Abstract
O-LED has been make from multilayer organic semiconductors materials to produce electroluminescence. The light emitted diode was synthesis by using organic compounds that are embedded in layers of thin films, poly (3, 4, -ethylene dioxythiophene) / polystyrene sulfonic acid (PEDOT: PSS), poly [(9, 9-dioctylfluorenyl-2, 7-diyl)-co-(4, 4′-(N-(4-secbutylphenyl) diphenylamine)] (TFB) and Tris (8-quinolinolato) aluminum (III) (Alq3), by the spin coating technique. The electroluminescence of O-LED was obtained by studying the effect of changing the forward bias voltages 8, 9, 10 V at room temperature. It was also observed by drawing the relationship between voltage and current (I-V) that the latter changes depending on the change in voltage with a zonal division in which the knee voltage was 0.55 V. These characteristics were invested to draw the broad spectrum of emission for a wavelength ranging from 350 to 750 nm, through which the color spaces were adopted and compared to the CIE 1931 system, and the correlated color temperature of the device, which ranges from 9500 to12700 K.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In previous decades, with the discovery of the transistor, inorganic materials such as silicon (Si) and germanium (Ge) and their compounds dominated the electronic industries trade because of the unique characteristics of semiconductors [1], until the late sixties, when the most important discovery of conductive polymers (CPs) was made by laureates Heeger, MacDiarmid and Shirakawa who deserved a Nobel prize for this discovery as they were able to describe the electrical conductivity of polyethylene [2]. Due to the great permeation of semiconductors in our daily lives, research and discoveries led to an effective control of new semiconductor materials with high efficiency in the world of electronics revolution, which is organic semiconductors [3, 4]. The tremendous progress in this field was accompanied by expectations for important applications such as LED, solar cells, flexible displays, detectors and sensors [5]. Organic light-emitting diodes (O-LED) have captured the attention of scientists and researchers for their various applications for solid-state lighting and flat screen displays, where efforts have been made on how to improve the efficiency of O-LED by raising performance based on basic factors as injection barriers between organic materials as well as the efficiency electrodes [6]. Organic materials are solid materials that form a structural structure of interconnected molecules or polymers, most of which are homogeneous atoms of carbon and hydrogen, and sometimes from heterogeneous atoms such as nitrogen and others. Organic semiconductors are characterized by an energy gap ranging between 2.5 and 4 eV [7], which can they are interpreted as insulating materials in their origin, not semiconductors, which become semiconductive by injecting charge carriers through electrodes or by doping. Light-emitting diodes produce light by electroluminescence of thin films of organic or mineral–organic materials sandwiched between two electrodes as thin layers [8], one of which must be transparent. The additional layer as hole transporting layer (HTL), hole injection layer (HIL), electron transporting layer (ETL) and electron injection layer (EIL) helps to inject and transfer the charge toward the light-emitting layer [9], as well as improving the performance of the device. In this research, PEDOT: PSS and TFB were used to improve the performance of holes transferring and holes injecting through the interlayers in organic light-emitting diodes and photovoltaic cells, as well as the presence of Alq3 as an important means of electrons transport or as an emissive layer (EL) due to its high thermal stability [10, 11]. O-LED technology guarantees high advantages for the manufacture of light-emitting devices with light weight, large area, high flexibility, and transparency through processing and low-cost. It depends on various thin film deposition techniques such as vacuum thermal evaporation or rotational coating, as we will talk about in detail in the practical part [4]. Previously, the following compounds PEDOT and Alq3 have been used in numerous researches to improve the optical properties of manufactured devices [12, 13].
Method
A number of preparatory steps have preceded the manufacture of the device shown in Fig. 1. The first step was to filter the PEDOT: PSS compound with a 0.45 μm PES filter with a dark package due to the damage of the compound and the change of its properties when exposed to light [14]. The second step is to dissolve 10 mg of TFB in 1 ml of an organic solvent (chloroform) and place it in a glass tube inside the ultrasound device for an hour to be ready for use [10].
