Abstract
Five novel europium(III) complexes with 2-[4-(dibutylamino)-2-hydroxybenzoyl]benzoic acid (DAHB) as primary ligand and bathophenanthroline (batho), 2,2’-bipyridyl (bipy), 5,6-dimethyl-1,10-phenanthroline (dmph), 1,10-phenanthroline (phen) as ancillary ligands were synthesized via solution precipitation method. The structural formulae of synthesized complexes were speculated to be Eu(DABH)3·2H2O (C1), Eu(DABH)3·batho (C2), Eu(DABH)3·bipy (C3), Eu(DABH)3·dmph (C4) and Eu(DABH)3·phen (C5) by elemental analysis, infrared spectroscopy and proton nuclear magnetic resonance spectroscopy (1H-NMR). The photoluminescent properties and thermal stability of the complexes were investigated by photoluminescent spectroscopy and thermogravimetric analysis (TG-DTG), respectively. The Commission Internationale de I’ Eclairage (CIE) color coordinates, Judd–Ofelt intensity parameter (Ω2), total quantum yield, intrinsic quantum efficiency and energy transfer dynamics of complexes were also explored. The excitation spectra of complexes are extended up to visible region. These complexes exhibit characteristic photoemission of Eu3+ metal ion with high color purity in red region attributed to efficient energy transfer from ligand to metal ion. The replacement of water molecules from coordination sphere of europium ion by ancillary ligands results in enhancement of luminescent properties of the C2–C5 complexes, indicating that ancillary ligands act as additional light harvesting centers in sensitization phenomenon. The thermal studies show that these complexes are suitable for meeting the requirement of temperature for fabrication of OLEDs devices.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The photoluminescent properties of trivalent lanthanide ions are very peculiar, characterized by broad spectral range, high color purity, long luminescence life time and high quantum yield [1]. Furthermore, they exhibit static luminescent location which depends only on the lanthanide ion and is independent of ligands around the lanthanide ion [2]. But the lanthanide metal ions have low absorption cross section due to Laporte forbidden f–f electronic transition leading to low absorption and emission spectral intensity. However, this drawback can be improved by coordinating chelating chromophoric ligand acting as sensitizer to central metal ion via antenna effect [3]. The organic ligand absorbs ultraviolet–visible (UV–Vis) light and efficiently transfers the energy from its excited triplet states to resonance levels of central metal ion, ultimately results into outstanding luminescent properties [4]. The organic ligands such as β-diketones, aromatic carboxylic acid, hydroxyl ketone derivatives with large π-conjugated system and strong binding site for lanthanide ion act as sensitizer. In recent years, rare earth carboxylate complexes have attracted much attention due to their excellent photoluminescent properties as well as good thermal stability. Aromatic carboxylic acid, after losing proton during complexation, coordinates with Ln(III) ions through oxygen donor atoms to form luminescent complexes. Hence, these rare earth complexes are of huge interest for a wide range of applications such as organic light emitting diode (OLEDs) [5], laser technology [6], fluoroimmunoassays [7] and telecommunication [8]. The organic ligands which do not gratify all the possible 8 or 9 coordination number of central metal ion, water molecules bind to the metal ion to complete the coordination sphere [9]. The vibrational modes of O–H oscillator in water molecules are effective quenchers of luminescence intensity by enhancing non-radiative decay process [10,11,12,13]. Hence, to avoid vibronic quenching, ternary rare earth complexes are prepared by introduction of ancillary ligands. Nitrogen-containing heterocyclic ancillary ligands act as energy donor and enhance the luminescence intensity of rare earth complexes; this effect is known as synergistic effect [14, 15]. They also fulfill the coordination number of central metal ion, making the complexes more rigid, hence more stable. The organolanthanide complexes of Eu(III), Tb(III), Sm(III) and Tm(III) emit light in visible region [16]. Among these complexes, Eu(III) complexes have commercial application in organic light emitting diodes (OLEDs) due to their strong and narrow emission band in red region at about 615 nm [17, 18]. The three primary colors, i.e., blue, green and red, are required for fabrication of white light emission sources.
Keep this in mind, aromatic carboxylic acid with large conjugated and rigid structure 2-[4-(dibutylamino)-2-hydroxybenzoyl]benzoic acid (DAHB) was synthesized. By using DAHB as main ligand and bathophenanthroline (batho), 2,2’-bipyridyl (bipy), 5,6-dimethyl-1,10-phenanthroline (dmph), 1,10-phenanthroline (phen) as ancillary ligand, five novel Eu(III) complexes C1-C5, i.e., Eu(DABH)3·2H2O (C1), Eu(DABH)3·batho (C2), Eu(DABH)3·bipy (C3), Eu(DABH)3·dmph (C4) and Eu(DABH)3·phen (C5) were prepared. The structure of complexes was confirmed by elemental analysis, infrared spectroscopy (IR) and proton nuclear magnetic resonance spectroscopy (1H-NMR). Their photophysical and thermal stability were investigated by UV–Visible absorption, photoluminescent (PL) spectroscopy and thermogravimetric analysis (TG-DTG). The Commission Internationale de I’ Eclairage (CIE) color coordinates, total quantum yield, intrinsic quantum efficiency and energy transfer mechanism of complexes were also thoroughly elucidated.
