Abstract
An aberrant right subclavian artery is a branching variation of the aortic arch. We encountered two female cadavers with an aberrant right subclavian artery during routine student dissection at our school. In both cases, the right subclavian artery was not a branch of the brachiocephalic trunk but originated directly from the distal part of the aortic arch as the last branch and ran between the esophagus and vertebral column, traveling to the upper limb. The right recurrent laryngeal nerve was absent, but a non-recurrent inferior laryngeal nerve branching from the vagus and traveling directly toward the larynx was observed. In the first case, the right and left common carotid arteries originated solely from the aortic arch as the first and second branches, respectively, whereas the right and left common carotid arteries formed a bicarotid trunk at their origin in the second case. A Kommerell diverticulum was present at the base of the aberrant right subclavian artery in the second case, but not in the first case. We analyzed the anatomical differences between the two cases and discussed the developmental aspects and potential clinical risks.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The aortic arch (AA) normally has three main branches: the brachiocephalic trunk (BCT), left common carotid artery (LCCA), and left subclavian artery (LSA). The BCT bifurcates into the right common carotid artery (RCCA) and right subclavian artery (RSA). An aberrant right subclavian artery (ARSA) or “arteria lusoria,” an atypical branching pattern of the AA, originates directly from the distal AA as the last branch. The incidence of ARSA varies in the literature and has been reported to be 0.2–1.6% in Japan (Iimura et al. 2017). ARSA is a congenital anomaly that is commonly seen together with cardiac anomalies such as tetralogy of Fallot and genetic disorders such as Downs’ syndrome (Jahangeer et al. 2018). A recent study showed severe cardiovascular malformations such as ARSA in Hoxa1 null mice (Makki and Capecchi 2012), suggesting the involvement of Hox genes in the molecular pathways of cardiovascular development (Roux and Zaffran 2016).
ARSA can be associated with other anomalies such as a bicarotid trunk (BiCT), which arises from the AA as a common trunk of the RCCA and LCCA, and Kommerell diverticulum (KD), a dilatation at the origin of ARSA. The recurrent laryngeal nerve (RLN) is a branch of the vagus nerve that provides motor and sensitive functions to the larynx. Normally, the right and left RLNs turn around the right RSA and AA, respectively. In the case of ARSA, the right non-recurrent laryngeal nerve (NRLN), which directly travels towards the larynx after branching from the cervical vagus, is present instead of the right RLN.
It has been reported that 90–93% of patients with ARSA are asymptomatic (Jahangeer et al. 2018). Nevertheless, ARSA and its associated anomalies may affect the surrounding structures and cause serious clinical conditions. Knowledge of ARSA and its associated variations is important not only for anatomical understanding but also for proper clinical procedures to avoid serious conditions. Here, we analyzed the anatomical features of the two ARSA cases found at our school and discussed its developmental and clinical implications.
Case report
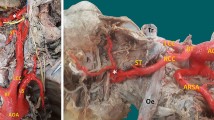
We identified ARSA in two cadavers during a student course of gross anatomy dissection at the Aichi Gakuin University School of Dentistry, one in 2016 and the other in 2017. The first cadaver was a 92-year-old woman who died of decrepitude. The RCCA, LCCA, and LSA branched independently from the AA sequentially from right to left (Fig. 1a, b, e). The origins of the three arteries were very close to each other. Closely distal to the LSA, the ARSA arose from the posterosuperior wall of the AA as the last branch and ran obliquely behind the esophagus. KD was absent, RLN was missing, and NRLN was observed on the right side. The left RLN normally branched from the left vagus nerve and looped posteriorly around the AA behind the arterial ligament (Fig. 1e).
Photographs of the two cases of aberrant right subclavian artery (ARSA). a, b Anterior views of ARSA in the first cadaver. In b, the aortic arch (AA) was retracted ventrolaterally to the left to show the ARSA arising from the posterior aspect of the AA and coursing posterior to the esophagus (ES) and trachea (TR). Anterior (c) and left side (d) views of ARSA in the second cadaver. Note that the second case is associated with the bicarotid trunk (BiCT) (c) and Kommerell diverticulum (KD) (d). Schematic diagrams of the first (e) and second (f) ARSA cases. AL arterial ligament, LCCA left common carotid artery, LMB left main bronchus, LSA left subclavian artery, LVN left vagus nerve, NRLN non-recurrent laryngeal nerve, PA pulmonary artery, RLN recurrent laryngeal nerve, RCCA right common carotid artery, RVN right vagus nerve, TG thyroid gland
The second cadaver was an 89-year-old woman who died from chronic heart disease. In this case, the first branch from the AA was a BiCT bifurcating into the RCCA and LCCA, and the second branch was the LSA (Fig. 1c, f). Approximately 1.1 cm distal to the origin of the LSA, ARSA was observed as the third and last branch of the AA and took a retroesophageal course. The BiCT and LSA originated from the superior surface of the AA, whereas the ARSA arose from the posterior wall of the AA. Furthermore, the base of the ARSA was associated with a bulbous segment of the KD (Fig. 1d, f). The right RLN was missing, and the right NRLN could not be found because both right and left vagus nerves had been destroyed by the students’ dissection before the ARSA was recognized.