The third step is to dissolve 30 mg of Alq3 in 3 ml of an organic solvent ethanol (this solvent does not affect or dissolve the TFB) and put it in a glass tube inside the ultrasound machine for an hour to be ready for use [15]. After dissolving the organic materials, the stage of manufacturing and installing the device begins on a layer of conductive ITO measuring 2 × 2 cm2 by installing it on the rotating coating device, where the filtered PEDOT:PSS material is deposited through the distillation and diffusion process, the device rotates at a speed of 1000 rpm, then the film is dried at a temperature of 100 °C for 15 min, After that, the process of deposition of a layer of TFB is repeated, and the coating device is rotated at 2000 rpm, then the film is dried at a temperature of 50 °C for an hour. The process is repeated by deposition of a layer of Alq3 on the coating device at a speed of 2000 rpm then it is dried at a temperature of 50 °C for an hour. The final film consists of three organic layers ready to deposit aluminum electrodes as shown below [16].
Operation mechanism of O-LED
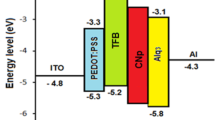
O-LEDs consist of organic layers sandwiched between positively and negatively biased electrodes. Indium tin oxide (ITO) is the electrode of the positive and transparent device through which, receive light the emission, in addition to the function of light passing through it, via this cathode is injected and transferred holes (h) in the valence band (HOMO) of organic semiconductors. On the other end of the device, aluminum (Al) metal is used as a negative electrode whose function is to facilitate the injection of electrons (e) into the conduction band (LUMO) of organic semiconductors [17]. As usual, if the device is composed of several organic layers, the barriers for each of the electrons and holes are not similar, so one type of carrier will be injected for each layer, and it will move freely within the layers with a forward bias process. When the forward applied voltage increases, electrons and holes diffuse through the organic layer (electrons for the conduction band and holes for the valence band). The concentration of holes increases in the HOMO and the concentration of electrons in the LUMO increases [18]. As a result of the Coulomb attraction, both e and h recombine, forming an excitons and exciplex under the influence of the applied electric field or the formation of an electron hole pair through which we get radiation and light emission, as in Fig. 2.
Measurements and results
Absorbance and photoluminescence
The absorbance spectrum experiment 300–800 nm is seen in Fig. 3a, b broadband absorption in the near-ultraviolet region (NUV). Beyond (NUV) region, the relation between the wavelength and absorbance is exponential decay. Where it was found that the highest absorption (wavelength excitation) peaks for each TFB and Alq3 of the 428 and 395 nm, respectively. Wherefore the PL measurements of TFB and Alq3 excited by 428 and 395 nm, as shown in Fig. 3a, b. The first peak of TFB centered at 477 nm, which is indicate to transition band to band between LUMO to HOMO for TFB (mean the energy gap), the energy gap was about 3 eV [10]; while, the first peak of Alq3 centered at 520 nm, which is indicate to transition band to band between LUMO to HOMO for Alq3 (mean the energy gap), the energy gap was about 2.73 eV [19].
Current–voltage characteristics
Characteristics of the current as a function of the voltage (I-V) for O-LED device at room temperature (RT) [20]. Figure 4 shows the mechanism for moving the current, as it is divided into three phases:
-
The phase 1, the current less than 1 V has a linear ohmic relationship as a function of the voltage (I α V) in which the flow of carriers is observed in low-voltage tunnels, where the knee voltage area is determined by 0.55 V.
-
The phase 2, (2–8 V), it was observed that the current increased dramatically and by exponential relationship as a function of the voltage (I α exp (0.33 V)) where the injected carriers movement is more freely as a result of the decrease in the resistance of the barrier with bias, which leads to filling the electronic vibrational energy levels. All levels are considered to be 8 V filled voltage levels (VLFL), we notice an expansion in phase 2 due to the low mobility of the carriers in the organic materials [15].
-
The phase 3, in which the current is greater than 8 V as a function of voltage with an energy relationship (I α V3.24) in which light emission begins, because increasing the bias voltage across (ITO/PEDOT: PSS/TFB/Alq3/Al) increases the concentration of the injected carriers density in the excited levels of the organic layers, thus increasing the Coulomb attraction, which leads to the emission of a spectrum of wavelengths [16].
