Abstract
Zeugodacus cucurbitae (Coquillett) is an economically significant destructive pest of many vegetable and fruit crops. Peptidase inhibitors are a class of plant proteins that cause protein degradation and decrease the supply of amino acids, hampering insect pest growth and survival. To investigate the role of peptidase inhibitors in the control of this pest, the midgut peptidase activities and the growth and development of Z. cucurbitae larvae was studied when they were exposed to partially purified peptidase inhibitor from Mucuna pruriens (L.) DC. The results obtained showed a decline in larval survival, pupal weight and nutritional indices. The results also revealed that after 24, 48 and 72 h of treatment, peptidase inhibitor partially purified from M. pruriens seeds inhibited the complete proteolytic activity of larvae. It exerted effect on midgut peptidases at a stage where Z. cucurbitae larvae feed voraciously thereby prolonging the larval cycle and reducing pupal weight. These results provide quantitative information on the ability of peptidase inhibitors to control Z. cucurbitae and other destructive pest, avoiding chemical pesticides.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Dipteran insects are major agricultural pests, due to characteristics like polyphagy and high fecundity. Zeugodacus cucurbitae, the melon fruit fly (Diptera: Tephritidae) is the most devastating insect-pest which reduces the marketability and value of horticultural crops by causing greater yield losses in global agricultural products (Qin et al. 2015). There are around 4000 species of fruit flies from 500 genera (White and Elson-Harris 1992). Approximately 1500 species of fruit fly feed on fruits and nearly about 250 species cause remarkable losses in economic important crops (Sarwar 2006; Qin et al. 2015). Various synthetic pesticides or insecticides are used worldwide for the control of different insect pests including Z. cucurbitae. Residues of these pesticides can be found in a wide range of daily consumable food stuffs like fruits, vegetables, water, refreshments, pulses and animal feeds (Chourasiya et al. 2015). In addition, the peeling and rinsing cannot clear the trace amounts of these pesticides entirely (Reiler et al. 2015) and therefore they adversely affect the human health (Alewu and Nosiri 2011; Pimentel and Burgess 2014; Zheng et al. 2016). Their inadvertent use has also contributed to the development of resistance in crops to insect pests and thus new compounds need to be investigated and discovered that are safe and environmentally friendly that can substantially reduce the use of organic pesticides for insect pest reduction.
A possible alternative is the utilization of proteins, called plant peptidase inhibitors (Dunaevskiĭ et al. 2005). These plant peptidase inhibitors play a key role in controlling aricultural pests, marine food sources, crops, insect life etc. (da Silva Bezerra et al. 2016). Peptidase inhibitor’s response mechanism involves suppression of digestive peptidases distributed in the midgut of the insect (Srinivasan et al. 2006). These inhibitors target peptidases by forming stoichiometric complexes, thereby blocking the active site of their respective peptidases (Volpicella et al. 2011). A variety of proteins from various plant origins like peptidase inhibitors have already been isolated till date. Such peptidase inhibitors include various plant components, such as leaves, flowers and seeds, which act as proteolytic activity managers and for protein storage (Stevens et al. 2013; Macedo et al. 2007).
The genus Mucuna, of the leguminaceae family which contains around 150 species of yearly and seasonal legumes, is widespread in tropical and subtropical areas of the world. Despite the high protein content, it is considered as a legitimate provider of diet proteins (Lampariello et al. 2012) and its palatability, is equivalent to other pulses like lima bean, soybean and rice bean (Gurumoorthi et al. 2003). The insecticidal potential of partially purified peptidase inhibitor from Mucuna pruriens (L.) DC. seeds have not been reported till date. Therefore, in this study, we examined the effect of peptidase inhibitor on the growth and development of melon fruit fly through bioassay, nutritional assay and enzyme assays. Antibacterial activity of partially purified M. pruriens peptidase inhibitor was also evaluated. The reports from this analysis may provide the basis for developing a new methodology for successful use of peptidase inhibitors to control population of various insect pests.
Materials and methods
Partial purification of peptidase inhibitor
M. pruriens seeds belonging to the leguminaceae family were procured from the local market of Amritsar, India. The seeds were identified and were given treatment of mercury chloride (0.01%) to eliminate any type of contamination, washed with distilled water, dried in air, and finely grinded in liquid nitrogen. The seed flour was solubilised in Millipore water (1:10,w/v) with continuous stirring at 4 °C for 3 h. The slurry obtained after stirring was centrifuged for 20 min at 4 °C at 5500 rpm and the supernatant called crude extract (CE) was collected. CE was subjected to protein fractionation by ammonium sulphate precipitation with different saturation ranges: 0–80%, 20–80%, 40–80% and 60–80% followed by centrifugation of each saturation mixture at 10,000 rpm for 30 min at 4 °C. The fraction obtained after dialysis (overnight against millipore water) possessing the highest trypsin inhibitory activity was classified as partially purified peptidase inhibitor that was further analysed for protein content and trypsin inhibitory activity. The partially purified peptidase inhibitor was then subjected to RP-HPLC (Shimadzu RP-HPLC system, LC-60 AD) with binary elution system (0.1% aqueous trifluoroacetic acid and 0–60% acetonitrile) at 30 °C for 15 min with a flow rate of 3 ml/min. The eluted fractions were monitored at 240 nm.
Protein estimation and trypsin inhibition assay
For each purification level the concentration of proteins was determined by employing bovine serum albumin (standard) as described by Bradford (1976). Peptidase inhibitory activity was calculated using N-α-benzoyl-DL-arginine p-nitroanilide (BApNA) as a substratum, using the method described by Samiksha et al. (2019a). There were three replicates per concentration for each experiment.