In both cases, the shape and position of the AA appeared normal, and both the left and right vertebral and internal thoracic arteries originated normally from the subclavian arteries.
Discussion
To date, several anatomical variations of the AA branches have been reported, and the prevalence of the presence of a variation differs from study to study (Tapia-Nañez et al. 2021). The two ARSAs presented in this report differ morphologically in the position and trajectory of the artery and other associated variations. According to a classification based on the most classical cadaver-based study by Adachi (1928), the first and second ARSA cases mentioned in this report belong to types G and H, respectively. Furthermore, it can be seen that KD exists at the base of the ARSA in the second case. Taking KD into consideration, ARSA patients were classified into four groups (groups 1–4) by Kieffer et al. (1994), and the second case in this report belongs to group 3. The two ARSA cases were found among a total of > 800 cadavers donated for student dissection at our school between 2012 and 2021. The frequency was, hence, calculated to be < 0.25%, which is within the reported range of 0.2–1.6% in Japan (Iimura et al. 2017). It has been reported that 19.2% and 14.9% of ARSA cases accompany BiCT and KD, respectively (Jahangeer et al. 2018). Therefore, the prevalence of the second case with the concomitant presence of both BiCT and KD with ARSA should be comparatively rare.
ARSA is formed as a result of altered development of primitive aortas and AAs (Sangam and Anasuya 2010). During 6–8 weeks of gestation, the right 4th AA, 7th intersegmental artery (ISA), and right dorsal aorta (DA) between them give rise to RSA (Fig. 2a, b). If the right 4th AA and right DA cranial to the right 7th ISA are regressed, the right 7th ISA affixed to the caudal portion of the right DA develops into ARSA (Fig. 2a, c). Thus, the proximal ARSA is derived from the right DA and is connected to the descending aorta distal to the left 7th ISA, which develops into the LSA and accounts for a retroesophageal portion. Using an intravascular dye-injection with nerve staining in whole mounted rat embryos, Aizawa et al. (1999) reported that the subclavian artery is derived from the DA as an independent branch of the 7th ISA. To determine the precise portions that persist or regress in each case, Kawai et al. (2011) showed detailed schematic diagrams using five ARSA cases, suggesting that various components of the embryonic AA complex are involved as the prototype just before the beginning of aberrant developmental processes. Based on their report, it is plausible that the disappearance of all of the right 4th AA and part of the right DA cranial to the 7th ISA occurred in both cases mentioned in the current paper. The evidence to support this notion was the fact that all the vertebral arteries normally branched from the subclavian artery, which was originally the 7th ISA, and entered the foramen transversarium of the 6th cervical vertebra. KD is considered a remnant of the right DA. The origin of the ARSA shifts cranially to get closer to the origin of the LSA. Distances between the ARSA and LSA origins differed between the two ARSA cases, perhaps reflecting those of the ARSA’s shift.
Schematic illustrations depicting the development of the aortic arches and branches. a Six paired primitive aortic arches (I–VI) formed at the 6th week of gestation. Normal (b) and aberrant (c) arterial regression at the 7th week of gestation. Dashed lines indicate the regressed portions. Note that the portions framed by the blue line in b and c give rise to the right subclavian artery (RSA) and aberrant right subclavian artery (ARSA), respectively. AS aortic sac, AT arterial trunk, DA dorsal aorta, ISA intersegmental artery, NRLN non-recurrent laryngeal nerve, RLN recurrent laryngeal nerve
Because of the regression of both the 5th and 6th AAs, the right RLN is sustained by the right 4th AA, which develops as a part of the RSA. However, due to the regression of the right 4th AA in ARSA, the right vagus branch heads directly toward the larynx without taking a recurrent course, resulting in right NRLN.
Both the RCCA and LCCA arise from the 3rd pair of AAs, which are connected to the right and left horns of the aortic sac. If the aortic sac fails to bifurcate, the LCCA is directly connected to the aortic sac and develops into a BiCT (Sangam and Anasuya 2010).
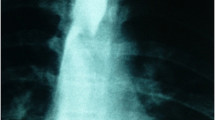
The anatomical features of ARSA are clinically significant. Due to its retroesophageal position, ARSA can compress the esophagus and/or trachea posteriorly to cause clinical symptoms such as dysphagia, dyspnea, stridor, and chest pain. Furthermore, because ARSA is in close contact with the esophagus, its presence carries a risk of arterial-esophageal fistula, which is usually fatal (Watanabe et al. 2016).
