Abstract
Purpose
Taking into consideration that the aberrant right subclavian artery (ARSA) is the commonest congenital aortic arch anomaly (prevalence 0.16–4.4%), our goal was to investigate its incidence in Greeks, with respect to location, course, and relationship to trachea and esophagus. Gender dimorphism, coexisting neural, and vascular variations were also examined. The clinical impact and embryological background of the variation are discussed.
Methods
Two hundred and sixty-seven (126 male and 141 female) formalin-embalmed Greek cadavers (mean age 59 ± 13 years) were examined.
Results
The ARSA was detected in 6 cadavers (2.2%), 4 females (2.8%) and 2 males (1.6%). The artery followed a retroesophageal course in 83%, an interesophageotracheal course in 16.7%, while no pretracheal ARSA was detected. The ARSA coexisted with a bicarotid trunk, a Kommerell diverticulum, and a combination of them in one cadaver. Α combination of the ARSA with a thyroidea ima artery and a bilateral abnormal origin of the internal mammary artery was also detected in one case (17%), while no other associated anomaly was detected in a single case (17%). All ARSAs were accompanied with a right non-recurrent laryngeal nerve.
Conclusion
The ARSA has a relative high incidence in Greeks and a female predominance. The aberrant vessel follows a retroesophageal course in most cases and only one case with a location between trachea and esophagus was detected. The retroesophageal ARSA justifies the wide variety of clinical manifestations and complications occurred.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The typical aortic arch branching pattern (incidence from 64.9 up to 94.3%) [29] consists of the brachiocephalic trunk, the left common carotid artery (LCCA) and the left subclavian artery (LSA). The brachiocephalic trunk is further divided into the right subclavian artery (RSA) and the right common carotid artery (RCCA). The aberrant right subclavian artery (ARSA) is a relatively rare aberration (incidence from 0.16 in up to 4.4%) in the general population [4, 41] with a female predominance [25]. Patients with congenital heart defects have an ARSA incidence up to 3%, while Down syndrome patients present the highest range (2.9–100%) [25].
The ARSA may follow a retroesophageal course (80–84%), a course between trachea and esophagus (12.7–15%), or a pretracheal route (4.2–5%) [26] (Fig. 1). The atypical vessel may compress on trachea and esophagus, when forming an incomplete vascular ring around them [22]. The retroesophageal ARSA is particularly susceptible to extrinsic compression and pressure necrosis secondary to nasogastric and endotracheal tube insertion, predisposing to arterio-esophageal fistula formation [7, 17].
Diagrammatic ventral views of various aberrant right subclavian artery (ARSA) patterns. Posterior to trachea (T) and esophagus (E) (I), between T and E (II), and in front of T (III). Aortic arch arrangement from proximal to distal are: the brachiocephalic trunk (BT), the left common carotid artery (LCCA) and the left subclavian artery (LSA)
The first description of the ARSA is attributed to Hunauld, in 1735. Fifty-nine years later, David Bayford correlated ARSA presence with the development of a peculiar form of dysphagia to solids, the so-called dysphagia lusoria [31]. The term “arteria lusoria” has been incorrectly used by some authors, when referring to the ARSA, even in asymptomatic cases.
Current retrospective study evaluates the incidence of the ARSA in the total sample, in males and females, emphasizing on vessel location, course and relationship to trachea and esophagus in a Greek population. Based on our findings, coexisting neural and vascular variations and congenital anomalies are also presented in order of frequency. The clinical impact and the embryological background of this variation are further discussed.
Materials and methods
Two hundred and sixty-seven (126 male and 141 female) formalin-embalmed Greek cadavers with age average of 59 ± 13 years were dissected from 1986 to 2016 for educational purposes. An informed consent was obtained from all donators before death. After careful dissection of the anterior part of the neck and thoracic cavity, the aortic arch branching pattern was thoroughly examined. In each case, the presence, origin and course of the ARSA and coexisting abnormalities were recorded. All cadavers showed no macroscopically evident abnormalities and their medical records were free of any congenital anomaly. Additionally, a database search (PubMed and Google Scholar) on cadaveric studies was performed using the keywords “aortic arch”, “aberrant right subclavian artery”, “arteria lusoria” and “variation”. Further relevant publications were extracted from the references of the included articles. The types of the included studies were: (a) cadaveric and (b) with clear, extractable prevalence data on the ARSA with respect to the course and associated variations. All selected studies were original articles, reviews, case reports, and conference abstracts based on exceptional ARSA cases. The excluded studies were: (a) studies reporting incomplete data and (b) studies on patients with trauma to the neck and thorax regions.