2 Experimental
2.1 General information
The lanthanide nitrate hexahydrate Ln(NO3)3·6H2O (Ln = Eu(III) and Gd(III)) were purchased from Sigma-Aldrich and used as received. All other chemicals used were obtained from commercial sources of analytical grade and used without further purification. Doubly distilled and deionized water was used for synthetic procedure.
The elemental analysis was carried out on PerkinElmer 2400 elemental analyzer. IR spectra of samples were obtained with KBr pellets using PerkinElmer spectrum 400 spectrometer, within 4000–400 cm−1 range. 1H-NMR spectra were recorded on Bruker Avance II 400 NMR spectrometer using CDCl3 as solvent. 1H-NMR spectra of all samples were referenced with tetramethylsilane (TMS). UV–Vis absorption spectra were measured with Shimadzu UV-3600 plus spectrophotometer. The photoluminescent studies of all complexes were performed on Hitachi F-7000 fluorescence spectrophotometer equipped with Xenon lamp. The total quantum yield of solid samples was calculated in Edinburg FLS980-D2D2 spectrometer quipped with xenon lamp by using F980 software, and BaSO4 was used as reference material. All measurements were carried out at room temperature. Thermogravimetric (TG) and differential thermogravimetric analysis (DTG) of the complexes were investigated with SDT Q600 at a heating range of 30 °C·min−1 under nitrogen atmosphere up to 900 °C.
2.2 Synthesis of ligand (Fig. 1)
The mixture of phthalic anhydride (1.0 mol) and anhydrous AlCl3 (2.2 mol) in CH2Cl2 and N,N-dibutyl-3-methoxyaniline (1 mol) was stirred at about 0 °C for 5 h. The reaction mixture was poured into equal volume of dilute HCl, stirred for 30 min and extracted with CH2Cl2. The extract was dried over MgSO4 and evaporated to give solid residue. The solid residue was recrystallized with ethanol to give 2-[4-(dibutylamino)-2-methoxybenzoyl]benzoic acid (A). BBr3 (2 mol) was added to the stirred solution of A (1 mol) in CH2Cl2 at room temperature and stirring was continued to 1 h. Then, the reaction was quenched with water and solvent was removed by evaporation to give ligand 2-[4-(dibutylamino)-2-hydroxybenzoyl]benzoic acid (DAHB) as crude product. The crude product was purified by recrystallization with ethanol to give DAHB as yellow solid. The progress of reaction was checked by thin layer chromatography (TLC) [19].
2.3 Synthesis of C1–C5 complexes (Fig. 1)
A solution of Eu(NO3)3·6H2O (1 mmol) in water was added dropwise to the stirred solution of DAHB (3 mmol) in ethanol, and the pH of solution was adjusted between 6.0 and 7.0 with aqueous NaOH solution. The reaction mixture was stirred for 4 h at room temperature (RT), and yellow precipitates were appeared. The precipitates were separated by vacuum filtration, purified by washing with water and alcohol and dried in vacuum desiccator at 80 °C to give C1 binary complex as yellow solid. The ternary C2–C5 complexes were synthesized by the same procedure for C1 complex, but the reaction mixture of DAHB (3 mmol), batho (1 mmol) and europium nitrate (1 mmol) for C2, DAHB (3 mmol), bipy (1 mmol) and europium nitrate (1 mmol) for C3, DAHB (3 mmol), dmph (1 mmol) and europium nitrate (1 mmol) for C4 and DAHB (3 mmol), phen (1 mmol) and europium nitrate (1 mmol) for C5 were used. In order to confirm energy transfer pathway and calculate the triplet energy state of ligand DAHB, the complex Gd(DAHB)3·2H2O (G1) was synthesized. The synthetic procedure was same as that of C1 (shown in the supplementary information).
3 Results and discussion
3.1 Structural characterization
The elemental analytical data of ligand DAHB and its complexes with Eu(III) ion are tabulated in Table 1. The content of Eu(III) is determined by ethylenediaminetetraacetic acid (EDTA) titration using Erichrome black T as indicator. The results of carbon, hydrogen and nitrogen (CHN) analysis and Eu(III) ion content are in good agreement with theoretical values, indicating that the composition of complexes and ligand are according to proposed structural formulas. All complexes are soluble in chloroform, dimethylsulfoxide (DMSO), methanol and little soluble in ethanol.