Effect of temperature and pH
Effect of temperature and pH on peptidase inhibitor from M. pruriens was conducted as described previously by Samiksha et al. (2019b). The peptidase inhibitor sample prepared in millipore water (1 mg/ml) was boiled at various temperatures (10–100 °C) for 30 min and then analysed for inhibitory activity assays. To evaluate pH stability, the peptidase inhibitor from M. pruriens (1 mg/ml) was mixed with an equivalent volume of different buffers (100 mM): phosphate buffer (pH 6–8), Tris–HCl buffer (pH 8–9), and carbonate–bicarbonate buffer (pH 9–11) for 30 min at 30 °C and then their residual inhibitory activity was assayed. There were three replicates per concentration for each experiment.
Effect of solvents, metal ions and detergents
Effect of various solvents on peptidase inhibitory activity was studied by incubating peptidase inhibitor from M. pruriens with different concentrations (10%, 20%, 30% and 40%) of the solvents at 25 °C for 30 min.
Effect of various metal ions (Ca2+, Co2+, Mg2+, Cu2+, Fe3+, Zn2+, Mn2+, Ni2+) on the inhibitory activity of peptidase inhibitor from M. pruriens was studied using 1, 5, 25 and 50 mM concentrations. The peptidase inhibitor from M. pruriens equilibrated with different metal ions were incubated at 25 °C for 30 min, and then evaluated for its peptidase inhibitory activity. Effect of various detergents like SDS, Triton X-100, CTAB, Tween-80, and Urea on the inhibitory activity of peptidase inhibitor from M. pruriens was analysed by incubating the peptidase inhibitor with each detergent at 0.1%, 0.5%, 1.0% and 2.0% concentrations for 30 min. There were three replicates per concentration for each experiment.
Stability of peptidase inhibitor
The partially purified peptidase inhibitor from M. pruriens seeds was stored at 4 °C and samples were collected at regular intervals (monthly) for residual trypsin inhibitory activity estimation. There were three replicates per concentration for each experiment.
Insect culture and diet
Melon fruit fly larvae were collected from Amritsar, India and supplemented with fresh wild culture every 6 months. Larvae were reared on pumpkin pieces and at second instar stage were subjected to different treatments. Dietary composition was (per 50 ml of diet): 3.00 g Casein (vitamin free), 0.04 g Cholesterol, 0.10 g Mc Collumns salt mixture, 0.10 g Ribonucleic acid, 0.10 g Chloramphenicol, 1.00 g Agar agar, 50.00 ml distilled water, 0.40–0.50 ml 10%KOH, 1.10 g Vitamin Sucrose mixture. Insects were reared at 25 ± 2 °C with 70–80% relative humidity and 14:10 dark and light photoperiod in insect culture room.
Effect of partially purified peptidase inhibitor on growth and diet consumption
Larvae were reared to second instar on pumpkin diet and then transferred to large sized test tubes having artificial diet incorporated with partially purified peptidase inhibitor at concentrations ranging from 200 ppm to 1000 ppm along with control. Different growth parameters like larval period, pupal period, total development period, percentage adult emergence and percent larval mortality were calculated. For the evaluation of nutritional indices separate experiments were carried out where larvae (n = 15) were weighed before being added to the diets and after 48 h were weighed again. The mean relative growth rate was obtained using the formula “MRGR (mg/mg/day) = (Final weight of larvae (mg) - Initial weight of larvae (mg))/Time (in days)” whereas food assimilated was calculated by using formula, “food assimilated (mg) = (Ti (Cf-Ci))/Ci+Tf-Ti” where, Ci = weight of larvae before feeding (control), Cf = weight of larvae after feeding (control), Ti = weight of larvae before feeding (treated) and Tf = weight of larvae after feeding (treated). For each concentration, there were six replications with fifteen larvae per replicate and each experiment was repeated thrice. One way ANOVA was used to compare the effect of different concentrations of partially purified peptidase inhibitor on the growth rate and nutritional indices followed by the Tukey HSD test for post hoc comparisons of means using 16.0 SPSS program (SPSS 2007).
Lethal concentration
The concentration which caused 50% larval mortality was calculated using probit analysis (Finney 1971).
Gut enzyme analysis
Preparation of gut extract
Z. cucurbitae larvae were given LC50 of partially purified peptidase inhibitor incorporated in artificial diet. Larvae were homogenized using electric homogenizer in 0.15 M NaCl and centrifuged at 4 °C for 10 min at 13,000 g. The supernatant was removed and stored at −20 °C for enzyme assays. There were six replications with fifteen larvae per replicate. Trypsin and chymotrypsin activity assay were done using a modified protocol (Christeller et al. 1990, 1992) in a total volume of 200 μl in wells of ELISA plate. Assays contained 50 μl of 0.1 M Tris-HCl (pH 8.2), 50 μl of sample and 100 μl of BAPNA (prepared in dimethyl sulfoxide) and casein (prepared in tris HCl buffer) as substrates for trypsin and chymotrypsin, respectively. The optical density at 405 nm was monitored at an interval of 1 min on a microplate reader (Bio-Rad Lab. Ltd). Appropriate blanks (water) were used and enzymatic activity was expressed in U/mg of total protein obtained by the Bradford assay. One unit of enzyme activity was defined as the amount of enzyme that liberates 1 mmol of p-nitroanilide from the substrate per minute.