Results
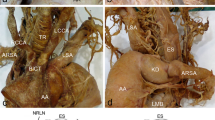
The ARSA was detected in six cadavers (2.2%), in four females (2.8%) and two males (1.6%) (Table 1). A female predominance was detected (female to male ratio 2:1). The ARSA followed a retroesophageal course in 83%, while in 16.7% the vessel coursed between trachea and esophagus adjacent to the right non-recurrent laryngeal nerve (NRLN) [26]. No pretracheal ARSA was found. In 33%, the ARSA was combined with a common stem of RCCA-LCCA, the so-called bicarotid trunk (BCT). Case 1: the retroesophageal ARSA coexisted with an abnormal bilateral origin of the internal mammary artery arising from the 3rd part of subclavian artery, while deep brachial artery atypically emanated from a common stem with the right subscapular artery [38]. Case 2: a thyroidea ima artery (TIA) emerged from the lower middle third of the RCCA in 17%, coursed upwards to supply the right lobe of the thyroid gland and the lower part of the isthmus [27]. Case 3: a single female cadaver with a retroesophageal ARSA had no associated anomaly (Fig. 2). Case 4: an ARSA coursing between trachea and esophagus coexisted with a BCT [26]. Case 5: the retroesophageal part of the ARSA was notably larger proximally, due to the presence of a Kommerell’s diverticulum (KD). The KD coexisted with a BCT [26]. Case 6: the ARSA originated from an aneurysmal KD, which connected to the left pulmonary artery through a left ligamentum arteriosum (Fig. 3). All examined right inferior laryngeal nerves were non-recurrent and originated directly from the vagus nerve at the laryngotracheal junction or above.
74-year-old female cadaver with a retroesophageal aberrant right subclavian artery (ARSA) and a right non-recurrent laryngeal nerve (white arrow) with no other associated anomaly detected. AA aortic arch, RCCA right common carotid artery, LCCA left common carotid artery, T trachea, E esophagus, LSA left subclavian artery and IJV internal jugular vein
72-year-old female cadaver with a retroesophageal aberrant right subclavian artery (ARSA). The aberrant artery emanated from an aneurysmal Kommerell diverticulum (asterisk symbol) which connected through a left ligamentum arteriosum to the left pulmonary artery, AA aortic arch, RCCA right common carotid artery, LCCA left common carotid artery, T trachea, E esophagus and LSA left subclavian artery
Discussion
The ARSA appears in 0.2–13.3% of the general population, as presented in Table 2. Similarly, to our results (male to female ratio of ARSA 1:2), a female predominance was found in cases of isolated ARSA and in cases of aortic coarctation with a pre-stenotic ARSA, while male predominance was detected in cases of aortic coarctation with post-stenotic ARSA. Equal gender distribution was found for the ARSA with aortic arch interruption [25, 31].