The characteristics IR absorption bands of free ligand DAHB and synthesized complexes with their respective assignments are given in Table 2. The absorption spectrum of free ligand DAHB exhibits single broadband centered at 3100 cm−1 which can be attributed to three overlapped bands of νO-H stretch (–COOH) at around 2500–3200 cm−1, alkenyl νCH stretch at 3000 cm−1 and νOH stretch (phenolic) at around 3200–3600 cm−1. The sharp bands at 1713 and 1633 cm−1 are assigned to νC=O stretch of carboxylic acid and ketonic group, respectively. The IR spectra of C1–C5 complexes are similar, but they appreciably differ from spectrum of ligand. The absence of broadband at 3100 cm−1 of DAHB and presence of one weak band at about 3460–3470 cm−1 (νOH, phenolic) and other sharp band at 2956 cm−1 (νCH, sharp) in spectra of complexes demonstrate the bonding of ligand with Eu(III) ion through carboxylate group. The band of DAHB at 1713 cm−1 completely vanishes in spectra of complexes and characteristics bands of νas(COO–) and νs(COO–) observe at 1588–1592 and 1401–1410 cm−1, respectively [20]. Meanwhile, appearance of band at 427–428 cm−1 in complexes belonged to νEu–O confirms the coordination of DAHB to Eu(III) ion through oxygen atoms of carboxylate group [21]. The additional bands at 1555–1565 cm−1 (νC=N) and 540–549 cm−1 (νEu–N) in C2–C5 ternary complexes as compared to C1 binary complex clearly reveal the introduction of nitrogen-containing ancillary ligand in coordination sphere of metal ion [22]. The IR spectra of ligand DABH and C3 complex as representative of C1–C5 complexes are depicted in Fig. 2.
The 1H-NMR spectra of europium complexes display noticeable change compared to free ligand DAHB. The NMR signal corresponding to proton of –COOH group of ligand at δ = 10.83 × 10−6 (chemical shift of protons in parts per million in NMR spectra of compounds) completely disappears in complexes, implying the coordination of ligand DAHB to europium ion through carboxylate group. The line broadening of certain peaks in complexes due to paramagnetic europium ion also suggests the complexation of ligand to metal ion [23]. More number of aryl proton signals at δ = 6.03–8.48 × 10−6 in C2–C5 complexes compared to that in DAHB and C1 complex indicates the presence of ancillary ligand in coordination sphere. The chemical shift values of aryl protons of ligand and ancillary ligand in complexes slightly shift to up field, which are ascribed to electron withdrawing inductive effect of coordination [24]. Hence, results of above techniques absolutely certify the structure of ligand, bonding mode of main ligand and ancillary ligands to metal ion and structure of all five complexes. The 1H-NMR spectra of ligand DAHB and C5 complex are shown in Fig. 3.
3.2 Thermal analysis
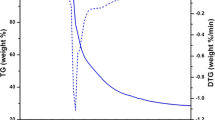
Figure 4 represents TG and DTG curve of C5 complex as representative of other complexes because all complexes exhibit similar thermal behavior and the curve is recorded at a heating rate of 30 °C·min−1 from room temperature to 900 °C under nitrogen atmosphere. TG/DTG curve of complex shows little mass loss of ~ 1.76% from 30 to 190 °C assigned to loss of moisture present in complex. The TG curve exhibits mass loss of 87.12% in region 190–610 °C which is due to removal of three ligand molecules DAHB and the ancillary ligand phen, which is also confirmed from peaks in DTG curve. The horizontal curve observed above 610 °C corresponds to formation of europium oxide as final residue. The total weight loss of complex is found close to theoretically calculated value. The thermal studies of complexes indicate that these complexes are suitable for meeting the requirement of temperature in fabrication of photoluminescent devices [25].
3.3 UV–Vis absorption spectra
The UV–Vis absorption spectra of DAHB, ancillary ligands and europium complexes in DMSO as solvent and reference are presented in Fig. 5. The absorbance spectrum of ligand DAHB lies in range of 250–400 nm, while that of ancillary ligands lies in comparatively narrow range from 258 to 350 nm. It suggests that absorption efficiency of ligand DAHB is more compared to that of the ancillary ligands; hence, DAHB is sole responsible for absorption of complexes. The absorption spectra of complexes in contrast to ligand DAHB do not show apparent change in shape of absorption bands, signifying that bonding of Eu(III) to ligand does not affect the singlet excited state of ligand [26, 27]. The absorption maxima of DAHB at 352 nm is referred to π → π* electronic transition in coordinated ligand. The absorption bands of complexes are hyperchromic shifted in ternary C2–C5 complexes with ancillary ligands compared to C1 binary complex.