Antibacterial assay
The antibacterial activity was evaluated using the cultures of different bacteria viz. Mycobacterium smegmatis, Pseudomonas aeruginosa, Bacillus thuringiensis and Escherichia coli procured from the department of Molecular Biology and Biochemistry, Guru Nanak Dev University, India. In vitro antibacterial activity of the partially purified peptidase inhibitor was tested using disc diffusion assay carried out in 100 X 20 mm petriplates each carrying 25-30 ml of Luria Bertani agar (LBA) after spreading about 100 μl suspension of each bacterial culture in different plates. The partially purified M. pruriens peptidase inhibitor was poured in each disc placed in LB agar plates upon sterilization by 0.2 mm millipore filter using different concentrations 25 μg/ml, 50 μg/ml, 75 μg/ml, 100 μg/ml. For the respective microorganism, the concentration which developed the inhibition zone equivalent to the positive control was taken as the minimum inhibitory concentration (MIC).
Statistical analysis
For each concentration of the bioassays, nutritional assays and enzyme assays, there were six replications with fifteen larvae per replicate. Each experiment was repeated thrice and the values were represented as Mean ± SE. The data were subjected to analysis of variance (ANOVA). Statistical differences were determined by Tukey’s post hoc test.
Results
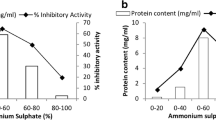
The dialysed fractions after ammonium sulphate precipitation (0–80%, 20–80%, 40–80% and 60–80%) of M. pruriens aqueous extract were evaluated for their trypsin inhibitory activity in a microplate reader using BAPNA as substrate. From the results obtained it was noticed that 0–80% among the entire fraction possessed maximum trypsin inhibitory activity. The chromatogram obtained using RP-HPLC given in Fig. 1. Partially purified peptidase inhibitor from M. pruriens seeds exhibited 3,510,720 total trypsin inhibitory units (TIUs) of protein with 180 TIU mg−1 protein specific activity (Table 1).
Effect of temperature and pH
The inhibitory activity of partially purified peptidase inhibitor from M. pruriens increased with increase in temperature but after 30 °C it declined but retained 39.67% of the inhibitory activity at 100 °C (Fig. 2a). Maximum trypsin inhibitory activity (84.30%) was observed at 30 °C. Partially purified peptidase inhibitor from M. pruriens exhibited stability at broad pH range (pH 6.0–11.0) and the maximum inhibitory activity (~80%) was noticed at pH 7.0 (Fig. 2b). It retained 48.68% of inhibitory activity at pH -11.0.
a Temperature stability of partially purified M. pruriens peptidase inhibitor. b pH stability of partially purified M. pruriens peptidase inhibitor. Data are mean ± Standard error, one way ANOVA and Tukey’s HSD. Temperature (F = 88.50**, HSD = 8.21), pH (F = 46.98**, HSD = 6.22). * and ** indicates significant at p < 0.05 and p < 0.01, respectively. F = F-ratio, HSD = Honestly Significant Difference
Effect of solvents, metal ions and detergents
The peptidase inhibitory activity of M. pruriens was increased in the presence of acetone, benzene, chloroform and DMSO but decreased with ethanol, ethylacetate, hexane, methanol and Petroleum ether all the four concentrations (Supplementary Fig. 1). The inhibitory activity of peptidase inhibitor from M. pruriens enhanced with Ca2+, Co2+, Cu2+ and Zn2+ but decreased with Fe2+, Mg2+, Mn2+ and Ni2+ from 1 mM to 50 mM when compared to control (Supplementary Fig. 2). The inhibitory activity of peptidase inhibitor from M. pruriens reduced with SDS, Tween-80 and Triton-X but increased with CTAB at all the concentrations when compared to control (Supplementary Fig. 3).
Stability of peptidase inhibitor
The partially purified peptidase inhibitor from M. pruriens seeds was found to be stable after 6 months, after that residual trypsin inhibitory activity started declining.
Bioassay
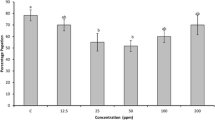
Bioassay results revealed that the larval (F6,35 = 93.76, P < 0.001), pupal (F6,35 = 4.67, P < 0.001) and total development period (F6,35 = 93.06 P < 0.001) of second instar larvae was significantly delayed when fed with different concentrations of partially purified M. pruriens peptidase inhibitor incorporated artificial diet. At 1000 ppm concentration, as compared with that of control, the different periods (larval, pupal and total development period) were prolonged maximally by 6.21, 2.52 and 2.5 days, respectively, (Fig. 3). Less number of adults emerged from the larvae with increase in concentration of partially purified peptidase inhibitor of M. pruriens supplemented in diet (F = 31.02, p < 0.01). Only 38.89% adults emerged at the highest concentration of 1000 ppm whereas 76.67% adults emerged from larvae reared on control diet. Decrease in percentage pupation was observed when 64-72 h old larvae were given partially purified peptidase inhibitor from M. pruriens supplemented diet (F = 99.43, p < 0.01). It reduced with increase in concentration. At highest concentration of 1000 ppm the percentage pupation reduced to 50.00% compared to 95.56% in control.
