Abstract
The role of neuronal nitric oxide synthase (nNOS) in cardiac ischemia–reperfusion (IR) and ischemia preconditioning (IP) is still controversial. Here, we focused on the possible roles of nNOS in cardiac IR and IP. Wild type C57BL/6 (WT) mice were subjected to coronary artery occlusion for 30 min followed by 24-h reperfusion (IR). Cardiac injury (infarct size and apoptotic cell number) was increased, associated with elevation of oxidative stress (lipid peroxidation) and nitrative stress (nitrotyrosine formation). A potent nNOS inhibitor, L-VNIO, and a superoxide dismutase mimetic and peroxynitrite scavenger, MnTBAP, significantly reduced IR-induced increases of oxidative/nitrative stress and cardiac injury. IR-induced cardiac injury in nNOS−/− (KO) mice was significantly lower than that in WT mice. MnTBAP markedly reduced IR-induced cardiac injury by suppression of oxidative/nitrative stress in KO mice. Cardiac IP was performed by three cycles of 5-min IR before 30-min ischemia followed by 24-h reperfusion. IP attenuated IR-induced cardiac injury in WT mice associated with reductions of oxidative/nitrative stress. IP-induced reduction of cardiac injury and oxidative/nitrative stress were eliminated by pretreatment with L-VNIO. In contrast with WT mice, IP had no protective effects in nNOS KO mice. In conclusion, nNOS played a dual role during cardiac IR and IP; nNOS exacerbated IR-induced injury by increasing oxidative/nitrative stress and contributed to IP-induced protection by inhibition of oxidative/nitrative stress.
Similar content being viewed by others
Introduction
Reactive oxygen species (ROS) such as superoxide (O2 −) and peroxynitrite (ONOO−) play an important role in the regulation of myocardial ischemia–reperfusion (IR) injury [1–5]. Nitric oxide synthase (NOS) and nitric oxide (NO) are protective in response to IR-induced injury [3–5], However, there is supportive evidence showing NOS is activated [6, 7] to increase ONOO−, a product of NO and O2 −, and these ROS are responsible for the detrimental effects of NO during cardiac IR [8, 9]. Furthermore, existing evidence suggests that exposure of endothelial NOS (eNOS) and neuronal NOS (nNOS) to oxidative stress increases enzymatic uncoupling and generation of O2 − [10, 11]. Critical roles of nNOS in regulation of cardiac functions such as heart rate, calcium cycling, sodium transport, and energy metabolism have been well documented [12–14]. Some observations have demonstrated that there were no significant differences in infarct size between wild type (WT) and nNOS−/− (KO) mice after a relative short time of ischemia and reperfusion in in-vitro and in-vivo hearts [15, 16]. In nNOS KO mice, it is suggested that several important factors, for example amounts of O2 − generation, myocardial oxygen consumption (MVO2), and sympathetic control, determine IR-induced cardiac injury. First, Khan et al. [17] observed that a large amount of O2 − generated in cardiac tissue attenuated sarcomere shortening and calcium transient in myocytes. Second, Kinugawa et al. [18] demonstrated that inhibitory control of MVO2 by NO derived from eNOS was attenuated. Finally, Choate et al. [19] demonstrated that positive chronotropic responses to sympathetic nerve activation and norepinephrine were reduced, resulting in suppression of IR-induced injury in nNOS KO mice. All these findings suggest direct antioxidant effect of nNOS in the reduction of IR-induced injury.
In contrast with the role of ROS in IR, it is well established that ROS also trigger the protective effects of ischemia preconditioning (IP) [1, 3–5]. There have been increasing observations related to the role of NOS and NO in cardiac IP, which has been divided into two distinct phases—a rapid developed phase mediating protective effects within 2–3 h and a delayed phase that became apparent 12–24 h later and lasted for approximately 72 h [20, 21]. In the delayed IP, Bolli et al. convincingly demonstrated that NOS, including two isoforms, inducible NOS (iNOS) and eNOS, and NO played pivotal roles both as triggers and a mediators [22, 23]. Recently, in the final stage of delayed IP, cardioprotective effects by nNOS in concert with cyclooxygenase-2 were observed in rabbits [24]. However, the involvement of nNOS and NO in early IP is still controversial. Although there are several reports showing that NO is not necessary for early IP [25–27], evidence also suggests that NO triggers reduction of a large amount of NO accumulated during IR [28]. Recently, Cohen et al. proposed the possibility that the NOS-cyclic GMP-protein kinase G cascade could contribute to early IP [29]. Although those observations indicated the possible involvement of NOS in early IP, there is still no direct evidence that nNOS is responsible for cardiac early IP.
In this study, first, we focused on the possible role of nNOS in cardiac IR and investigated the effects of acute administration of an nNOS inhibitor or an ONOO− radical scavenger, and genetic deletion of nNOS, on IR-induced cardiac injury. Second, we focused on the possible roles of nNOS in cardiac IP. To do this, we investigated the effects of acute administration of an nNOS inhibitor and genetic deletion of nNOS on IP-induced cardiac protection. Our results indicated that nNOS played a dual role in cardiac IR and IP; nNOS exacerbated IR-induced injury by increasing oxidative/nitrative stress and contributed to IP-induced protection by inhibition of oxidative/nitrative stress.