3.4 Photoluminescent properties
The excitation spectra of prepared complexes in solid state are obtained by monitoring emission transition 5D0 → 7F2 of Eu(III) ion at 615 nm in range of 200–500 nm, as shown in Fig. 6. Their spectra display broadband covering UV-violet indigo visible region from 250 to 450 nm. All complexes exhibit some prominent bands at around 360, 398 and 412 nm ascribed to ligand transition while weak peak at 465 nm assigned to 7F0 → 5D2 transition of Eu(III) ion [28]. This suggests that observed luminescence is corollary of sensitization of Eu(III) ion by energy transfer from ligands. The most intense band in C5 ternary complex at 412 nm as a result of rigid and large conjugated system of DAHB and ancillary ligand phen makes it excited by visible light. It results in prevention of photodecomposition of complex by UV irradiation in photoluminescent applications [29].
The solid-state emission spectra of complexes ranging from 500 to 700 nm upon excitation with wavelength corresponding to maxima in excitation spectra are presented in Fig. 7. Their spectra show characteristics narrow emission bands of europium (III) ion corresponding to the 5D0 → 7FJ (J = 0, 1, 2) transitions at about 580, 593 and 615 nm, respectively, while very weak emission bands corresponding to 5D0 → 7F3 and 5D0 → 7F4 transition are not discernible [30]. The emission band at 580 nm is weak since 5D0 → 7F0 transition is forbidden in both electric and magnetic dipole scheme. The relatively strong band at 593 nm is due to parity allowed magnetic dipole transition 5D0 → 7F1 which is not affected by ligand field [31]. The most intense band at 615 nm responsible for characteristic red emission of Eu3+ in C1–C5 complexes indicates the presence of highly polarizable chemical environment around central metal ion because 5D0 → 7F2 transition is electric dipole transition, and its intensity is sensitive to ligand field [32, 33]. The experimental result also suggests that absorbed energy is efficiently transferred from ligands to central metal ion due to strong coordinative interaction between antenna ligands and metal ion. The relative intensity ratio of electric dipole transition to magnetic dipole transition (I2/I1 = 5D0 → 7F2/5D0 → 7F1) signifies that coordination environment around Eu3+ is without inversion center [30, 34, 35]. The intensity ratios are consistent with pure red emission and low symmetry coordination environment of europium ion. The photoluminescent intensity of ternary C2–C5 complexes is higher than that of C1 binary complex being devoid of vibronic quenching induced by O–H oscillator of water molecules in coordination sphere of C1 complex. It points out that ancillary ligands in C2–C5 complexes act as additional antenna ligands which further increase the efficiency of sensitization phenomenon.
To further elucidate the luminescent properties of europium complexes in solid state, decay curve of 5D0 excited state was recorded by monitoring the most intense band (5D0 → 7F2) centered at 615 nm (Fig. 8, Table 3). The lifetime values (τ) of C1–C5 complexes are 0.343, 0.474, 0.934, 0.974 and 1.05 ms, respectively, which are derived by fitting decay curves with single exponential function, indicating the presence of single luminescent center [36]. The longer lifetime values for C2–C5 complexes than that for C1 complex confirm the replacement of water molecules from coordination sphere of europium ion by ancillary ligands. The highest decay time value of C5 complex suggests that the ancillary ligand phen enhances the luminescence stability of overall coordination system compared to other ancillary ligands.
The CIE (Commission Internationale de I’ Eclairage) color coordinates of complexes obtained from their corresponding PL spectra by using MATLAB software are given in Fig. 9 and Table 3. Figure 9 demonstrates that the chromaticity coordinates (x, y) of C1–C5 complexes are shifted toward pure red region due to higher red/orange ratio as consequences of introduction of ancillary ligands in coordination sphere. The color coordinates of complexes are close to NTSC (National Television System Committee, 1987), suggesting that these complexes can be employed as red component in OLEDs.
The Judd–Ofelt theory is widely used to interpret f–f electronic transition by determining intensity parameters, Ωλ, (λ = 2, 4) from following equation:
where ħ is Planck’s constant over 2π, c is velocity of light, e is the electronic charge, ω is the angular frequency [37, 38], χ is the Lorentz local field correction given by χ = n 20 (n 20 + 2)2/9 where n0 is the average index of refraction equal to 1.5. The value of square reduced matrix elements are < 5D0║U(2)║ 7F2 > 2 = 0.0032, < 5D0║U(4)║ 7F4 > 2 = 0.0023, and A0–λ is coefficient of spontaneous emission corresponding to 5D0 → 7Fλ transition [37]. The intensity parameter Ω2 is highly sensitive to symmetry and sequence of ligand field around europium ion and also related to the degree of covalency in coordination sphere of europium ion [39]. The values of intensity parameter Ω2 of C1–C5 complexes estimated from their emission spectra based on 5D0 → 7F2 electronic transition are tabulated in Table 3, and intensity parameter Ω4 is not determined because 5D0 → 7F4 transition is not discernible in emission spectra of complexes. The result indicates that the chemical environment around europium ion is asymmetric and associated with covalence characteristic of ligand field in these complexes, also larger value of Ω2 for C5 complex compared to other complexes reflects more asymmetric chemical environment around Eu3+ in C5 complex.
