Abstract
Sustainable aquaculture of the Japanese eel Anguilla japonica requires an understanding of the physiological conditions of the fish under culture conditions. Therefore, we examined the effects of tentative stressors such as background color and rearing density on stress-related hormones in juvenile Japanese eel. In Experiment 1, fish were divided into white- or black-coated tanks and reared for 35 days. Plasma cortisol levels were significantly higher in the white-acclimated fish on day 35. No significant differences were observed between the groups in hypothalamic CRH mRNA levels. In Experiment 2, fish were divided into three rearing density groups (0.5 kg/m2, 1.2 kg/m2, and 2.4 kg/m2) and reared for 28 days. Plasma cortisol levels were significantly lower in the low-density-acclimated fish than in the medium- and high-density-acclimated fish. However, no significant differences were observed in hypothalamic CRH mRNA levels. Evaluation of plasma cortisol levels indicates that a white background and high rearing density induce more stress for juvenile Japanese eel.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Corticotropin-releasing hormone (CRH) is a key activator of the hypothalamic-pituitary-interrenal (HPI) axis in fish. Stress stimulates the hypothalamus to release CRH, which in turn promotes the synthesis of pro-opiomelanocortin in the pituitary and its cleavage to adrenocorticotropic hormone (ACTH). ACTH promotes cortisol release from the interrenal tissue, following which cortisol-induced gluconeogenesis results in higher plasma levels of glucose to cope with the stress (Pankhurst 2011). Thus, plasma cortisol levels can be good indicators of stress response.
The Japanese eel Anguilla japonica is one of the most primitive living teleost species and is a commercially important aquaculture fish in many regions of East Asia, including Japan. Japanese eel aquaculture largely depends on the supply of glass eels, although a recently established technique allows artificial production of glass eels from parent fish (Tanaka et al. 2003; Kagawa et al. 2005).
To improve the sustainability of Japanese eel aquaculture, it is necessary to understand the physiological state of the fish under aquaculture conditions. Eels are generally solitary and territorial in the wild, and have high tolerance for numerous environmental stressors such as temperature, hypoxia, hypercapnia, ammonia and pH levels (Wilson, 2014). In a still-water pond aquaculture, eels are reared under stocking densities at 1–5 kg/m2 (Cultured Aquatic Species Information Programme 2005). Although eels have high stress tolerance, high rearing density may function as a stressor and negatively affect their physiology. Indeed, high rearing densities have been reported to negatively affect somatic growth in various fish species such as the turbot Scophthalmus maximus (Irwin et al. 1999), Atlantic halibut Hippoglossus hippoglossus L. (Kristiansen et al. 2004), Solea solea (Palermo et al. 2008; Schram et al. 2006), S. senegalensis (Salas-Leiton et al. 2010), Nile tilapia Oreochromis niloticus L. (Azaza et al. 2013), and zebrafish Danio rerio (Ribas et al. 2017). The effects of high rearing densities on somatic growth and stress-related hormones have not been reported in Japanese eel.
It has been suggested that background color influences stress response in some teleost fish species (Barton 2002). For example, juveniles of the carnivorous freshwater catfish Lophiosilurus alexandri had higher plasma cortisol levels when placed against a black background than against yellow, brown, and blue backgrounds (Costa et al. 2017). Plasma cortisol levels in goldfish Carassius auratus were higher against a red background than against white and black backgrounds (Eslamloo et al. 2015). Since cortisol secretion is regulated by the HPI axis, it can be hypothesized that background color also affects CRH levels in the brain. However, to date there is no information regarding the effect of background color on CRH levels in teleost fish.
We have recently identified the Japanese eel CRH by isolating its cDNA from the fish brain: CRH cDNA encodes a signal peptide, a cryptic peptide, and CRH (41 amino acids). Moreover, we have clarified that some CRH-immunoreactive (IR) fibers originating from the hypothalamus project to ACTH cells in the rostral pars distalis of the pituitary in the Japanese eel (Amano et al. 2014).
The aim of this study was to understand the endocrinological states of the juvenile Japanese eel under stressful conditions. Therefore, we first established a quantitative reverse-transcription polymerase chain reaction (qRT-PCR) protocol for eel CRH. Next, we investigated the effects of tentative stressors such as background color and rearing density on hypothalamic CRH mRNA levels, and plasma levels of cortisol and glucose in the juvenile Japanese eel.
Materials and methods
Experimental fish
Japanese glass eels were commercially purchased from a local commercial supplier (Yoshida, Shizuoka, Japan) in April 2012. The fish were reared in a stock tank (120 L) covered by a polyvinyl chloride plate and water temperature was maintained at 28 ± 1 °C by a thermostat. The fish were fed a commercial eel diet (First, Chubu Shiryo Co. Ltd, Nagoya, Aichi, Japan) for the first 3 weeks and then were fed a commercial eel diet (Royal Feed S, Nosan Corporation, Yokohama, Kanagawa, Japan) six times a week. The experiment was performed following the guidelines of the animal care committee of Kitasato University.