Methods
Animal preparation for cardiac IR and IP
Mice homozygous for targeted disruption of the nNOS gene were purchased from Jackson Laboratories (Bar Harbor, Maine, USA) and littermate age-matched C57BL/6 mice (Clea, Japan) were used as WT in this study. All surgical and experimental procedures were performed according to the guidelines for the care and use of animals established by Kagawa University and conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). Mouse cardiac IR and IP models were followed as previously described, with modification [30]. Briefly, animals were anesthetized with sodium pentobarbital (50 mg/kg, i.p.) and artificially ventilated with room air (type 845, Harvard Apparatus, Kent, UK). Left thoractomy was performed to expose the heart. Ligation of the left anterior descending (LAD) coronary artery was performed 2 mm from the apex by means of a 7-0 nylon suture (Nescosuture, Osaka, Japan). Successful LAD coronary artery occlusion was confirmed under the microscope. Thirty minutes before the start of IR or IP, nNOS inhibitor N5-(1-imino-3-butenyl)ornithine (L-VNIO, A.G. Scientific, San Diego, CA, USA; 60 μg/kg) [31] and superoxide dismutase mimetic and peroxynitrite scavenger Mn(III)-tetrakis (4-benzoic acid)porphyrin (MnTBAP, Sigma–Aldrich, St Louis, USA; 1 mg/kg) [32] were administrated by intravenous infusion into the carotid vein. IP groups were obtained by three cycles of 5-min ischemia and 5-min reperfusion before 30-min ischemia. Sham-operated mice underwent the same surgery without ligation of the coronary artery. Mouse heart rate and blood pressure were measured before the experiment and after 24-h reperfusion by the tail cuff method under conscious conditions. After measurements of blood pressure, mouse hearts were removed to evaluate the area at risk and infarct size, and embedded in optimal tissue compound to measure caspase-3 activity and NOS isoform protein expression.
Determination of myocardial infarct size
At the end of reperfusion, the ratio of infarct size to risk area was determined as previously described [30]. In brief, with the LAD coronary artery occlusion, hearts were perfused with fluorescence polymer microspheres solution. Risk areas were identified under ultraviolet light. The slices were then incubated with 2% 2,3,5-triphenyltetrazolium chloride (TTC; Sigma–Aldrich, Salt Lake, USA) solution at 37°C for 25 min. The infarct areas were demarcated as white areas, while viable tissue stains brick-red. The risk area and infarct size were determined via computerized planimetry by use of the NIH software Image 1.0. Results are presented as the percentage of infract size to risk area.
Determination of myocardial apoptosis
Detection of apoptotic cells was carried out using the terminal deoxynucleotidyl transferase-mediated dUTP nickend labeling (TUNEL) assay kit. Tissues from the area at risk were fixed in optimal cutting temperature compound and 4 μm thick slices were stained using the in-situ cell death detection kit (Calbiochem, Darmstadt, Germany) according to the manufacturer’s instructions. Fluorescence staining was viewed with a confocal microscope (Bio-Rad, California, USA). Three sections from each myocardial sample were randomly selected and ten microscopic fields per section were evaluated by two independent observers. In each field, nuclei were counted and the mean percentage of TUNEL-positive nuclei was calculated. Apoptosis in cardiac tissue was also confirmed by caspase-3 activity assay (Chemicon International, USA), which was performed according to the manufacturer’s instructions. Results are presented as mean units per μg protein.
Determination of cardiac lipid peroxidation
In ischemic left ventricular (LV) tissue, thiobarbital-reactive substances (TBARS) levels were measured as previously reported [30, 33]. Briefly, LV tissues were homogenized (5% w/v) in a solution containing 0.15 mol/L KCl and 0.02 mol/L Tris HCl (pH 7.4). The homogenate was mixed with 15% trichloroacetic acid and 0.375% thiobarbituric acid. Butylated hydroxytoluene (0.01%) was added to the assay mixture to prevent autoxidation of the sample, and the mixture was heated at 100°C for 15 min. After cooling, the mixture was centrifuged at 3500 rpm for 20 min, the absorbance of the organic phase was measured at 535 nm, and results are expressed as nmol per g wet tissue.
Immunoblotting for nitrotyrosine
Tissue protein concentrations were determined by protein assay kit (Bio-Rad, California, USA). Equal amounts of protein (50 μg) from tissue homogenates were separated on SDS–PAGE gels, transferred to nitrocellulose membranes, which were incubated overnight with primary antibodies against nitrotyrosine (Alpha Diagnostic International, San Antonio, USA). Membranes were then incubated with HRP-conjugated secondary antibody (Cell Signaling Technology, USA) for 1 h and visualized with an enhanced chemiluminescence system (ECL kit, AmerSham Pharmacia, GE Healthcare, UK). Membranes then were re-probed with an antibody against β-actin (Sigma Chemical, St Louis, MO, USA) as an indicator for equal loading of samples. Western blotting data were quantified by densitometric analysis (NIH Image software). Density of blot was analyzed with NIH image software as previous reported [30]. Density of nitrotyrosine blot was analyzed in the whole lane in each sample. Results are expressed as relative differences after normalization to β-actin.
