of okra. Seed Research, 6, 161–164.
Sintim, H. O., Tashiro, T., & Motoyama, N. (2009). Response of the cutworm Spodoptera litura to sesame leaves or crude extracts in diet. Journal of Insect Science, 9, 1–13.
Sosa, M. E., Tonn, C. E., Guerreiro, E., & Giordano, O. S. (2000). Bioactividad de flavonoides sobre larvas de Tenebrio monitor. Revista de la Sociedad Entomológica Argentina, 59, 179–184.
Sree, K. S., & Padmaja, V. (2008). Oxidative stress induced by destruxin from Metarhizium anisopliae (Metch.) involves changes in glutathione and ascorbate metabolism and instigates ultrastructural changes in the salivary glands of Spodoptera litura (Fab.) larvae. Toxicon, 51, 1140–1150.
Srinivasan, P. M., & Gowder, R. B. (1960). A preliminary note on the control of bhendi shoot and fruit borer. Indian Journal of Agricultural Sciences, 30, 57.
Subramanian, S., & Mohankumar, S. (2006). Genetic variability of the bollworm, Helicoverpa armigera, accruing on different host plants. Journal of Insect Science, 6, 26.
Thiboldeaux, R. L., Lindroth, R. L., & Tracy, J. W. (1998). Effects of juglone (5-hydroxy-1,4- naphthoquinone) on midgut morphology and glutathione status in Saturniid moth larvae. Comparative Biochemistry and Physiology, 120, 481–487.
Whitaker, J. R. (1981). Naturally occurring peptide and protein inhibitors of enzymes. In J. C. Ayres & J. Kirschman (Eds.), Impact of toxicology on food processing (pp. 57–104). Westport, CT, USA: Avi Publishing.
Wood, E., Casabe, N., Melgar, F., & Zerba, E. (1986). Distribution and properties of glutathione-S-transferase from Triatoma infestans. Comparative Biochemistry and Physiology, 84, 607–618.
Wood, E. J., Melgar, F., & Zerba, E. N. (1990). Inhibitors of the glutathione S-transferases from Triatoma infestans. Anales de la Asociacion Quimica Argentina, 78, 251–260.
Zanden, J. J. V., Geraets, S., Wortelbier, H. M., Bladeren, P. J. V., Rietjens, I. M. C. M., & Cnubben, N. H. P. (2004). Structural requirements for the flavonoid mediated modulation of glutathione S-transferase P1-1 and GS-X pump activity in MCF-7 breast cancer cells. Biochemical Pharmacology, 67, 1607–1617.
Zibaee, A., & Bandani, A. R. (2010a). Effects of Artemisia annua L. (Asteracea) on digestive enzymes profiles and cellular immune reactions of the sunn pest, Eurygaster integriceps (Heteroptera: Scutellaridae), against Beauvaria bassiana. Bulletin of Entomological Research, 100, 185–196.
Zibaee, A., & Bandani, A. R. (2010b). A study on the toxicity of a medicinal plant, Artemisia annua L. (Asteracea) extracts to the sunn pest, Eurygaster integriceps Puton (Heteroptera: Scutelleridae). Journal of Plant Protection Research, 50, 48–54.
Zucker, W. V. (1983). Tannins: Does structure determine function? An ecological perspective. American Naturalist, 121, 335–365.
Acknowledgment
The authors thank Entomology Research Institute, Loyola College, India, for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baskar, K., Muthu, C. & Ignacimuthu, S. Effect of pectolinaringenin, a flavonoid from Clerodendrum phlomidis, on total protein, glutathione S-transferase and esterase activities of Earias vittella and Helicoverpa armigera . Phytoparasitica 42, 323–331 (2014). https://doi.org/10.1007/s12600-013-0363-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12600-013-0363-4