Associated vascular and neural anatomic variations
BCT is the most frequent aortic arch branching pattern variation and coexists with an ARSA in 0.16–19.2% [14, 20, 26]. The current study revealed a lower incidence of up to 0.8%. The BCT is the commonest abnormality in patients with congenital heart defects (septal defects, valvular pulmonary stenosis and atrial septal defect) and genetic disorders (trisomy 21, Williams and CHARGE syndromes). Another congenital abnormality was described by Kommerell in 1936, as a dilated segment at the ARSA origin. The root of the ARSA had a broad base, formed by a remnant of the persisting right aorta, the so-called KD that coexisted with the ARSA in 14.9% [20]. Although KD is primarily asymptomatic, cases of extreme dilation may result to aortic aneurysm [35], dissection, or rupture [23]. In our study, a single case of KD (1.7%) was found. The right NRLN is a rare embryological variant (0.3–1.6%) where the nerve enters the larynx directly off the cervical portion of the vagus nerve. Its occurrence strongly indicates the ARSA presence and more rarely a coexistent BCT [10]. Level of origin of the right NRLN according to Toniato’s classification [39] highlights the ascending, vertical, and descending course of the atypical nerve. The ARSA-right NRLN coexistence is important to be recognized in patients who may require thyroidectomy, parathyroidectomy and endarterectomy [2] due to the high risk of nerve injury particularly when it is partially exposed [10]. In such cases, the preoperative ultrasonography truly preserves the right NRLN integrity [15]. The appearance of a left NRLN in 0.04% is associated with pathology, like situs inversus.TIA or Neubauer’s artery is a thin inconstant vessel (0.16–12.2%) that supplies thyroid gland, usually arising from the RCCA [27] or from the common carotid artery, the internal thoracic, the pericardiophrenic, the subclavian, the inferior thyroid and the suprascapular arteries, the thyrocervical trunk, or rarely from the aortic arch (0.16%) [25, 27]. In our study, the rare combination ARSA-TIA [27] was detected in 0.4%, where TIA emerged from the RCCA. Although TIA presence is asymptomatic, its identification is of paramount importance, since missed adenomas or hyperplastic gland lesions may be revealed, during parathyroid arteriography, if TIA is present [27]. Keiffer and co-authors [20] have emphasized on the coexistence ARSA-abnormal right vertebral artery origin (from the aortic arch or the RCCA), the presence of a common carotid trunk, a right-sided thoracic duct, a right NRLN and a TIA. Loukas et al. [22] highlighted the combination of a retrotracheal ARSA with a common trunk of a right vertebral artery and a TIA arising from the left aortic arch. Unique cases of ARSA coexistence with a persistent left superior vena cava and a thoracic duct ending in the right jugulosubclavian junction [20] were also reported. Our retrospective study highlights the 1st case of an ARSA combination with an abnormal bilateral origin of the internal mammary artery arising from the 3rd part of subclavian artery and the coexistence of an atypical common origin of the deep brachial artery with the right subscapular artery [38].
Coexisting malformations
A wide range of the ARSA incidence in fetuses (7.9–29.6%) and adults (1.6–35.7%) with Down syndrome exists. An incidence of 20% was detected in fetuses with congenital heart disease [6]. Other congenital cardiovascular malformations (conotruncal anomalies, septal defects, congenital aortic insufficiency, rhabdomyoma, endocardial fibroelastosis, right pulmonary artery from aorta, total anomalous pulmonary venous connection, single ventricle, conjoined hearts of twins, pulmonary venous stenosis and the patent ductus arteriosus) associated with the ARSA presence in 68–91% increase the suspicion of visceral anomalies detection (esophageal atresia, trachea-esophageal fistula, abnormalities of lung lobation, anal atresia, gall bladder agenesis, asplenia, double uterus and vagina, renal anomalies and sacral spina bifida) [40]. The ARSA may also coexist with Edwards’ (55%), Patau (50%), Turner (43%), DiGeorge (14%), Noonan, Postrubella and Potter syndromes [7, 40]. Our study did not reveal any coexisted malformation.
Embryology and associated pathologic conditions
The proximal RSA part derives from the right 4th arch, while the distal part is formed after the involution of the right dorsal aorta and the right 7th intersegmental artery. When the right 4th arch is absent, the 7th intersegmental artery remains attached to the descending aorta and from its persistence derives the ARSA. The arterial wall abnormalities may explain why the ARSA is the subject of specific anomalies and pathologies [8]. Vessel aneurysms just after its origin (12.8%) [20] are explained by the muscular type of subclavian artery (low amount of elastic fibers in tunica media) and the progressive degradation of elastic and collagen fibers. The ARSA may also coexist with a Stanford type-A acute aortic dissection [36]. The accompanied right NRLN usually results from partial regression of the 4th pharyngeal arch, resulting in the retroesophageal ARSA. The atypical vascular pattern permits the nerve to migrate freely into the neck and directly enters the larynx, as the fetus grows longitudinally. In 14.9–60%, the ARSA may originate from a small aneurysmal aortic arch dilatation, the KD [31]. The KD, a remnant of the right dorsal aorta, was detected with an incidence of 20%, in the current study. It is difficult to explain the courses of the ARSA anterior to the trachea or between trachea and esophagus.