Statistical analysis
Results are presented as mean ± SEM. Data were analyzed by ANOVA with SPSS software followed by the post-hoc test for differences between the experimental groups. A value of P < 0.05 was considered to be statistically significant.
Results
Blood pressure and heart rate after cardiac IR and IP
Among all the groups in WT mice there were no significant differences in body weight and heart weight. Age-matched nNOS KO mice showed smaller body weight and heart weight compared with WT mice (P < 0.05). Under conscious conditions, the systolic and diastolic blood pressure (SBP, DBP), and heart rate did not differ among all the groups before and after IR or IP. Pretreatment with L-VNIO or MnTBAP also did not affect SBP, DBP, and heart rate 24 h after IR or IP between each group. (Table 1).
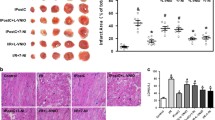
Effects of L-VNIO and MnTBAP on IR-induced cardiac infarct size (Fig. 1)
In WT mice, the mean percentage of infarct size to risk area in the IR group was 55.13 ± 2.54 (P < 0.05 compared with the wild Sham group). L-VNIO significantly reduced the percentage to 30.68 ± 2.03 (P < 0.05 compared with the WT IR group). MnTBAP further reduced it to 16.92 ± 1.30 (P < 0.05 compared with the WT IR + L-VNIO group and the WT IR group).
Effects of L-VNIO and MnTBAP on IR-induced cardiac infarct size. Cardiac infarct size was measured as described in “Methods”. Results are expressed as mean percentage of infarct size to risk area. WT denotes wild type and KO denotes nNOS−/−. Open circles denote individual mice whereas solid circles denote mean ± SEM. *P < 0.05 compared with the WT Sham group, ¶P < 0.05 compared with the WT IR group, †P < 0.05 compared with the WT IR + LVNIO group, §P < 0.05 compared with the KO IR group
In KO mice, the mean percentage of infarct size to risk area in the IR group was 35.02 ± 2.00 (P < 0.05 compared with the KO Sham group), but it was significantly lower than that in the WT IR group (35.02 ± 2.00 vs. 55.13 ± 2.54, P < 0.05). Pretreatment with MnTBAP further decreased it to 20.07 ± 1.05 (P < 0.05 compared with the KO IR group).
Effects of L-VNIO and MnTBAP on IR-induced cardiac apoptosis (Fig. 2)
Cardiac apoptosis was evaluated by TUNEL staining and caspase-3 activity. Representative TUNEL-positive staining for each group is shown in Fig. 2a. Green spots indicate positive apoptotic nuclei whereas blue indicates all nuclei.
Effects of L-VNIO and MnTBAP on IR-induced cardiac apoptosis. a Representative TUNEL-positive staining for each group, as described in “Methods”. Green spots indicate positive apoptosis nuclei and blue indicates individual nuclei. b Percentage of positive apoptosis nuclei and total nuclei expressed as mean ± SEM. c Caspase-3 activities were measured as described in “Methods”, and are expressed as mean ± SEM. White bars represent WT mice groups; black bars represent KO groups. *P < 0.05 compared with the wild Sham group, ¶P < 0.05 compared with the WT IR group, †P < 0.05 compared with the WT IR + LVNIO group, §P < 0.05 compared with the KO IR group
In WT mice, mean percentage of TUNEL-positive cells and caspase-3 activity significantly increased in the WT IR group compared with the WT Sham group (14.51 ± 0.81 vs. 1.50 ± 0.50 and 311.49 ± 23.26 vs. 168.40 ± 18.60 units per μg protein, P < 0.05). Pretreatment with L-VNIO markedly reduced the mean percentage of TUNEL-positive cells to 5.30 ± 0.80 and mean caspase-3 activity to 178.89 ± 34.57 units per μg protein (P < 0.05 compared with the WT IR group). MnTBAP further reduced the mean percentage of TUNEL-positive cells to 3.61 ± 0.70 and mean caspase-3 activity to 154.34 ± 13.67 units per μg protein (P < 0.05 compared with the WT IR + L-VNIO group and the WT IR group).
In KO mice, the KO Sham group did not show any difference in the percentage of TUNEL-positive cells and caspase-3 activity compared with the WT Sham group (2.00 ± 0.50 and 178.82 ± 35.61 vs. 1.50 ± 0.50 and 168.40 ± 18.60 units per μg protein). Although IR significantly increased the TUNEL-positive cells and caspase-3 activity to 9.41 ± 0.91 and 255.83 ± 24.57 units per μg protein, respectively, both increases were significantly smaller than those in the WT IR group (P < 0.05). MnTBAP treatment significantly reduced mean percentage of TUNEL-positive cells and caspase-3 activity compared with those in the KO IR group (3.73 ± 0.53 vs. 9.41 ± 0.91, 157.83 ± 32.72 vs. 255.83 ± 24.57 units per μg protein, P < 0.05).