The emission quantum efficiency of the 5D0 emitting level of Eu3+ in C1–C5 complexes can be calculated by using respective emission spectral data and luminescence lifetime of complexes (Table 3). The intrinsic quantum efficiency (ΦLn) can be expressed as ratio of radiative rate constant (Arad) to overall rate constant (Atot), by assuming that only radiative and non-radiative processes are responsible for depopulation of excited state (Atot = Arad + Anrad).
The denominator in Eq. (2) is determined from luminescence lifetime data by following equation:
where Anrad is the rate constant for non-radiative decay process. In case of europium luminescence, the magnetic dipole transition 5D0 → 7F1 being independent of ligand field is used as reference to estimate the radiative rate constant Arad, which is calculated by summing over the A0J for each transition of europium ion and A0J is given by the equation as below:
where I0J and I01 are integrated intensities, while ν0J and ν01 are energy barycenter of 5D0 → 7FJ and 5D0 → 7F1 transition, respectively, and A01 is the Einstein’s coefficient of spontaneous emission of 5D0 → 7F1 transition with value of ~ 50 s−1 in the air [40, 41]. The C1–C5 complexes exhibit intrinsic quantum efficiency in range of 6.82%–32.59%. The total quantum yield of complexes (Φtot) is related to the efficiency of ligand sensitization (ηsen) and intrinsic quantum efficiency of complexes according to the following equation:
The total quantum yield of complexes is found to be in range of 2.81%–17.9%; thereafter, the sensitization efficiency of complexes is calculated and the quantum yield-related parameters are enlisted in Table 4. The results of quantum yield data agree with luminescence intensity of complexes corresponding to 5D0 → 7F2 transition except in C4 complex, and it may be attributed to the lower intrinsic quantum efficiency of C4 complex due to vibronic quenching of additional C–H bonds of methyl groups in dmph.
4 Energy transfer dynamics
In order to elucidate sensitization phenomenon in europium complexes, energy values of the lowest excited singlet state (E(S1)) and triplet state (E(T1)) of ligand DAHB are calculated by referencing the absorbance edge of UV–Vis absorption spectra of ligand (Fig. 5) and lower emission edge wavelength of phosphorescence spectra of G1 complex (Fig. 10) and the value of S1 and T1 of ligand is found to be 24,844 and 21,978 cm−1, respectively [30, 42,43,44,45]. The lowest excited state of Gd3+ located at about 32,000 cm−1 is much higher than the triplet state of ligand, making sensitization of Gd3+ ion by DAHB ligand impossible; hence, phosphorescence spectrum of G1 complex is due to emission of ligand [46]. Among ancillary ligands, basic structural framework of dmph is intermediate of phen and batho, while bipy has different structural frameworks; therefore, proposed energy transfer mechanism of C2, C3 and C5 complexes is depicted in Figs. S1, S2 and Fig. 11. The singlet and triplet excited states of ancillary ligands phen (31,000, 22,100 cm−1), bipy (29,900, 22,900 cm−1) and batho (29,000, 21,000 cm−1) are calculated and matched with Ref. [29]. The energy value of S1 and T1 states of ligand DAHB and ancillary ligands phen, bipy and batho are also tabulated in Table 5.
The energy transfer processes in these complexes are supported by the thermal de-excitation theory and Dexter electron exchange theory which involves excitation of ligands on irradiation of UV–Vis light from ground state S0 to excited singlet state Sn via S0 → Sn electronic transitions, which dropped to S1 state through non-radiative transition, and then, the energy is transferred to triplet state of ligands via intersystem crossing (ISC). The empirical rule is that energy gap of 5000 cm−1 between S1 and T1 states of ligand should be required for effective intersystem crossing [32]. The energies at S1 state of ligand DAHB, phen, bipy and batho are 2866, 8900, 7000 and 8000 cm−1, higher than those at their respective triplet state, indicating that the energy is transferred from singlet state to triplet state of ligands; but according to empirical rule, the energy gap of 2866 cm−1 between S1 and T1 states of ligand DAHB indicates that the energy transfer process from singlet state to triplet state is less efficient in ligand DAHB. Subsequently, the energy is transferred from triplet state of ligands to emitting levels of europium ion through internal conversion. The empirical rule of Latva suggests that the optimal energy transfer may take place from T1 of ligands to emitting levels of europium ion, when the energy gap ∆E (T1–Ln3+) is in range of 2500–5000 cm−1 [47, 48]. The triplet energy level of ligand DAHB is compatible for efficient energy transfers to 5D0 (∆E0 = 4725 cm−1) and 5D1 (∆E1 = 3252 cm−1) emitting levels of europium ion, suggesting that the ligand DAHB can efficiently sensitize europium ion. The energy gap (∆E1) between triplet state of ancillary ligands and 5D1 level of europium ion in C5, C3 and C2 complex is 3374, 4174 and 2274 cm−1, while energy gap (∆E0) between triplet state and 5D0 level is 4847, 5647 and 3747 cm−1, respectively. Although, higher excited states relax to lowest excited 5D0 state through non-radiative transition, hence, indirectly populating the 5D0 state. However, according to thermal de-excitation theory, the ∆E1 of 2274 cm−1 in C2 complex facilitates the inverse energy transfer process, responsible for comparatively low population of 5D0 state due to less energy transfer from 5D1 to 5D0 state, leading to low luminescence intensity in C2 complex compared to those in C3 or C5 complexes [49, 50]. As aforesaid Latva’s rule, high ∆E0 of 5647 cm−1 in C3 complex is responsible for lower intensity of C3 complex than C5. The luminescence intensity of C4 complex is slightly lower than that of C5 complex, which may be due to vibronic quenching of additional C-H bonds of methyl groups in dmph. Speculated interpretation based on various rules is justified by calculated ηsen of C1–C5 complexes. The result of quantum yield data and energy transfer dynamics clearly reveal that the ancillary ligands act as additional lumophore by increasing the efficiency of sensitization phenomenon in C2–C5 complexes, leading to high luminescence intensity of these complexes than that of C1 binary complex.