Larval period, pupal period, total developmental period, percent pupation and percent adult emergence of melon fruit fly when 64-72 h larvae were fed on artificial diets incorporated with different concentrations of partially purified M. pruriens peptidase inhibitor. Data are mean ± Standard error, one way ANOVA and Tukey’s HSD. * and ** indicates significant at p < 0.05 and p < 0.01, respectively. F = F-ratio, HSD = Honestly Significant Difference
Pupal weight
To analyze how larvae feeding on different concentrations of partially purified peptidase inhibitor supplemented diets affected pupal weight, pupae obtained from larval growth experiments were weighed after initial and final treatment. Significant reduction in pupal weight (F = 52.32, p < 0.01) was noticed for larvae that fed on partially purified peptidase inhibitor supplemented diets compared to larvae that fed on the control diet. Maximum decline (7.64 mg) was observed at highest concentration of 1000 ppm as compared with that in control pupae (12.27 mg) (Fig. 4).
Pupal weight of melon fruit fly when 64-72 h larvae were fed on artificial diets incorporated with different concentrations of partially purified peptidase inhibitor from M. pruriens. Data are mean ± Standard error, one way ANOVA and Tukey’s HSD. * and ** indicates significant at p < 0.05 and p < 0.01, respectively. F = F-ratio, HSD = Honestly Significant Difference
Nutritional assay
The mean relative growth rate of 64-72 h old larvae of melon fruit fly when given diet incorporated with different concentrations of partially purified M. pruriens peptidase inhibitor depicted a significant (F6,35 = 15.25, p < 0.01) reduction when compared with that of control. Amended diet resulted in 67.93% reduction in mean relative growth rate over the control at 1000 ppm concentration (Fig. 5). The treated larvae consumed less food than the control larvae as concentration dependent significant decline (F6,35 = 18.34, p < 0.01) in food assimilated was noticed. The highest concentration of 1000 ppm caused 33.26% reduction in food assimilated over the 200 ppm.
Food assimilated and mean relative growth rate of melon fruit fly when 64-72 h larvae were fed on artificial diets incorporated with different concentrations of partially purified peptidase inhibitor from M. pruriens. Data are mean ± Standard error, one way ANOVA and Tukey’s HSD. * and ** indicates significant at p < 0.05 and p < 0.01, respectively. F = F-ratio, HSD = Honestly Significant Difference
Enzyme assay
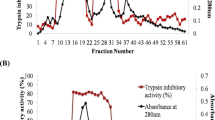
Evaluation of gut extract showed decline in trypsin activity in all treatment intervals (24, 48 and 72 h) when 64-72 h old larvae of melon fruit fly were given diet supplemented with LC50 (Fig. 6) of partially purified M. pruriens peptidase inhibitor. The chymotrypsin activity also declined in all the time intervals i.e. 24 h, 48 h, and 72 h when second instar larvae of melon fruit were fed LC50 concentration of partially purified M. pruriens peptidase inhibitor incorporated in artificial diet. Maximum decline was observed at 72 h interval (Fig. 7).
Effect of partially purified M. pruriens peptidase inhibitor against Larval Mortality. The LC50 of partially purified M. pruriens peptidase inhibitor was 1008.08 ppm. Data are mean ± Standard error, one way ANOVA and Tukey’s HSD. * and ** indicates significant at p < 0.05 and p < 0.01, respectively. F = F-ratio, HSD = Honestly Significant Difference
Effect of partially purified M. pruriens peptidase inhibitor on digestive peptidases (trypsin and chymotrypsin) of second instar larvae of melon fruit fly. Data are mean ± Standard error, one way ANOVA and Tukey’s HSD. * and ** indicates significant at p < 0.05 and p < 0.01, respectively. F = F-ratio, HSD = Honestly Significant Difference
Antibacterial assay
The antibacterial activity of partially purified M. pruriens peptidase inhibitor was evaluated using an agar well diffusion assay (Table 2). The partially purified M. pruriens peptidase inhibitor was detected to have antibacterial activity against P. aeruginosa, B. thuringiensis and E. coli while no inhibitory activity was noticed against M. smegmatis (Supplementary Fig. 4).
Discussion
A few trials have already been conducted to validate the function of plant peptidase inhibitors on insect, where the results showed that in vitro peptidase inhibitors are effective in decreasing the proteolytic activity of a large number of insect species (Medel et al. 2015; Zhao et al. 2019; Mendonça et al. 2020). Peptidase inhibitors in natural and artificial diets have been shown to delay the growth and development of many pest insects, serving as an efficient control mechanism (Pilon et al. 2018, Mendonça et al. 2020). The response to peptidase inhibitors can be framed in terms of digestive enzyme production, survival and performance of the insect. In the present study partially purified peptidase inhibitor resulted in delayed larval, pupal and total development period. Similar results have been observed by Mendonça et al. 2020 when they fed Anticarsia gemmatalis larvae with different soybean peptidase inhibitors (soybean Kunitz trypsin inhibitor, SKTI and soybean bowman birk inhibitor, SBBI) and reported increased larval stage duration as compared to the control. A significant prolongation in larval, pupal and total development period of S. litura larvae was observed when treated with different concentrations of partially purified peptidase inhibitor from Cassia occidentalis seeds (Vasudev and SOHAL 2016). A concentration dependent effect was observed on Plutella xylostella (Lepidoptera: Plutellidae) larvae along with delayed larval, pupal, adult, larva-adult duration as compared to control when given Nα-p-methyl sulfonyl-L-lysine chloromethylketone (TLCK), Nα-methyl sulfonyl-L- phenylalanine chloromethyl ketone (TPCK), soybean trypsin inhibitor (STI) (Zhao et al. 2019). Delayed growth and reduced weight gain can raise the exposure time to natural field enemies which affect reproduction (Zhu-Salzman and Zeng 2015). Pompermayer et al. (2001) observed inhibition in growth and development of larvae of Diatraea saccharalis when treated with soybean inhibitor supplemented artificial diet. Generally, the peptidase inhibitors do not have an immediate devastating effect on insects, but their consequences are impaired development, reproductive capacity, and development due to a decrease in the supply of essential amino acids (Wang et al. 1995).