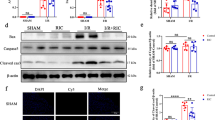
Effects of L-VNIO and MnTBAP on IR-induced TBARS and nitrotyrosine levels (Fig. 3)
In WT mice, IR markedly increased cardiac TBARS levels (nmol MDA per mg weight) compared with the WT Sham group (27.6 ± 3.89 vs. 11.34 ± 1.56, P < 0.05). Pretreatment with L-VNIO significantly reduced TBARS levels to 18.60 ± 3.67 (P < 0.05 compared with the WT IR group). MnTBAP further reduced TBARS levels to 12.40 ± 2.78 (P < 0.05 compared with the WT IR + LVNIO group and the WT IR group). A similar pattern to TBARS levels was observed in nitrotyrosine levels. IR increased nitrotyrosine levels to 6.45 ± 0.54-fold that in the WT Sham group (P < 0.05). This increase was markedly inhibited by L-VNIO and MnTBAP to 1.34 ± 0.25 and 1.45 ± 0.34-fold that in the WT Sham group, respectively (P < 0.05 compared with the WT IR group).
Effects of L-VNIO and MnTBAP on IR-induced TBARS and nitrotyrosine levels. a Cardiac tissue TBARS levels were measured as described in “Methods” and results are expressed as mean ± SEM. Cardiac nitrotyrosine levels were determined as described in “Methods”. b The upper band is β-actin; lower bands for nitrotyrosine from each group were shown as described in “Methods”. c Results were normalized to multiples of the result for the WT Sham group, and are expressed as mean ± SEM. White bars represent WT mice groups; black bars represent KO groups. *P < 0.05 compared with the WT Sham group, ¶P < 0.05 compared with the WT IR group, †P < 0.05 compared with the WT IR + LVNIO group, §P < 0.05 compared with the KO IR group
In KO mice, the KO Sham group showed higher TBARS levels than those in the WT Sham group (21.56 ± 3.67 vs. 11.34 ± 1.56, P < 0.05). Although IR tended to increase TBARS levels to 24.78 ± 4.36, it was not significant compared with the KO Sham group. Pretreatment with MnTBAP significantly reduced TBARS levels to 15.78 ± 2.89 (P < 0.05 compared with the KO IR group). The KO Sham group showed higher nitrotyrosine levels than in the WT Sham group (6.78 ± 1.08-fold that in the wild Sham group). IR did not further increase nitrotyrosine levels compared with the KO Sham group (6.05 ± 0.65-fold that in the WT Sham group), but MnTBAP significantly reduced nitrotyrosine levels to 1.67 ± 0.57-fold that in the WT Sham group (P < 0.05 compared with the KO IR group).
Effects of pharmacologic inhibition of nNOS by L-VNIO and genetic deletion of nNOS on IP-induced reduction of cardiac infarct size (Fig. 4)
In WT mice, IP treatment reduced the mean percentage of infarct size to risk area compared with the IR group (16.76 ± 1.23 vs. 55.13 ± 2.54, P < 0.05). Pretreatment with L-VNIO markedly increased the mean percentage value to 39.86 ± 3.34 (P < 0.05 compared with the WT IP group).
Effects of pharmacologic inhibition of nNOS by L-VNIO and genetic deletion of nNOS on IP-induced reduction of cardiac infarct size. Cardiac infarct size was measured as described in “Methods”. Data are expressed as mean percentage of infarct size to risk area. Open circles represent individual mice whereas solid circles represent mean ± SEM. ¶P < 0.05 compared with the WT IR group, †P < 0.05 compared with the WT IP group
In KO mice, IP treatment did not reduce mean percentage of infarct size to risk area compared with the KO IR group (46.66 ± 2.37 vs. 35.02 ± 2.00).
Effects of pharmacologic inhibition of nNOS by L-VNIO and genetic deletion of nNOS on IP-induced reduction of cardiac apoptosis (Fig. 5)
In WT mice, IP reduced the mean percentage of TUNEL-positive cells and caspase-3 activity (4.50 ± 0.80, 165.70 ± 25.70 units per μg protein, P < 0.05 compared with the WT IR group); but L-VNIO treatment reversed IP-induced reduction of mean percentage of TUNEL-positive cells and caspase-3 activity to 15.40 ± 1.21 and 321.54 ± 35.86 units per μg protein, respectively. (P < 0.05 compared with the WT IP group).
Effects of pharmacologic inhibition of nNOS by L-VNIO and genetic deletion of nNOS on IP-induced reduction of cardiac apoptosis. a Representative TUNEL-positive staining for each group as described in “Methods”. Green spots indicate positive apoptosis nuclei whereas blue indicates individual nuclei. b Percentage of positive apoptosis nuclei and total nuclei expressed as mean ± SEM. c Caspase-3 activities were measured as described in “Methods”, and are expressed as mean ± SEM. White bars represent WT mice groups; black bars represent KO groups. ¶P < 0.05 compared with the WT IR group, †P < 0.05 compared with the WT IP group
In KO mice, IP did not show any reduction of percentage of TUNEL-positive cells and caspase-3 activity (16.43 ± 0.73 and 342.65 ± 31.7 units per μg protein, respectively).