5 Conclusion
In this work, five new europium complexes were synthesized and characterized by elemental analysis, IR, 1H-NMR, TG/DTG, UV–Vis and photoluminescent spectroscopy. The excitation spectra of complexes are extended up to visible region. The emission spectra of C1–C5 complexes display characteristic red emission of europium ion, indicating that europium ion is efficiently sensitized by ligand DAHB and ancillary ligands act as additional light harvesting centers in sensitization phenomenon. The above fact is also justified by quantum yield parameters and energy transfer dynamics. The decay curve and Judd–Ofelt intensity parameter (Ω2) of the complexes suggest that there is only one luminescent center in these complexes and europium ion is in asymmetric chemical environment. The CIE color coordinates of the complexes present high red color purity. The TG/DTG curve shows that these complexes have good thermal stability. In a word, the europium complexes with ligand DAHB and ancillary ligands exhibit high luminescent performance and high thermal stability which make them promising materials for development of photoluminescent devices.
References
Gallardo H, Conte G, Bortoluzzi AJ, Bechtold IH, Pereira A, Quirino WG, Legnani C, Cremona M. Synthesis, structural characterization, and photo and electroluminescence of a novel terbium(III) complex: {tris(acetylacetonatonate)[1,2,5]thiadiazolo[3,4-f][1,10]phenanthroline}terbium(III). Inorg Chim Acta. 2011;365(1):152.
Wang L, Li B, Zhang L, Li P, Jiang H. An optical anion chemosensor based on a europium complex and its molecular logic. Dyes Pigm. 2013;97(1):26.
Song X, Wang L, Zheng Q, Liu W. Synthesis, crystal structure and luminescence properties of lanthanide complexes with a new bridging furfurylsalicylamide ligand. Inorg Chim Acta. 2012;391:171.
Souza AP, Paz FAA, Freire RO, Calos LD, Malta OL, Alves S Jr, de Sa GF. Synthesis, crystal structure, and modelling of a new tetramer complex of europium. J Phys Chem B. 2007;111(31):9228.
Tsaryuk V, Zolin V, Zhuravlev K, Kudryashova V, Legendziewicz J, Szostak R. Blocking effect of ligand spacer groups on the luminescence excitation of europium aromatic carboxylates. J Alloys Compd. 2008;451(1):153.
Kuriki K, Koike Y, Okamoto Y. Plastic optical fiber lasers and amlifiers containing lanthanide complexes. Chem Rev. 2002;102(6):2347.
Rasanen M, Takalo H, Rosenberg J, Makela J, Haapakka K, Kankare J. Study on photophysical properties of Eu(III) complexes with aromatic β-diketones-role of charge transfer states in the energy migration. J Lumin. 2014;146(25):211.
Jiu HF, Liu GD, Zhang ZJ, Fu YH, Chen JC, Fan T, Zhang LX. Fluorescence enhancement of Tb(III) complex with a new β-diketone ligand by 1,10-phenanthroline. J Rare Earths. 2011;29(8):741.
Mwaura JK, Mathai MK, Chen C, Papadimitrakopoulos F. Light emitting diodes prepared from terbium-immobilized polyurea chelates. J Macromol Sci A. 2003;40(12):1253.
Whan RE, Crosby GA. Luminescence studies of rare earth complexes: benzoylacetonate and dibenzoylmethide chelates. J Mol Spectrosc. 1962;8(1–6):315.
Rao CN, Ferraro JR, editors. Spectroscopy in Inorganic Chemistry. New York: Academic Press; 1971. 2.
Lasker IR, Chen TM. Tuning of wavelengths: synthesis and photophysical studies of iridium complexes and their applications in organic light emitting devices. Chem Mater. 2004;16(1):111.
Bao Z, Lovinger AJ, Brown J. New air-stable n-channel organic thin film transistors. J Am Chem Soc. 1998;120(1):207.