Electroluminesces measurements
The EL at a bias voltage of 8 V, for light that was obtained approximately through (ITO/PEDOT: PSS/TFB/Alq3/AL), a spectrum of light emission for the hybrid device was recorded by utilize a photo-multiplier detector at room temperature. Due to the highest efficiency among conductive organic thermoelectric materials and transparent of light PEDOT: PSS, it was deposited as an additional layer to increase the transport of carriers [18]; while, the transporting and injecting organic layers were as follows, TFB and Alq3 were used as organic layers that contribute to the injection and transport of holes and electrons, respectively [21]. The anode ITO is a passage for injection and diffusion of holes to HOMO of the TFB, conversely, Aluminum cathodes inject and transfer electrons to the LOMO of Alq3, as a result of the process of injecting both the holes and electrons through the organic layers, which leads to the fusion process as a result of Coulombic attraction, as shown in Fig. 5. Subsequently, holes and electrons their fusion was the reason for the formation of excitons and exciplex, so the outcome of this process is the generation of light with multiple wavelength.
The emission at 331 nm is due to the band to band transition from S1 for TFB to S0 for Alq3 anther emission 389, 390, 400 nm transitions between S1 for TFB to sublevels for grand state of Alq3 (S01, S02, S03) [2]. The blue emission of 411 nm corresponds to the emission of TFB, because the band gap of TFB is 3.1 eV [6] and emission of 435 to 494 nm corresponds to the transitions exited state (S1) of TFB to (S01, S02 and S03) [22]. The green, yellow and red emission of 514, 550, 596, 602 nm corresponds to the transitions triplet state (T1) of TFB to S0, S01, S02 and S03 according to the available information, the convergence of the energy levels LUOM of Alq3 with the energy levels triplet of TFB and because of the electric field applied to the electronic injection, these levels will be occupied by electrons [1] or generations exciplex between LOMO of Alq3 and HOMO of TFB [2].
Some these peaks appear when increases bias voltage where an Auger-assisted energy because of the spread of electronic injection to the upper levels of LUMO for TFB (S1 to S0, S01, S02 and S03), the amount of non-radiative energy resulting from the fusion between holes and electrons is given to another electron, and it moves to the higher levels [14]. It was noticed that the temperature of the samples increased significantly because of high injection, so we used some methods to cool them. All these transitions are appearing in Fig. 6. It was observed that the color temperature changed depending on the applied voltage differences the color of light is located by the CIE 1931 Chromaticity Coordinate as Fig. 7. The Chromaticity Coordinates x y on CIE 1931 Chromaticity Coordinate is found from X, Y and Z:
where the X, Y and Z are area under the curve for red, green and blue are regions in EL spectrum, respectively. The correlated color temperature (CCT) of light can be found by using McCamy’s approximation polynomial to estimate the CCT from the x and y chromaticity coordinates as in Eq. 3 [9, 22, 23]:
where
The all result can be tabulated in Table 1
Conclusion
The research can be summarized as the possibility of manufacturing a multi-layered thin film of organic materials (PEDOT:PSS/TFB/Alq3) to obtain a polychromatic luminescence by shedding an appropriate forward bias voltage as a result of the transition between the single and triple electronic levels within a package LUMO and HOMO of the organic matter resulting from been excitons or exciplex. As well as the chromaticity can be controlled through the change of the applied potential difference.
Data availability
Data sharing is not applicable to this article as no datasets were created or analyzed during the current study.