Present study depicted decline in adult emergence, pupal weight and increase in larval mortality with increase in concentration of the partially purified peptidase inhibitor from M. pruriens. Velmani et al. (2019) had noticed a significant reduction in pupal, larval and adult weight of S. litura along with decline in adult emergence and percent pupation after its larvae were administered peptidase inhibitor isolated from Adenanthera pavonina seeds. They also reported considerable increase in larval mortality as compared to the control larvae. Considerable decline in pupal weight of S. litura was noticed at higher concentrations when given Subabul trypsin inhibitor supplemented artificial diet (Vasudev and Sohal 2015). El-latif (2015) noted antagonistic effects on larval mortality, percent pupation, larval weight and pupal weight of S. littoralis larvae when treated with serine peptidase inhibitor purified from soybean seeds. Vasudev et al. (2016) also reported significant reduction in percentage of adults emerged of S. litura when fed with different concentrations of partially purified peptidase inhibitor from Cassia occidentalis seeds. Mendonça et al. (2020) observed marked decline in pupal weight of Anticarsia gemmatalis larvae when given different soybean peptidase inhibitors (SKTI and SBBI) concentrations compared to the control. Plutella xylostella larvae when treated with Nα-p-methyl sulfonyl-L-lysine chloromethylketone (TLCK), Nα-methyl sulfonyl-L-phenylalanine chloromethyl ketone (TPCK), soybean trypsin inhibitor (STI), resulted in lower pupation rate, weight of pupae formed compared to the control group (Zhao et al. 2019). They also reported concentration dependent effect on the P. xylostella larvae as with increase in concentration the effect of the peptidase inhibitors prolonged accordingly. Wu et al. (2013) reported prolonged growth, reduced larval and pupal weight and delayed generation time of S. litura when larvae were treated with soybean trypsin inhibitor. Udamale et al. (2013) studied the effect of peptidase inhibitors of Okra and its wild relatives on Helicoverpa armigera (Hubner) larvae and reported various effects like decline in pupation rate, pupal weight, formation of malformed pupae and adult, increased pupal mortality as compared to control. The lowered pupal weight of the larvae fed on inhibitor demonstrated diminished reproductive ability since there was a positive correlation between adult weight and fertility (Tammaru et al. 1996).
Different adult deformities of melon fruit fly were observed when larvae were reared on partially purified peptidase inhibitor incorporated diet (Fig. 8). Similar results has been observed by Telang et al. (2003) where they fed S. litura larvae with peptidase inhibitor purified from bitter gourd. These deformities were due to the lack of proteins which are required during metamorphosis (Velmani et al. 2019). Babu et al. (2012) reported 10–20% distorted morphometry in adult and pupal stages of H. armigera when the larvae were treated with diet incorporated with peptidase inhibitor purified from Acacia nilotica seeds.
Various nutritional parameters like food assimilated and mean relative growth rate of melon fruit fly larvae were also affected negatively when given partially purified peptidase inhibitor from M. pruriens seeds. Similar results have been reported by Vasudev and Sohal (2015) where they reported reduction in nutritional indices when S. litura larvae were fed peptidase inhibitor partially purified from Subabul. Kaur and Sohal (2013) showed reduction in mean larval growth rate, larval weight gain and FA when second instar larvae of melon fruit fly were given partially purified peptidase inhibitor from peas. Study of nutritional indices can form the basis for understanding the metabolic and behavioural aspects of plant insect interactions (Lazarević and Perić-Mataruga 2003). Peptidase inhibitors have anti-nutritional consequences that can result in breaking the equilibrium between peptidases, halting the digestion and ultimately, disrupting the reproduction and growth of the insect (Chougule et al. 2003).
In dipteran pests, serine peptidases (trypsin and chymotrypsin) are the main digestive enzymes active at a pH of 9–11 (alkaline) (Gomes et al. 2005). Peptidase inhibitors inhibit peptidase function by binding to their active sites (Zhao et al. 2019). Few studies in serine peptidase inhibitors are shown to be used for monitoring herbivorous insects to devise efficient anti-pest strategies (Tamhane et al. 2007). The Bowman–Birk inhibitors purified from Clitoria fairchildiana seeds demonstrated considerable inhibitory activity against larvae of Heliothis virescens, Diatraea saccharalis and Anagasta kuehniella (Dantzger et al. 2015). After 12 h or 24 h treatment of H. armigera larvae with SKTI and TLCK (trypsin inhibitor), a considerable decline in trypsin activity was observed as compared to control whereas Chymotrypsin activity was reduced only for larvae fed on the phenylmethylsulfonyl fluoride (PMSF) incorporated diet (Kuwar et al. 2020). The insects periodically adjust their intestinal peptidases to respond to the inhibitory effects of peptidase inhibitors by making a shift in the peptidase activity. When larvae ingested long-term elevated concentrations of peptidase inhibitors, then overall peptidase activity and non-alkaline trypsin activity decreased (Wang et al. 1995). Peptidase inhibitors intervene with the production and excretions of midgut peptidases of insects, and thus obstruct their growth and development (Chougule et al. 2005).