Parra DF, Mucciolo A, Brito HF. Green luminescence system containing a Tb3+-β-diketonate complex doped in the epoxy resin as sensitizer. J Appl Polym Sci. 2004;94(3):865.
Bala M, Kumar S, Taxak VB, Boora P, Khatkar SP. Terbium(III) complexes sensitized with β-diketone and ancillary ligand: synthesis, elucidation of photoluminescence properties and mechanism. J Mater Sci-Mater Electron. 2016;27(9):9306.
Feng J, Zhang H. Hybrid materials based on lanthanide organic complexes: a review. Chem Soc Rev. 2013;42(1):387.
Yang Y, Zhang SY. Study of lanthanide complexes with salicylic acid by photoacoustic and fluorescence spectroscopy, Spectrochim. Acta A. 2004;60(8–9):2065.
Nakayama T, Tsutsui T. Enhancement in 1-D optical resonator devices with organic films. Synth Met. 1997;91(1–3):57.
Kamino S, Horio Y, Komeda S, Minoura K, Ichikawa H, Horigome J, Tatsumi A, Kaji S, Yamaguchi T, Usami Y, Hirota S, Enomoto S, Fujita Y. A new class of rhodamine luminophores: design, synthesis and aggregation-induced emission enhancement. Chem Commun. 2010;46(47):9013.
Liu JY, Ren N, Zhang JJ, Zhang CY. Preparation, thermodynamics property and antimicrobial activity of some rare earth (III) complexes with 3-bromo-5-iodobenzoic acid and 1,10-phenanthroline. Thermochim Acta. 2013;570:51.
Tian L, Ren N, Zhang JJ, Sun SJ, Ye HM, Bai JH, Wang RF. Synthesis, crystal structure, and thermal decomposition kinetic of the complex of dysprosium benzoate with 2,2’-bipyridine. J Chem Eng Data. 2009;54(1):69.
Devi R, Chahar S, Khatkar SP, Taxak VB, Boora P. Relative study of luminescent properties with Judd-Ofelt characterization in trivalent europium complexes comprising ethyl-(4-fluorobenzoyl) acetate. J Fluoresc. 2017;27(4):1349.
Yang C, Luo J, Ma J, Lu M, Liang L, Tong B. Synthesis and photoluminescent properties of four novel trinuclear europium complexes based on two tris-β-diketones ligands. Dyes Pigm. 2012;92(1):696.
Bhacca NS, Selbin J, Wander JD. Nuclear magnetic resonance spectra of 1:1 adducts of 1,10-phenanthroline and α, α′-bipyridyl with tris[2,2,6,6-tetramethylheptane-3,5-dionato] complexes of the lanthanides. J Am Chem Soc. 1972;94(25):8719.
Utochnikova VV, Solodukhin NN, Aslandukov AN, Marciniak L, Bushmarinov IS, Vashchenko AA, Kuzmina NP. Lanthanide tetrafluorobenzoates as emitters for OLEDs: New approach for host selection. Org Elec. 2017;44:85.
Shao G, Li Y, Feng K, Gan F, Gong M. Diphenylethyne based β-diketonate europium(III) complexes as red phosphor applied in LED. Sens Actuators B. 2012;173(1):692.
Wang H, He P, Yan H, Gong M. Synthesis, characteristics and luminescent properties of a new europium(III) organic complex applied in near UV LED. Sens Actuators, B. 2011;156(1):6.
Gusev AN, Hasegawa M, Shul’gin VF, Nishchymenko G, Linert W. Photophysical studies on ternary mixed ligand europium complexes containing pyridyltriazolylmethane and 1,3-diketonate. Inorg Chim Acta. 2014;414:71.
Liu S, Su W, Pan R, Zhou X. Red emission of Eu(III) complex based on 1-(7-(tert-butyl)-9-ethyl-9H-carbazol-2-yl)-4,4,4-trifluorobutane-1,3-dione excited by blue light. Chin J Chem Phys. 2012;25(6):697.
Francis B, Ambilli Raj DB, Reddy MLP. Highly efficient luminescent hybrid materials covalently linking with europium(III) complexes via a novel fluorinated β-diketonate ligand: synthesis, characterization and photophysical properties. Dalton Trans. 2010;39(34):8084.
Wang YB, Zheng XJ, Zhuang WJ, Jin LP. Hydrothermal synthesis and characterization of novel lanthanide 2,2-diphenyldicarboxylate complexes. Eur J Inorg Chem. 2003;2003(7):1355.
Yu J, Deng R, Sun L, Li Z, Zhang H. Photophysical properties of a series of high luminescent europium complexes with fluorinated ligands. J Lumin. 2011;131(2):328.
Zucchi G, Murugesan V, Tonfelier D, Aldakov D, Jeon T, Yang F, Thuery P, Ephritikhine M, Geffroy B. Solution, solid state, and film properties of a structurally characterized highly luminescent molecular europium plastic material excitable with visible light. Inorg Chem. 2011;50(11):4851.