Clinical manifestations
The ARSA occurrence is clinically evident in 90–93% of the cases. Symptoms appear in 10–33% of the ARSA cases due to the course of the aberrant vessel in a limited anatomical space. In adults, clinical manifestations have been associated with age-related morphological alterations of the vessels and adjacent tissues (atheromatosis, aortic elongation, fibromuscular dysplasia of paratracheal and esophageal tissues) and aneurysmal dilatation [31] due to the progressive degradation of elastic and collagen fibers of the connective tissue [12]. The onset of symptoms differs between males (44.9 years) and females (54 years of age). Dysphagia is the commonest symptom (71.2%) [20, 32, 34], in cases of a retroesophageal ARSA [31]. Dyspnea (18.7%) is more often associated to a retrotracheal ARSA [20]. The increased frequency of pulmonary infections in pediatric population is due to tracheomalacia [31]. Other symptoms in order of frequency are: stridor, retrosternal pain (17.0%), cough (7.6%), feeding difficulties accompanied with weight loss (5.9%), recurrent pulmonary infections, stomach-ache, back pain and numbness of the right upper limb [26, 32]. Moreover, the coexistence retroesophageal ARSA-BCT more frequently limits trachea-esophageal mobility, accounting for symptoms in cases of aneurysm absence at the origin of the aberrant vessel [33]. In addition, cases of stenosis, tortuosity, and kinking of the ARSA may lead to unequal upper extremity blood pressure, right arm claudication, splinter hemorrhages, or vertebrobasilar ischemia [27].
Diagnosis
The ARSA detection usually remains an incidental finding [8, 18, 26, 28]. Diagnosis may be posed by chest roentgenography. Barium-contrast oesophagography can provide a more accurate depiction of the oblique defect or indentation along the posterior esophageal wall, while the Color Doppler is extremely useful to trace the abnormal vessel course. A computed tomography (CT) or magnetic resonance (MR) angiography is considered the gold standard for the diagnosis, as it offers a detailed visualization of the arch anatomy and the ARSA, especially in cases of aneurysms and depicts the degree of tracheal compression [19]. Differential diagnosis may include the azygos vein which may follow a course behind trachea before entering the superior vena cava [6], the thoracic outlet syndrome [18] and in esophageal dysphagia caused by the ARSA, motility disorders, mechanical and inflammatory diseases [3]. When an ARSA coexists with an esophageal carcinoma, dysphagia is causally attributed to the tumor growth and the aberrant vessel may be missed on imaging [30].
Conclusion
In Greeks, the ARSA has a relative high incidence and a female predominance. The aberrant vessel follows a retroesophageal course in the majority of cases and only one case coursing between trachea and esophagus was detected. The retroesophageal ARSA justifies the wide variety of reported clinical manifestations and complications.
Change history
14 September 2017
An erratum to this article has been published.
References
Adachi B (1928) Das Arteriensystem der Japaner, vol 1, 1st edn. Verlag der Kaiserlich-Japanischen Universitat, Kenyusha Press, Kyoto, pp 29–41
Atay Y, Engin C, Posacioglu H, Ozyurek R, Ozcan C, Yagdi T, Ayik F, Alayunt EA (2006) Surgical approaches to the aberrant right subclavian artery. Tex Heart Inst J 33:477–481
Barone C, Carucci NS, Romano C (2016) A rare case of esophageal dysphagia in children: aberrant right subclavian artery. Case Rep Pediatr 2016:2539374. doi:10.1155/2016/2539374
Bergman RA, AWW AK, Miyauchi R (1985–2002) Illustrated encyclopedia of human anatomic variation online. http://www.anatomyatlases.org/AnatomicVariants/Cardiovascular/Text/Arteries/Aorta.shtml and http://www.anatomyatlases.org/AnatomicVariants/Cardiovascular/Text/Arteries/Subclavian.shtml. Accessed 8 Dec 2015