Numerous findings have shown peptidase inhibitors alter the membrane permeability of microorganisms by inhibiting their peptidase activity, implying that it is a strong antibacterial agent (Bacha et al. 2017; Arulpandi and Sangeetha 2012). Martins et al. (2018) purified a Bowman Birk peptidase inhibitor from Luetzelburgia auriculata seeds and depicted strong inhibitory activity against Staphylococcus aureus. Costa et al. (2014) isolated a trypsin inhibitor from Jatropha curcas and reported its anti bacterial activity against Salmonella enteric and Staphylococcus aureus. Dabhade et al. (2016) purified and characterized trypsin inhibitor from Albizia amara and noticed that it inhibited the activity of Bacillus subtilis and Pseudomonas aeruginosa, Alternaria tenuissima, Alternaria alternata, and Candida albicans. Bacha et al. (2017) isolated and purified serine peptidase inhibitor from Rhamnus frangula and reported that it possesses strong antibacterial activity against Bacillus cereus, B. subtilis, Klebsiella pneumonia, Staphylococcus epidermidis, Staphylococcus aureus, Salmonella enteric, Enterococcus faecalis, P. aeruginosa, E. coli with no inhibitory activity against gram negative bacteria. Hence, peptidase inhibitor can be used for control of various microorganisms, thus reducing the use of chemical spraying.
Conclusion
In the present study, we investigated the impact of partially purified peptidase inhibitor from M. pruriens seeds on the growth and development as well as peptidase activity of Z. cucurbitae larvae. We found that peptidase inhibitor had differing levels of diminishing effect on peptidase activity that prolonged the growth and development cycle of Z. cucurbitae. The peptidase inhibitor from M. pruriens seeds also possessed antibacterial activity which demonstrated its importance in designing environment safe pest management programmes. The next potential step may be to use peptidase inhibitors as potential biological pesticides to regulate Z. cucurbitae or to pass the gene of peptidase inhibitor to the plants which would limit the feeding of Z. cucurbitae, preventing its growth and mitigating the damage to the host plant.
References
Alewu, B., Nosiri, C. (2011) Pesticides and human health. In: Stoytcheva M, editor. Pesticides in the Modern World – Effects of Pesticides Exposure. InTech, 231–50. Available from: http://www.intechopen.com/ books/pesticides-in-the-modern-world-effects-of-pesticides-exposure/ pesticide-and-human-health.
Arulpandi, I., & Sangeetha, R. (2012). Antibacterial activity of fistulin: a protease inhibitor purified from the leaves of Cassia fistula. ISRN pharmaceutics, 2012.
Babu, S. R., Subrahmanyam, B., & Santha, I. M. (2012). In vivo and in vitro effect of Acacia nilotica seed proteinase inhibitors on Helicoverpa armigera (Hübner) larvae. Journal of Biosciences, 37(2), 269–276.
Bacha, A. B., Jemel, I., Moubayed, N. M., & Abdelmalek, I. B. (2017). Purification and characterization of a newly serine protease inhibitor from Rhamnus frangula with potential for use as therapeutic drug. 3 Biotech, 7(2), 148.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72(1–2), 248–254.
Chougule, N. P., Hivrale, V. K., Chhabda, P. J., Giri, A. P., & Kachole, M. S. (2003). Differential inhibition of Helicoverpa armigera gut proteinases by proteinase inhibitors of pigeonpea (Cajanus cajan) and its wild relatives. Phytochemistry, 64(3), 681–687.
Chougule, N. P., Giri, A. P., Sainani, M. N. & Gupta, V. S. (2005). Gene expression patterns of Helicoverpa armigera gut proteases. Insect Biochemistry and Molecular Biology, 35(4), 355–367.
Chourasiya, S., Khillare, P. S., & Jyethi, D. S. (2015). Health risk assessment of organochlorine pesticide exposure through dietary intake of vegetables grown in the periurban sites of Delhi, India. Environmental Science and Pollution Research, 22(8), 5793–5806.
Christeller, J. T., Laing, W. A., Shaw, B. D., & Burgess, E. P. J. (1990). Characterization and partial purification of the digestive proteases of the black field cricket, Teleogryllus commodus (Walker): Elastase is a major component. Insect Biochemistry, 20(2), 157–164.
Christeller, J. T., Laing, W. A., Markwick, N. P., & Burgess, E. P. J. (1992). Midgut protease activities in 12 phytophagous lepidopteran larvae: Dietary and protease inhibitor interactions. Insect Biochemistry and Molecular Biology, 22(7), 735–746.
Costa, H. P. S., Oliveira, J. T. A., Sousa, D. O., Morais, J. K. S., Moreno, F. B., Monteiro-Moreira, A. C. O., et al. (2014). JcTI-I: A novel trypsin inhibitor from Jatropha curcas seed cake with potential for bacterial infection treatment. Frontiers in Microbiology, 5, 5.
da Silva Bezerra, C., de Oliveira, C. F. R., Machado, O. L. T., de Mello, G. S. V., da Rocha Pitta, M. G., de Melo Rêgo, M. J. B., et al. (2016). Exploiting the biological roles of the trypsin inhibitor from Inga vera seeds: A multifunctional Kunitz inhibitor. Process Biochemistry, 51(6), 792–803.
Dabhade, A. R., Mokashe, N. U., & Patil, U. K. (2016). Purification, characterization, and antimicrobial activity of nontoxic trypsin inhibitor from Albizia Amara Boiv. Process Biochemistry, 51(5), 659–674.
Dantzger, M., Vasconcelos, I. M., Scorsato, V., Aparicio, R., Marangoni, S., & Macedo, M. L. R. (2015). Bowman–Birk proteinase inhibitor from Clitoria fairchildiana seeds: Isolation, biochemical properties and insecticidal potential. Phytochemistry, 118, 224–235.