Effects of pharmacologic inhibition of nNOS by L-VNIO and genetic deletion of nNOS on IP-induced reduction of TBARS and nitrotyrosine levels (Fig. 6)
In WT mice, cardiac IP significantly reduced TBARS and nitrotyrosine levels to 13.75 ± 4.35 and 1.37 ± 0.54-fold that in the WT Sham group (P< 0.05 compared with the WT IR group). Pretreatment with L-VNIO reversed the reduction of TBARS and nitrotyrosine levels to 24.75 ± 4.35 and 7.54 ± 1.04-fold that in the WT Sham group (P < 0.05 compared with the WT IP group).
Effects of pharmacologic inhibition of nNOS by L-VNIO and genetic deletion of nNOS on IP-induced reduction of TBARS and nitrotyrosine levels. a Cardiac tissue TBARS levels were measured as described in “Methods” and results are expressed as mean ± SEM. Cardiac nitrotyrosine levels were determined as described in “Methods”. b The upper band is β-actin; lower bands for nitrotyrosine were shown for each group as described in “Methods”. c Results were normalized to multiples of the result for the WT Sham group, and are expressed as mean ± SEM. White bars represent WT mice groups; black bars represent KO groups. ¶P < 0.05 compared with the WT IR group, †P < 0.05 compared with the WT IP group
In KO mice, IP treatment did not reduce TBARS and nitrotyrosine levels (27.89 ± 5.78 and 8.75 ± 2.19-fold that in the WT Sham group).
Discussion
In this study we have made several important observations in cardiac IR and IP by pharmacologically inhibiting nNOS and genetically deleting nNOS. First, the IR-induced increase of cardiac injury was attenuated by the ROS scavenger and by pharmacological nNOS inhibition. Second, the IP-induced attenuation of cardiac injury was reversed by pharmacological nNOS inhibition and genetic nNOS deletion. These results indicate that nNOS is involved both in IR-induced injury and in IP-induced protection, mediated through oxidative/nitrative stress-related mechanisms.
It is well recognized that ROS formation is greatly increased in cardiac IR and serves as a critical central mechanism of IR injury. At low levels NO, a relatively stable ROS, exerts a number of regulatory and cytoprotective actions; at higher levels NO is potentially toxic because of its oxidative reaction products, for example ONOO−, which has been demonstrated to contribute to cardiac IR injury [4, 5]. Our current results clearly demonstrated that a marker of ONOO− production, nitrotyrosine, was increased after cardiac IR (Fig. 3), accompanied by cardiac injury; this is consistent with previous studies [8, 9]. MnTBAP, a potent ONOO− scavenger, markedly reduced cardiac injury, strongly supporting the suggestion that the increase of nitrative stress is involved in cardiac injury during IR.
Recently, Liang et al. [34] revealed that iNOS expression was increased 3 h after IR, and was responsible for increases of nitrative stress and enhanced IR injury, whereas eNOS reduced nitrative stress and reduced apoptosis. Here, we observed that ONOO− scavenger treatment further reduced cardiac nitrative stress and injury. This suggested that iNOS-dependent NO might be involved in the increase of nitrative stress and cardiac injury. It should also be noted that NO generated by reduction of nitrite may also be responsible for the increase of cardiac nitrative stress and injury [35]; this needs further investigation.
In addition to our current observations, the following mechanisms might be involved in the low injury in nNOS KO mice in response to cardiac IR: impairments of cardiac contractility, calcium transients, and LV ejection fraction [14], reduction of positive chronotropic responses to sympathetic stimulation [13], and NO-dependent inhibitory control of MVO2 [18]. Nevertheless, there have been reports of no protective effects in response to myocardial IR both in in-vitro [16] and in in-vivo hearts [15] in nNOS KO models. The discrepancy among previous studies and ours in the role of nNOS in cardiac injury of nNOS KO mice may be attributable to the differences in models and durations of ischemia and reperfusion.
The role of NOS in delayed IP has been fully documented by Bolli et al. [20, 22, 23]. Although there is much evidence in support of the idea that NO is necessary to trigger early IP [28, 36], the precise mechanisms by which NOS provides cardioprotection in the early phase have not been clearly established.
It has been shown that early IP is able to preserve eNOS protein expression and function in the IR myocardium, and the resulting acceleration of complete recovery of endothelial function and stimulation of the basal production of NO could result in cardioprotection [37]. Bell et al. [38] reported that eNOS was not essential for robust early IP (more than four cycles), but eNOS might contribute to early IP by lowering the IP threshold. It was also demonstrated that eNOS did not modulate infarct size in the non-preconditioned state and was not necessary for the cardioprotective effects of early IP in mice [27].