References
Brütting, Wolfgang. Introduction to the physics of organic semiconductors. Phys Organic Semicond. 2005; 1–14
Lakshmanan R, Kiruba Daniel SCG.Engineered nanomaterials for organic light-emitting diodes (OLEDs). In Handbook of nanomaterials for industrial applications. Amsterdam: Elsevier 2018; p. 312-323
B. Lüssem, M. Riede, K. Leo, Doping of organic semiconductors. Physica Status Solidi (a) 210 1, 9–43 (2013)
He, Haiping, Applications in OLED and QLED." In Solution Processed Metal Oxide Thin Films for Electronic Applications, 2020; 141–154. Elsevier
Will, Paul-Anton, and Sebastian Reineke. "Organic light-emitting diodes." In Handbook of Organic Materials for Electronic and Photonic Devices 2019; p. 695–726. Woodhead Publishing
M.C. Gather, A. Köhnen, K. Meerholz, White organic light-emitting diodes. Adv. Mater. Mater. 23(2), 233–248 (2011)
B. Geffroy, P. Le Roy, C. Prat, Organic light-emitting diode (OLED) technology: materials, devices and display technologies. Polymer Int. 55(6), 572–582 (2006)
P. Li, Z.H. Lu, Interface engineering in organic electronics: energy-level alignment and charge transpor. Small Sci. 1(1), 2000015 (2021)
G.M. Farinola, R. Ragni, Electroluminescent materials for white organic light emitting diodes. Chem Soc Rev Soc Rev. 40(7), 3467–3482 (2011)
A.R. Rodrigues, W. Renzi, N.J.A. Cordeiro, E. Laureto, I.F. Dias, J.L. Duarte, Study of energy transfer in polymer blends of TFB P3HT. Semina Exact Technol Sci. 38(1), 91–100 (2017)
V.A. Dediu, L.E. Hueso, I. Bergenti, C. Taliani, Spin routes in organic semiconductors. Nat. Mater. 8(9), 707–716 (2009)
L.A. Essa, R.K. Jamal, Polluted water sensor based on carbon quantum Dots/Alq3 using drop casting and spin coating techniques. Iraqi J Phys. 21(4), 103–112 (2023)
M.W. Kadhim, R.K. Jamal, Fabrication of a novel solar cell using copper phthalocyanine tetrasulfonic acid tetrasodium salt / graphene oxide. J. Opt. 52(3), 1507–1512 (2022)
Mohammed HR, Ibrahim OA. Electroluminescence of Light-Emitting Organic Semiconductor/Europium Oxide Nanoparticle Hybrid Junction. Iraqi J Sci. 2020; p. 1952–1959
H.R. Mohammed, O.A. Ibrahim, Preparation of hybrid junction light emitting device from cadmium sulphite quantum dot/poly-TPD junction. Univ Thi-Qar J Sci. 8(2), 100–103 (2021)
H.R. Mohammed, Fabrication of electroluminescence device for PEDOT: PSS/ploy TPD/Eu2O3 nanoparticles junction. Iraqi J Phys. 17(43), 33–40 (2019)
Omar Adnan Ibrahim. White light generation LAP LAMBERT Academic publishing, (2017)
J. Kido, M. Kimura, K. Nagai, Multilayer white light-emitting organic electroluminescent device. Science 267(5202), 1332–1334 (1995)
T. Hoshi, K.-I. Kumagai, K. Inoue, S. Enomoto, Y. Nobe, M. Kobayashi, Electronic absorption and emission spectra of Alq3 in solution with special attention to a delayed fluorescence. J. Lumin.Lumin 128(8), 1353–1358 (2008)
Mohammed AH, Naje AN. Photoconductive detector based on graphene doping with silver nanoparticles. Iraqi J Sci. 2022; p. 5218–5231
M. Auer-Berger, R. Trattnig, T. Qin, R. Schlesinger, M.V. Nardi, G. Ligorio, C. Christodoulou et al., All-solution-processed multilayer polymer/dendrimer light emitting diodes. Org. Electron. 35, 164–170 (2016)
J.-H. Lee, C.-H. Chen, P.-H. Lee, H.-Y. Lin, M.-K. Leung, T.-L. Chiu, C.-F. Lin, Blue organic light-emitting diodes: current status, challenges, and future outlook. J Mater Chem C 7(20), 5874–5888 (2019)
P. Xiao, T. Dong, J. Xie, D. Luo, J. Yuan, B. Liu, Emergence of white organic light-emitting diodes based on thermally activated delayed fluorescence. Appl Sci Sci. 8(2), 299 (2018)
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
Each co-author has made unique contributions to the work. The author HRM prepared the thin films and contributed to writing the article draft and wrote the program for optical properties and contributed to the analysis of the results. As the author ANN, She supervised the work and reviewed the article draft.
Corresponding author
Ethics declarations
Conflict of interest
The authors have not disclosed any competing interests.
Ethical approval
The authors would like to declare that they do not have any conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mohammed, H.R., Naje, A.N. Electroluminescence of organic poly-TFB/Alq3 light-emitting device. J Opt (2024). https://doi.org/10.1007/s12596-024-01663-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12596-024-01663-x