Dunaevskiĭ, I., Elpidina, E. N., Vinokurov, K. S., & Belozerskiĭ, M. A. (2005). Protease inhibitors: Use to increase plant tolerance to insects and pathogens. Molekuliarnaia Biologiia, 39(4), 702–708.
El-latif, A. O. (2015). Protease purification and characterization of a serine protease inhibitor from Egyptian varieties of soybean seeds and its efficacy against Spodoptera littoralis. Journal of Plant Protection Research.
Finney, D. J. (1971). Probit analysis. New York: Cambridge University Press.
Gomes, C.E., Barbosa, A.E., Macedo, L.L., Pitanga, J.C., Moura, F.T., Oliveira, A.S., Moura, R.M., Queiroz, A.F., Macedo, F.P., Andrade, L.B., Vidal, M.S. Effect of trypsin inhibitor Gomes, C. E., Barbosa, A. E., Macedo, L. L., Pitanga, J. C., Moura, F. T., Oliveira, A. S., ... & Vidal, M. S. (2005). Effect of trypsin inhibitor from Crotalaria pallida seeds on Callosobruchus maculatus (cowpea weevil) and Ceratitis capitata (fruit fly). Plant Physiology and Biochemistry, 43(12), 1095–1102.
Gurumoorthi, P., Pugalenthi, M., & Janardhanan, K. (2003). Nutritional potential of five accessions of a south indian tribal pulse Mucuna pruriens var utilis: Ii. Investigations on total free phenolics, tannins, trypsin and chymotrypsin inhibitors, phytohaemagglutinins, and in vitro protein digestibility. Tropical and Subtropical Agroecosystems, 1(2–3), 153–158.
Kaur, A. P., & Sohal, S. K. (2013). Biopotency of partially purified protease inhibitor from peas on the larval growth, development and enzyme system of Bactrocera cucurbitae (Diptera: Tephritidae). International Journal of Tropical Insect Science, 33(1), 82–90.
Kuwar, S. S., Pauchet, Y., & Heckel, D. G. (2020). Effects of class-specific, synthetic, and natural proteinase inhibitors on life-history traits of the cotton bollworm Helicoverpa armigera. Archives of Insect Biochemistry and Physiology, 103(4), e21647.
Lampariello, L. R., Cortelazzo, A., Guerranti, R., Sticozzi, C., & Valacchi, G. (2012). The magic velvet bean of Mucuna pruriens. Journal of Traditional and Complementary Medicine, 2(4), 331–339.
Lazarević, J. M., & Perić-Mataruga, V. D. (2003). Nutritive stress effects on growth and digestive physiology of Lymantria dispar larvae. Jugoslovenska medicinska biohemija, 22(1), 53–59.
Macedo, M. L. R., Freire, M. D. G. M., da Silva, M. B. R., & Coelho, L. C. B. B. (2007). Insecticidal action of Bauhinia monandra leaf lectin (BmoLL) against Anagasta kuehniella (Lepidoptera: Pyralidae), Zabrotes subfasciatus and Callosobruchus maculatus (Coleoptera: Bruchidae). Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 146(4), 486–498.
Martins, T. F., Vasconcelos, I. M., Silva, R. G., Silva, F. D., Souza, P. F., Varela, A. L., ... & Oliveira, J. T. (2018). A Bowman–Birk inhibitor from the seeds of Luetzelburgia auriculata inhibits Staphylococcus aureus growth by promoting severe cell membrane damage. Journal of Natural Products, 81(7), 1497–1507.
Medel, V., Palma, R., Mercado, D., Rebolledo, R., Quiroz, A., & Mutis, A. (2015). The effect of protease inhibitors on digestive proteolytic activity in the raspberry weevil, Aegorhinus superciliosus (Guerin)(coleoptera: Curculionidae). Neotropical Entomology, 44(1), 77–83.
Mendonça, E. G., de Almeida Barros, R., Cordeiro, G., da Silva, C. R., Campos, W. G., de Oliveira, J. A., & de Almeida Oliveira, M. G. (2020). Larval development and proteolytic activity of Anticarsia gemmatalis Hübner (Lepidoptera: Noctuidae) exposed to different soybean protease inhibitors. Archives of Insect Biochemistry and Physiology, 103(1), e21637.
Pilon, A. M., Campos, W. G., Silva, C. R., Cordeiro, G., Silva, C. R., & Oliveira, M. G. A. (2018). Protease inhibitory, insecticidal and deterrent effects of the trypsin-inhibitor benzamidine on the velvetbean caterpillar in soybean. Anais da Academia Brasileira de Ciências, 90(4), 3475–3482.
Pimentel, D., & Burgess, M. (2014). Environmental and economic costs of the application of pesticides primarily in the United States. In Integrated pest management (pp. 47–71). Springer, Dordrecht.
Pompermayer, P., Lopes, A. R., Terra, W. R., Parra, J. R. P., Falco, M. C., & Silva-Filho, M. D. C. (2001). Effects of soybean proteinase inhibitor on development, survival and reproductive potential of the sugarcane borer, Diatraea saccharalis. Entomologia Experimentalis et Applicata, 99(1), 79–85.
Qin, Y., Paini, D. R., Wang, C., Fang, Y., & Li, Z. (2015). Global establishment risk of economically important fruit fly species (Tephritidae). PLoS One, 10(1).
Reiler, E., Jørs, E., Bælum, J., Huici, O., Caero, M. M. A., & Cedergreen, N. (2015). The influence of tomato processing on residues of organochlorine and organophosphate insecticides and their associated dietary risk. Science of the Total Environment, 527, 262–269.