Cairney J (1925) The anomalous right subclavian artery considered in the light of recent findings in arterial development; with a note on two cases of an unusual relation of the innominate artery to the trachea. J Anat 59:265–296
Chaoui R, Thiel G, Heling KS (2006) Prevalence of an aberrant right subclavian artery (ARSA) in fetuses with chromosomal aberrations. Ultrasound Obstet Gynecol 11:414
Chavda HS, Varlekar PD, Khatri CR, Saiyad SS, Bhatt RH (2014) Abnormal origin of right subclavian artery—a cadaveric study. Int J Med Sci Public Health 3:85–88
Davies M, Guest JP (2003) Developmental abnormalities of the great vessels of the thorax and their embryological basis. Br J Radiol 76:491–502
DeGaris CF (1932) Aortic axillary collaterals and the pattern of arm arteries in anomalous right subclavian artery. Am. J. Anat. 51:189–213
Dolezel R, Jarosek J, Hana L, Ryska M (2015) Clinical relevance and surgical anatomy of non-recurrent laryngeal nerve: 7-year experience. Surg Radiol Anat 37:321–325
Evans PR (1950) Cardiac anomalies in Mongolism. Br Heart J 12:258–262
Godlewski J, Widawski T, Michalak M, Kmieć Z (2010) Aneurysm of the aberrant right subclavian artery—a case report. Pol J Radiol 75:47–50
Goldbloom AA (1922) The anomalous right subclavian artery and its possible clinical significance. Surg Gynecol Obstet 34:378–384
Hartyánszky IL, Lozsadi K, Marcsek P, Huttl T, Sapi E, Kovacs AB (1989) Congenital vascular rings: surgical management of 111 cases. Eur J Cardiothorac Surg 3:250–254
Henry BM, Vikse J, Graves MJ, Sanna S, Sanna B, Tomaszewska IM, Tubbs RS, Tomaszewski KA (2016) Extralaryngeal branching of the recurrent laryngeal nerve: a meta-analysis of 28,387 nerves. Langenbecks Arch Surg 401:913–923
Holtzapfel G (1899) Ungewöhnlicher Ursprung und Verlauf der Arteria subclavia dextra. Anat Hefte 12:369–523
Inman JC, Kim P, McHugh R (2008) Retroesophageal subclavian artery—esophageal fistula: a rare complication of a salivary bypass tube. Head Neck 30:1120–1123
Janssen M, Baggen MG, Veen HF, Smout AJ, Bekkers JA, Jonkman LG, Ouwendijk RJ (2000) Dysphagia lusoria: clinical aspects, manometric findings, diagnosis, and therapy. Am J Gastroenterol 95:1411–1416
Karcaaltincaba M, Haliloglu M, Ozkan E, Kocak M, Akinci D, Ariyurek M (2009) Non-invasive imaging of aberrant right subclavian artery pathologies and aberrant right vertebral artery. Br J Radiol 82:73–78
Kieffer E, Bahnini A, Koskas F (1994) Aberrant subclavian artery: surgical treatment in thirty-three adult patients. J Vasc Surg 19:100–111
Liechty JD, Shields TW, Anson BJ (1957) Variations pertaining to the aortic arches and their branches. Q. Bull. Northwestern University Medical School 31:136–143
Loukas M, Louis RG, Gaspard J, Fudalej M, Tubbs RS, Merds W (2006) A retrotracheal right subclavian artery in association with a vertebral artery and thyroidea ima. Folia Morphol 65:236–241
Luciano D, Mitchell J, Fraisse A, Lepidi H, Kreitmann B, Ovaert C (2015) Kommerell diverticulum should be removed in children with vascular ring and aberrant left subclavian artery. Ann Thorac Surg 100:2293–2297
McDonald JJ, Anson BJ (1940) Variations in the origin of arteries derived from the aortic arch, in American whites and negroes. Am J Phys Anthrop 27:91–107
Molz G, Burri B (1978) Aberrant subclavian artery (arteria lusoria): sex differences in the prevalence of various forms of the malformation. Evaluation of 1378 observations. Virchows Arch A Pathol Anat Histol 380:303–315
Myers PO, Fasel JH, Kalangos A, Gailloud P (2010) Arteria lusoria: developmental anatomy, clinical, radiological and surgical aspects. Ann Cardiol Angeiol (Paris) 59:147–154