Furthermore, iNOS [39] and nNOS [40] have been demonstrated to play an essential role by promoting ischemic tolerance after IP in a middle cerebral artery occlusion model. Our data clearly revealed that pretreatment with nNOS inhibitor reversed the protective effects in early IP, suggesting the involvement of nNOS in early IP. Although in this study we did not obtain direct evidence of nNOS activation in early IP, possible mechanisms were proposed by Csonka et al. [28]; NO-triggered early IP in turn attenuated NO accumulation by down-regulation of nNOS and iNOS expression.
Increases of cardiac oxidative/nitrative stress and myocardial injury after IP in nNOS KO mice were larger than after IP in WT mice, also supporting the possibility that nNOS participated in early IP-induced cardiac protective effects. We speculate that three mechanisms may be involved. First, neuronal NOS is distributed to mitochondria (mitochondrial NOS; mtNOS) [41, 42] and the cardiac sarcoplasmic reticulum (nNOS) [43], and thus compartmentalized release of NO is believed to play a key role in regulating intracellular and mitochondrial calcium kinetics and, in turn, myocardial contractility. Therefore, deletion of nNOS not only affected the regulation of ROS generation, but also may affect IP-induced lowering of mitochondria calcium overload [44]. Second, the activation of iNOS might also play a role in increased cardiac injury in IP of nNOS KO mice, because iNOS was demonstrated to be involved in IR-induced injury. Finally, IP cycles may affect the results presented here, because different IP cycles provided different experimental results [29, 38].
A limitation of this study should be noted. Direct measurements of NOS activities after IR and IP at different time points might supply more convincing evidence. Without this measurement, because of complex roles of nNOS in regulation of cardiac biological function in different phenotypes, for example nNOS KO and WT mice, it might be difficult to evaluate the role of nNOS by simply comparing the response to IR and IP between these phenotypes.
Our current results suggest that nNOS is involved in IR-induced cardiac injury by increasing oxidative/nitrative stress. nNOS also participated in IP-induced cardiac protection, which might be mediated through the mechanisms of inhibition of oxidative/nitrative stress. In conclusion, nNOS played opposite roles in cardiac IR-induced injury and IP-induced protection in mice.
References
Ferdinandy P, Schulz R, Baxter GF (2007) Interaction of cardiovascular risk factors with myocardial ischemia/reperfusion injury, preconditioning, and postconditioning. Pharmacol Rev 59:418–458. doi:10.1124/pr.107.06002
Buja LM (2005) Myocardial ischemia and reperfusion injury. Cardiovasc Pathol 14:170–175. doi:10.1016/j.carpath.2005.03.006
Becker LB (2004) New concepts in reactive oxygen species and cardiovascular reperfusion physiology. Cardiovasc Res 15:461–470. doi:10.1016/j.cardiores.2003.10.025
Saini HK, Machackova J, Dhalla NS (2004) Role of reactive oxygen species in ischemic preconditioning of subcellular organelles in the hearts. Antioxid Redox Signal 6:393–404. doi:10.1089/152308604322899468
Ferdinandy P, Schulz R (2003) Nitric oxide, superoxide, and peroxynitrite in myocardio ischemia-reperfusion injury and preconditioning. Br J Pharmacol 138:532–543. doi:10.1038/sj.bjp.0705080
Depre C, Fierain L, Hue L (1997) Activation of nitric oxide synthase by ischaemia in the perfused heart. Cardiovasc Res 33:82–87. doi:10.1016/S0008-6363(96)00176-9
Kawahara K, Hachiro T, Yokokawa T, Nakajima T, Yamauchi Y, Nakayama Y (2006) Ischemia/reperfusion-induced death of cardiac myocytes: possible involvement of nitric oxide in the coordination of ATP supply and demand during ischemia. J Mol Cell Cardiol 40:35–46. doi:10.1016/j.yjmcc.2005.06.020
Wang P, Zweier JL (1996) Measurement of nitric oxide and peroxynitrite generation in the postischemic heart. Evidence for peroxynitrite-mediated reperfusion injury. J Biol Chem 271:29223–29230. doi:10.1074/jbc.271.46.29223
Yasmin W, Strynadka KD, Schulz R (1997) Generation of peroxynitrite contributes to ischemia-reperfusion injury in isolated rat hearts. Cardiovasc Res 33:422–432. doi:10.1016/S0008-6363(96)00254-4