Normally, the origins of BCT and LCCA are approximately 4 cm apart. In the first ARSA cadaver, the origins of the RCCA and LCCA were contiguous with each other anterior to the trachea, indicating that they may possibly have been compressing the trachea. In the case of coexistence of BiCT with ARSA as seen in the second cadaver, because BiCT can form a V configuration of vessels in front of the trachea, the compression may be enhanced (Klinkhamer 1966). Furthermore, KD can cause compression of the trachea and/or esophagus and may further aggravate the symptoms. Pathological examination of the resected KD specimens revealed histological abnormalities, indicating vulnerability of the region (Suzuki et al. 2019). Therefore, if a KD progresses to an aneurysm, it can cause fatal complications, including aortic dissection and rupture (Jahangeer et al. 2018).
The existence of ARSA, if not recognized, may put it at risk of injury during surgery of the esophagus or trachea. Because the right NRLN is almost always accompanied by ARSA, it can be injured during surgery such as thyroidectomy (Bakalinis et al. 2018). Furthermore, in case of coexistence of ARSA and BiCT, the latter can be misinterpreted as BCT if the presence of the ARSA is missed, and the surgery would result in life-threatening complications. The management of ARSA and its concomitant variations such as BiCT and KD should be considered depending on multiple factors such as age, symptoms, and anatomical features, including location, size, course, and relationship to adjacent structures. The data presented in this report will contribute to understanding the anatomy of ARSA with its associated variations and avoiding potential clinical risks in the thorax and neck region.
References
Adachi B (1928) Das Arteriensystem der Japaner, Bd. 1. Maruzen, Kyoto, pp 35–41
Aizawa Y, Isogai S, Izumiyama M, Horiguchi M (1999) Morphogenesis of the primary arterial trunks of the forelimb in the rat embryos: the trunks originate from the lateral surface of the dorsal aorta independently of the intersegmental arteries. Anat Embryol 200:573–584
Bakalinis E, Makris I, Demesticha T, Tsakotos G, Skandalakis P, Filippou D (2018) Non-recurrent laryngeal nerve and concurrent vascular variants: a review. Acta Med Acad 47:186–192
Iimura A, Oguchi T, Tou M, Matsuo M (2017) The retroesophageal right subclavian artery—a case report and review. Okajimas Folia Anat Jpn 94:75–80
Jahangeer S, Bashir M, Harky A, Yap J (2018) Aberrant subclavian: new face of an old disease. J vis Surg 4:108
Kawai K, Honma S, Kumagai Y, Koba Y, Koizumi M (2011) A schematic diagram showing the various components of the embryonic aortic arch complex in the retroesophageal right subclavian artery. Anat Sci Int 86:135–145
Kieffer E, Bahnini A, Koskas F (1994) Aberrant subclavian artery: surgical treatment in thirty-three adult patients. J Vasc Surg 19:100–111
Klinkhamer AC (1966) Aberrant right subclavian artery. Clinical and roentgenologic aspects. Am J Roentgenol Radium Ther Nucl Med 97:438–446
Makki N, Capecchi MR (2012) Cardiovascular defects in a mouse model of HOXA1 syndrome. Hum Mol Genet 21:26–31
Roux M, Zaffran S (2016) Hox genes in cardiovascular development and diseases. J Dev Biol 4:14
Sangam MR, Anasuya K (2010) Arch of aorta with bi-carotid trunk, left subclavian artery, and retroesophageal right subclavian artery. Folia Morphol (warsz) 69:184–186
Suzuki K, Sasaki T, Kunugi S et al (2019) Resection of Kommerell’s diverticulum in an infant with prenatal diagnosis of right aortic arch. Surg Case Rep 5:172
Tapia-Nañez M, Landeros-Garcia GA, Sada-Treviño MA et al (2021) Morphometry of the aortic arch and its branches. A computed tomography angiography-based study. Folia Morphol (warsz) 80:575–582
Watanabe M, Suzuki K, Fujinaga K et al (2016) Postmortem diagnosis of massive gastrointestinal bleeding in a patient with aberrant right subclavian artery–esophageal fistula. Acute Med Surg 3:139–142
Acknowledgements
The authors sincerely thank those who donated their bodies to science so that anatomical research could be performed. Results from such research can potentially increase mankind's overall knowledge that can then improve patient care. Therefore, these donors and their families deserve our highest gratitude. We thank Editage (www.editage.com) for the English language editing of our paper.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by TI, AI, and YI. The first draft of the manuscript was written by YI, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ito, T., Itoh, A., Kiyoshima, D. et al. Anatomical characteristics of two cases of aberrant right subclavian artery. Anat Sci Int 97, 423–427 (2022). https://doi.org/10.1007/s12565-022-00658-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12565-022-00658-7