Samiksha, Singh, D., Kesavan, A. K., & Sohal, S. K. (2019a). Exploration of anti-insect potential of trypsin inhibitor purified from seeds of Sapindus mukorossi against Bactrocera cucurbitae. Scientific Reports, 9(1), 1–14.
Samiksha, Singh, D., Kesavan, A. K., & Sohal, S. K. (2019b). Purification of a trypsin inhibitor from Psoralea corylifolia seeds and its influence on developmental physiology of Bactrocera cucurbitae. International Journal of Biological Macromolecules, 139, 1141–1150.
Sarwar, M. U. H. A. M. M. A. D. (2006). Occurrence of insect pests on guava (Psidium guajava) tree. Pakistan Journal of Zoology, 38(3), 197.
SPSS Inc. Released (2007). SPSS for windows, version 16.0. Chicago, SPSS Inc.
Srinivasan, A., Giri, A. P., & Gupta, V. S. (2006). Structural and functional diversities in lepidopteran serine proteases. Cellular & Molecular Biology Letters, 11(1), 132–154.
Stevens, J. A., Dunse, K. M., Guarino, R. F., Barbeta, B. L., Evans, S. C., West, J. A., & Anderson, M. A. (2013). The impact of ingested potato type II inhibitors on the production of the major serine proteases in the gut of Helicoverpa armigera. Insect Biochemistry and Molecular Biology, 43(2), 197–208.
Tamhane, V. A., Giri, A. P., Sainani, M. N., & Gupta, V. S. (2007). Diverse forms of pin-II family proteinase inhibitors from Capsicum annuum adversely affect the growth and development of Helicoverpa armigera. Gene, 403(1–2), 29–38.
Tammaru, T., Kaitaniemi, P., & Ruohomäki, K. (1996). Realized fecundity in Epirrita autumnata (Lepidoptera: Geometridae): relation to body size and consequences to population dynamics. Oikos, 407–416.
Telang, M., Srinivasan, A., Patankar, A., Harsulkar, A., Joshi, V., Damle, A., ... & Birah, A. (2003). Bitter gourd proteinase inhibitors: Potential growth inhibitors of Helicoverpa armigera and Spodoptera litura. Phytochemistry, 63(6), 643–652.
Udamale, S. K., Moharil, M. P., Ugale, T. B., & Mankar, J. M. (2013). Differential inhibition of Helicoverpa armigera (Hubner) gut proteinases by proteinase inhibitors of okra and It's wild relatives. ISRN biotechnology, 2013, 1–10.
Vasudev, A., & Sohal, S. K. (2015). Evaluation of partially purified subabul protease inhibitors as bio insecticidal tool with potential for the control of Spodoptera litura. International Journal of Current Research and Review, 7(18), 31.
Vasudev, A., & SOHAL, S. (2016). Partially purified Glycine max proteinase inhibitors: Potential bioactive compounds against tobacco cutworm, Spodoptera litura (Fabricius, 1775)(Lepidoptera: Noctuidae). Turkish Journal of Zoology, 40(3), 379–387.
Velmani, S., Shanthi, M., Chinniah, C., & Vellaikumar, S. (2019). Antimetabolic effect on Spodoptera litura due to acute feeding of Adenanthera pavonina proteinase inhibitor. IJCS, 7(4), 980–986.
Volpicella, M., Leoni, C., Costanza, A., De Leo, F., Gallerani, R., & Ceci, L. (2011). Cystatins, serpins and other families of protease inhibitors in plants. Current Protein and Peptide Science, 12(5), 386–398.
Wang, C. I., Yang, Q., & Craik, C. S. (1995). Isolation of a high affinity inhibitor of urokinase-type plasminogen activator by phage display of ecotin. Journal of Biological Chemistry, 270(20), 12250–12256.
White, I. M., & Elson-Harris, M. M. (1992). Fruit flies of economic significance: Their identification and bionomics. CAB international.
Wu, G. Z., Zhu, K. Y., & Zeng, R. S. (2013). Effects of soybean trypsin inhibitor on growth and development phase of Spodoptera litura (F.). Journal of Ecology and Environmental Sciences, 22, 1335–1340.
Zhao, A., Li, Y., Leng, C., Wang, P., & Li, Y. (2019). Inhibitory effect of protease inhibitors on larval midgut protease activities and the performance of Plutella xylostella (Lepidoptera: Plutellidae). Frontiers in Physiology, 9, 1963.
Zheng, S., Chen, B., Qiu, X., Chen, M., Ma, Z., & Yu, X. (2016). Distribution and risk assessment of 82 pesticides in Jiulong River and estuary in South China. Chemosphere, 144, 1177–1192.
Zhu-Salzman, K., & Zeng, R. (2015). Insect response to plant defensive protease inhibitors. Annual Review of Entomology, 60, 233–252.
Acknowledgements
The grant received from UGC, UPE, New Delhi for conducting the research work is gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: Samiksha and SKS. Performed the experiments: Samiksha, DS. Analysed the data: Samiksha, SKS, DS and AKK. Wrote the manuscript: Samiksha and DS.
Corresponding author
Ethics declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 412 kb)
Rights and permissions
About this article
Cite this article
Samiksha, Singh, D., Kesavan, A.K. et al. Peptidase inhibitor from Mucuna pruriens seeds inhibits the growth and development of Zeugodacus cucurbitae larvae. Phytoparasitica 49, 645–657 (2021). https://doi.org/10.1007/s12600-021-00901-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12600-021-00901-3