Zou MH, Shi C, Cohen RA (2002) Oxidation of the zinc-thiolate complex and uncoupling of endothelial nitric oxide synthase by peroxynitrite. J Clin Invest 109:817–826
Sun J, Druhan LJ, Zweier JL (2008) Dose dependent effects of reactive oxygen and nitrogen species on the function of neuronal nitric oxide synthase. Arch Biochem Biophys 471:126–133. doi:10.1016/j.abb.2008.01.003
Kanai AJ, Pearce LL, Clemens PR, Birder LA, VanBibber MM, Choi SY, Groat WC, Peterson J (2001) Identification of a neuronal nitric oxide synthase in isolated cardiac mitochondria using electrochemical detection. Proc Natl Acad Sci USA 98:14126–14131. doi:10.1073/pnas.241380298
Barouch LA, Harrison RW, Skaf MW, Rosas GO, Cappola TP, Kobeissi ZA, Hobai IA, Lemmon CA, Burnett AL, O’Rourke B, Rodriguez ER, Huang PL, Lima JA, Berkowitz DE, Hare JM (2002) Nitric oxide regulates the heart by spatial confinement of nitric oxide synthase isoforms. Nature 416:337–339
Sears CE, Bryant SM, Ashley EA, Lygate CA, Rakovic S, Wallis HL, Neubauer S, Terrar DA, Casadei B (2003) Cardiac neuronal nitric oxide synthase isoform regulates myocardial contraction and calcium handling. Circ Res 92:52–59. doi:10.1161/01.RES.0000064585.95749.6D
Jones SP, Girod WG, Huang PL, Lefer DJ (2000) Myocardial reperfusion injury in neuronal nitric oxide synthase deficient mice. Coron Artery Dis 11:593–597. doi:10.1097/00019501-200012000-00004
Sumeray MS, Rees DD, Yellon DM (2000) Infarct size and nitric oxide synthase in murine myocardium. J Mol Cell Cardiol 32:35–42. doi:10.1006/jmcc.1999.1050
Khan SA, Lee K, Minhas KM, Gonzalez DR, Raju SV, Tejani AD, Li D, Berkowitz DE, Hare JM (2004) Neuronal nitric oxide synthase negatively regulates xanthine oxidoreductase inhibition of cardiac excitation–contraction coupling. Proc Natl Acad Sci USA 101:15944–15948. doi:10.1073/pnas.0404136101
Kinugawa S, Huang H, Wang Z, Kaminski PM, Wolin MS, Hintze TH (2005) A defect of neuronal nitric oxide synthase increases xanthine oxidase-derived superoxide anion and attenuates the control of myocardial oxygen consumption by nitric oxide derived from endothelial nitric oxide synthase. Circ Res 96:355–362. doi:10.1161/01.RES.0000155331.09458.A7
Choate JK, Murphy SM, Feldman R, Anderson CR (2007) Sympathetic control of heart rate in nNOS knockout mice. Am J Physiol Heart Circ Physiol 294:H354–H361. doi:10.1152/ajpheart.00898.2007
Bolli R (2007) Preconditioning: a paradigm shift in the biology of myocardial ischemia. Am J Physiol Heart Circ Physiol 292:H19–H27. doi:10.1152/ajpheart.00712.2006
Zhao ZQ, Vinten-Johansen J (2002) Myocardial apoptosis and ischemic preconditioning. Cardiovasc Res 55:438–455. doi:10.1016/S0008-6363(02)00442-X
Guo Y, Jones WK, Xuan YT, Tang XL, Bao W, Wu WJ, Han H, Laubach VE, Ping P, Yang Z, Qiu Y, Bolli R (1999) The late phase of ischemic preconditioning is abrogated by targeted disruption of the inducible NO synthase gene. Proc Natl Acad Sci USA 96:11507–11512. doi:10.1073/pnas.96.20.11507
Xuan YT, Guo Y, Zhu Y, Wang OL, Rokosh G, Bolli R (2007) Endothelial nitric oxide synthase plays an obligatory role in the late phase of ischemic preconditioning by activating the protein kinase C epsilon p44/42 mitogen-activated protein kinase pSer-signal transducers and activators of transcription1/3 pathway. Circulation 116:535–544. doi:10.1161/CIRCULATIONAHA.107.689471
Wang Y, Kodani E, Wang J, Zhang SX, Takano H, Tang XL, Bolli R (2004) Cardioprotection during the final stage of the late phase of ischemic preconditioning is mediated by neuronal NO synthase in concert with cyclooxygenase-2. Circ Res 95:84–91. doi:10.1161/01.RES.0000133679.38825.a6
Post H, Schulz R, Behrends M, Gres P, Umschlag C, Heusch G (2000) No involvement of endogenous nitric oxide in classic ischemic preconditioning in swine. J Mol Cell Cardiol 32:725–733. doi:10.1006/jmcc.2000.1117
Nakano A, Liu GS, Heusch G, Downey JM, Cohen MV (2000) Exogenous nitric oxide can trigger a preconditioned state through a free radical mechanism, but endogenous nitric oxide is not a trigger of classic ischemic preconditioning. J Mol Cell Cardiol 32:1159–1167. doi:10.1006/jmcc.2000.1152
Guo Y, Li Q, Wu WJ, Tan W, Zhu X, Mu J, Bolli R (2008) Endothelial nitric oxide synthase is not necessary for the early phase of ischemic preconditioning in the mouse. J Mol Cell Cardiol 44:496–501. doi:10.1016/j.yjmcc.2008.01.003