Natsis K, Didagelos M, Manoli SM, Papathanasiou E, Sofidis G, Anastasopoulos N (2011) A bicarotid trunk in association with an aberrant right subclavian artery. Report of two cases, clinical impact, and review of the literature. Folia Morphol (Warsz) 70:68–73
Natsis K, Lazaridis N, Gkiouliava A, Didagelos M, Piagkou M (2015) A retroesophageal right subclavian artery in association with a thyroid ima artery: case report, clinical impact and review of the literature. Folia Morphol (Warsz). doi:10.5603/FM.a2015.0080
Natsis KI, Tsitouridis IA, Didagelos MV, Fillipidis AA, Vlasis KG, Tsikaras PD (2009) Anatomical variations in the branches of the human aortic arch in 633 angiographies: clinical significance and literature review. Surg Radiol Anat 31:319–323
Nayak SR, Pai MM, Prabhu LV, D’Costa S, Shetty P (2006) Anatomical organization of aortic arch variations in the India: embryological basis and review. Jornal Vasc Bras 5:95–100
Pantvaidya GH, Mistry RC, Ghanekar VR, Upasani VV, Pramesh CS (2005) Injury of an aberrant subclavian artery: a rare complication of video assisted thoracoscopic esophagectomy. Ann Thorac Cardiovasc Surg 11:35–37
Polguj M, Chrzanowski Ł, Kasprzak JD, Stefanczyk L, Topol M, Majos A (2014) The aberrant right subclavian artery (arteria lusoria): the morphological and clinical aspects of one of the most important variations—a systematic study of 141 reports. Sci World J 2014:292734. doi:10.1155/2014/292734
Quain R (1844) The anatomy of the arteries of the human body. Taylor and Walton, London, pp 152–155
Rogers AD, Nel M, Eloff EP, Naidoo NG (2011) Dysphagia lusoria: a case of an aberrant right subclavian artery and a bicarotid trunk. ISRN Surg. doi:10.5402/2011/819295
Takahashi Y, Sasaki Y, Kato Y, Motoki M, Bito Y, Morisaki A, Miyabe M, Inno G (2015) Less-invasive endovascular treatment of arch aneurysm with aberrant right subclavian artery. Ann Thorac Surg 100:1089–1091
Tanaka Y, Kitamura T, Horai T, Miyaji K (2016) Two-stage operation for Stanford type A acute aortic dissection originating from Kommerell’s diverticulum. Interact CardioVasc Thorac Surg 22:695–697
Thomson A (1893) Third annual report of the committee of selective investigation of the Anatomical Society of Great Britain and Ireland for the year 1891–1892. J Anat Physiol 27:183–194
Toniato A, Mazzarotto R, Piotto A, Bernante P, Pagetta C, Pelizzo MR (2004) Identification of the nonrecurrent laryngeal nerve during thyroid surgery: 20-year experience. World J Surg 28:659–661
Tsikaras P, Natsis K, Agiabasis A, Gigis P (1996) The clinical significance of the atypical origin and course of the right subclavian artery and its branches. Case report in a female cadaver. Conference proceedings of the 11th Medical Conference of Northern Greece. University Studio Press, vol B, p 596–600
Van Son JA, Mierzwa M, Mohr FW (1999) Resection of atherosclerotic aneurysm at origin of aberrant right subclavian artery. Eur J Cardiothorac Surg 16:576–579
Williams GD, Aff HM, Schmeckebier M, Edmonds HW, Grand EG (1932) Variations in the arrangement of the branches arising from the aortic arch in the American whites and negroes. Anat Rec 54:247–251
Zapata H, Edwards JE, Titus JL (1993) Aberrant right subclavian artery with left aortic arch: associated cardiac anomalies. Pediatr Cardiol 14:159–161
Funding
No funding was received for this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Informed consent
Informed consent was obtained from all body donors included in the study.
Additional information
An erratum to this article is available at https://doi.org/10.1007/s00276-017-1922-z.
Rights and permissions
About this article
Cite this article
Natsis, K., Didagelos, M., Gkiouliava, A. et al. The aberrant right subclavian artery: cadaveric study and literature review. Surg Radiol Anat 39, 559–565 (2017). https://doi.org/10.1007/s00276-016-1796-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00276-016-1796-5