Divya V, Biju S, Verma RL, Reddy MLP. Highly efficient visible light sensitized red emission from europium tris[1-(4-biphenoyl)-3-(2-fluoroyl)propanedione](1,10-phenanthroline) complex grafted on silica nanoparticles. J Mater Chem. 2010;20(25):5220.
Li HF, Yan PF, Chen P, Wang Y, Xu H, Li GM. Highly luminescent bis-diketone lanthanide complexes with triple-stranded dinuclear structure. Dalton Trans. 2012;41(3):900.
Brito HF, Malta OL, Souza LR, Menezes JFS, Carvalho CAA. Luminescence of the films of europium(III) with thenoyltrifluoroacetonate and macrocyclics. J Non-Cryst Solids. 1999;247(1–3):129.
Zhang X, Zhou F, Shi J, Gong M. Sr3.5Mg0.5Si3O8Cl4:Eu2+ bluish-green-emitting phosphor for NUV-based LED. Mater Lett. 2009;63(11):852.
Teotonio EES, Brito HF, Felinto MCFC, Kodaira CA, Malta OL. Luminescence investigations on Eu(III) thenoyltrifluoroacetonate complexes with amide ligands. J Coord Chem. 2003;56(10):913.
Ferreira RAS, Nobre SS, Granadeiro CM, Nogueira HIS, Carlos LD, Malta OL. A theoretical interpretation of the abnormal 5D0 → 7F4 intensity based on the Eu3+ local coordination in the Na9[EuW10O36]·14H2O polyoxometalate. J Lumin. 2006;121:561.
Carlos LD, Messaddeq Y, Brito HF, Ferreira RAS, Bermudez VD, Rebeiro SJL. Full-color phosphors from europium(III)-based organosilicates. Adv Mater. 2000;12(8):594.
Ferreira R, Pires P, de Castro B, Ferreira RAS, Carlos LD, Pischel U. Zirconium organophosphonates as photoactive and hydrophobic host materials for sensitized luminescence of Eu(III), Tb(III), Sm(III), and Dy(III). New J Chem. 2004;28(12):1506.
Xu H, Yin K, Huang W. Synthesis, photophysical and electroluminescenct properties of a novel bright light-emitting Eu3+ complex based on a fluorene-containing bidentate aryl phosphine oxide. Syn Met. 2010;160(19–20):2197.
Taha ZA, Ajlouni AM, Al-Hassan KA, Hijaki AK, Faiq AB. Syntheses, characterization, biological activity and fluorescence properties of bis-(salicylaldehyde)-1,3-propylenediimine Schiff base ligand and its lanthanide complexes. Spectrochim Acta A Mol Biomol Spectrosc. 2011;81(1):317.
Gawryszewska P, Sokolnicki J, Legendziewicz J. Photophysics and structure of selected lanthanide compounds. Coord Chem Rev. 2005;249(21–22):2489.
Greenham NC, Samuel IDW, Hayes GR, Philips RT, Kessener YARR, Moratti SC, Holmes AB, Friend RH. Measurement of absolute photoluminescence quantum effiencies in conjugated polymers. Chem Phys Lett. 1995;241(1–2):89.
Zhang A, Zhang J, Pan Q, Wang S, Jia H, Xu B. Synthesis, photoluminescence and intramolecular energy transfer model of a dysprosium complex. J Lumin. 2012;132(4):965.
Reddy MLP, Divya V, Pavithran R. Visible-light sensitized luminescent europium(III)-β-diketonate complexes: bioprobes for cellular imaging. Dalton Trans. 2013;42(43):15249.
Latva M, Takalo H, Mukkala VM, Matachescu C, Ubis JCR, Kankare J. Correlation between the lowest triplet state energy level of the ligand and lanthanide(III) luminescence quantum yield. J Lumin. 1997;75(2):149.
Ilmi R, Hasan N, Liu J, Mara D, Deun RV, Iftikhar K. Effect of 2,4,6-tri(2-pyridyl)-1,3,5-triazine on visible and NIR luminescence of lanthanide tris(trifluoroacetylacetonates). J Photochem Photobiol A. 2017;347:116.
Dutra JDL, Gimenez IF, da Costa Junior NB, Freire RO. Theoretical design of highly luminescent europium(III) complexes: a factorial study. J. Photochem. Photobio. A. 2011;217(2–3):389.
Acknowledgements
This work was financially supported by the Junior Research Fellowship (JRF) from University Grants Commission, New Delhi, India (No. 2061410096).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dhankhar, P., Devi, R., Devi, S. et al. Synthesis and photoluminescent performance of novel europium (III) carboxylates with heterocyclic ancillary ligands. Rare Met. 41, 1342–1352 (2022). https://doi.org/10.1007/s12598-019-01261-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-019-01261-y