Csonka C, Szilvássy Z, Fülöp F, Páli T, Blasig IE, Tosaki A, Schulz R, Ferdinandy P (1999) Classic preconditioning decreases the harmful accumulation of nitric oxide during ischemia and reperfusion in rat hearts. Circulation 100:2260–2266
Cohen MV, Yang XM, Downey JM (2006) Nitric oxide is a preconditioning mimetic and cardioprotectant and is the basis of many available infarct-sparing strategies. Cardiovasc Res 70:231–239. doi:10.1016/j.cardiores.2005.10.021
Kimura S, Zhang GX, Nishiyama A, Shokoji T, Yao L, Fan YY, Rahman M, Suzuki T, Maeta H, Abe Y (2005) Role of NAD(P)H Oxidase- and mitochondria-derived reactive oxygen species in cardioprotection of ischemic reperfusion injury by angiotensin II. Hypertension 45:860–866. doi:10.1161/01.HYP.0000163462.98381.7f
Deng Y, Kaufman S (2001) Splenorenal reflex regulation of arterial pressure. Hypertension 38:348–352
Levrand S, Vannay-Bouchiche C, Pesse B, Pacher P, Feihl F, Waeber B, Liaudet L (2006) Peroxynitrite is a major trigger of cardiomyocyte apoptosis in vitro and in vivo. Free Radic Biol Med 41:886–895. doi:10.1016/j.freeradbiomed.2006.04.034
Zhang GX, Kimura S, Nishiyama A, Shokoji T, Rahman M, Abe Y (2004) ROS during the acute phase of Ang II hypertension participates in cardiovascular MAPK activation but not vasoconstriction. Hypertension 43:117–124. doi:10.1161/01.HYP.0000105110.12667.F8
Liang F, Gao E, Tao L, Liu H, Qu Y, Christopher TA, Lopez BL, Ma XL (2004) Critical timing of L-arginine treatment in post-ischemic myocardial apoptosis-role of NOS isoforms. Cardiovasc Res 62:568–577. doi:10.1016/j.cardiores.2004.01.025
Zweier JL, Wang P, Samouilov A, Kuppusamy P (1995) Enzyme-independent formation of nitric oxide in biological tissues. Nat Med 1:804–809. doi:10.1038/nm0895-804
Rakhit RD, Edwards RJ, Mockridge JW, Baydoun AR, Wyatt AW, Mann GE, Marber MS (2000) Nitric oxide-induced cardioprotection in cultured rat ventricular myocytes. Am J Physiol Heart Circ Physiol 278:H1211–H1217
Muscari C, Bonafé F, Gamberini C, Giordano E, Tantini B, Fattori M, Guarnieri C, Caldarera CM (2004) Early preconditioning prevents the loss of endothelial nitric oxide synthase and enhances its activity in the ischemic/reperfused rat heart. Life Sci 74:1127–1137. doi:10.1016/j.lfs.2003.10.001
Bell RM, Yellon DM (2001) The contribution of endothelial nitric oxide synthase to early ischaemic preconditioning: the lowering of the preconditioning threshold. An investigation in eNOS knockout mice. Cardiovasc Res 52:274–280. doi:10.1016/S0008-6363(01)00394-7
Cho S, Park EM, Zhou P, Frys K, Ross ME, Iadecola C (2005) Obligatory role of inducible nitric oxide synthase in ischemic preconditioning. J Cereb Blood Flow Metab 25:493–501. doi:10.1038/sj.jcbfm.9600058
Atochin DN, Clark J, Demchenko IT, Moskowitz MA, Huang PL (2003) Rapid cerebral ischemic preconditioning in mice deficient in endothelial and neuronal nitric oxide synthases. Stroke 34:1299–1303. doi:10.1161/01.STR.0000066870.70976.57
Kanai AJ, Pearce LL, Clemens PR, Birder LA, VanBibber MM, Choi SY (2001) Identification of a neuronal nitric oxide synthase in isolated cardiac mitochondria using electrochemical detection. Proc Natl Acad Sci USA 98:14126–14131. doi:10.1073/pnas.241380298
Elfering SL, Sarkela TM, Giulivi C (2002) Biochemistry of mitochondrial nitric-oxide synthase. J Biol Chem 277:38079–38086. doi:10.1074/jbc.M205256200
Xu KY, Huso DL, Dawson TM, Bredt DS, Becker LC (1996) Nitric oxide synthase in cardiac sarcoplasmic reticulum. Proc Natl Acad Sci USA 96:657–662. doi:10.1073/pnas.96.2.657
Wang L, Cherednichenko G, Hernandez L, Halow J, Camacho SA, Figueredo V, Schaefer S (2001) Preconditioning limits mitochondrial Ca(2+) during ischemia in rat hearts: role of K(ATP) channels. Am J Physiol Heart Circ Physiol 280:H2321–H2328
Acknowledgments
This work was supported in part by a grand-in-aid for Scientific Research NO.197901905001 from the Ministry of Education, Science and Culture, Japan.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Lu, XM., Zhang, GX., Yu, YQ. et al. The opposite roles of nNOS in cardiac ischemia–reperfusion-induced injury and in ischemia preconditioning-induced cardioprotection in mice. J Physiol Sci 59, 253–262 (2009). https://doi.org/10.1007/s12576-009-0030-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12576-009-0030-1