Experiment 1
On May 20, for acclimation, 40 fish (approximate total length (TL) 24.9 cm) were randomly selected from the stock tank and divided among four 60-L glass tanks (30 × 32 × 60 cm, 10 fish/tank). Each tank was placed in a light- and temperature-controlled room, where the fish were reared under controlled light–dark conditions (lights on: 08:00–20:00) at 25 °C. The tanks were continuously supplied with filtered and well-aerated tap water that had been dechlorinated with activated charcoal. Fish were fed a commercial eel diet (Unagimaru SS, Scientific Feed Laboratory Co. Ltd, Tokyo, Japan) once a day at 10:00 to satiety, and the remaining food was recovered at 12:00. On June 23, 28 fish were randomly selected from the 60-L glass tanks and were equally divided among four 60-L glass tanks (30 × 32 × 60 cm, seven fish/tank), which were internally covered with either white (two tanks) or black (two tanks) plastic plates. Shelters were not placed in all the tanks. Fish were reared under the same conditions described above. On the same day, six fish out of the remaining 12 fish were used for initial sampling.
The fish were sampled on June 23 (initial), July 14 (day 21) and July 28 (day 35) between 11:30 and 12:30. Fish were not fed on the day before sampling. No fish died during the experiment. On days 21 and 35, all the fish from one white tank and one black tank were sampled. Fish were anesthetized in 0.33% 2-phenoxyethanol, as plasma cortisol levels of eels are not influenced by this anesthetic (Chiba et al. 2006). Photographs of the whole body were taken to evaluate the brightness of the body. To minimize the effect of handling stress on plasma cortisol levels, blood was promptly collected from the caudal vessels using a heparinized syringe (25 G), and kept on ice for measurement of plasma cortisol levels via a time-resolved fluoroimmunoassay (TR-FIA) (Yamada et al. 2002) and glucose levels. Subsequently, TL and body weight (BW) were measured, and the weight of the collected blood was added to BW. The hypothalamus was dissected out, immediately frozen on dry ice, and stored at −80 °C until RNA extraction. Blood samples were centrifuged at 2500g for 15 min at 4 °C, and the plasma was stored at −35 °C until analysis. The gonads were dissected out to verify the sex. Since all the fish were at an immature stage, both sexes were pooled for measurement and analysis.
Experiment 2
On October 17, 21 fish (mean TL 25.6 cm, mean BW 14.7 g) were randomly selected from the stock tank and divided equally among three semitransparent plastic containers of different sizes, each having small holes to allow the passage of water. The plastic containers were small (28 × 15.5 × 15 cm, 434 cm2, 6.5 L), medium (36 × 24 × 14 cm, 864 cm2, 12.1 L), and large sized (52 × 37 × 20.5 cm, 1924 cm2, 39.4 L). Seven fish were placed in each plastic container. Approximate initial rearing densities were 0.5 kg/m2 (2.6 kg/m3), 1.2 kg/m2 (8.5 kg/m3), and 2.4 kg/m2 (15.8 kg/m3) for low-, medium-, and high-density groups, respectively. These containers were lidded and suspended inside a larger tank (90 × 180 × 60 cm) covered by a polyvinyl chloride plate in order to ensure that rearing conditions in each container (except rearing density) remained similar across groups. Shelters were not placed in all the containers. The water temperature was maintained at 25 °C by a thermostat and the tank was continuously supplied with filtered and well-aerated tap water dechlorinated with activated charcoal. Fish were fed the commercial eel diet (Unagimaru SS) once a day at 10:00 to satiety, and the remaining food was recovered at 12:00. One fish died in the low-density group (large-sized container) during the rearing period. The fish were sampled on November 14 (day 28) using the same sampling procedures as in Experiment 1. Since differences of the body color were not observed among the three density groups, the brightness of the body was not evaluated.
Evaluation of body color brightness
Whole body photographs were converted to TIFF images using Preview 11.0 on Mac OS 11.2.3 (Apple Inc. Cupertino, CA, USA). The images were then analyzed using ImageJ 1.53i (Schneider et al. 2012). A rectangular area was selected from the middle position on the left side of the trunk, with the vertical side from the lateral line to the base of the dorsal fin and the horizontal side being 1 cm. The average gray value of the selected area was determined as the brightness of the body of each individual using the "measure" tool of ImageJ without knowledge of the origin of the sample.
qRT-PCR protocol for the measurement of CRH mRNA levels in eels
Total RNA was extracted from the fish hypothalamus using Isogen (Nippon Gene, Toyama, Japan) and the RNeasy Mini Kit (QIAGEN, Germantown, MD, USA). The RNA was then treated with the TURBO DNA-free kit (Thermo Fisher Scientific, Waltham, MA, USA) for 24 h at 37 °C. After DNase inactivation, the total RNA was stored at −80 °C until analysis.
The partial cDNA clone for CRH was amplified from 5 ng of hypothalamic total RNA using the One Step PrimeScript RT-PCR Kit (Takara, Otsu, Japan). Oligonucleotide primers, 5′-CAACCGTAAGTGGGAGAGGA-3′ (forward) and 5′-GATCAGACTGTGGACCAGGA-3′ (reverse), were designed based on eel CRH mRNA sequence (LC010940.1). All primers for the qRT-PCR were synthesized at Eurofins Genomics GmBH (Ebersberg, Germany). The amplified cDNA fragment of CRH was subcloned into the pGEM-T Easy Vector (Promega, Madison, WI, USA). The nucleotide sequence was confirmed with a Big Dye Terminator v3.1 Cycle Sequencing Kit (Thermo Fisher Scientific) and an ABI 3130xl Genetic Analyzer (Thermo Fisher Scientific). The plasmid clone was digested with Nco I at the 3′-terminal of the inserts and used as a template to synthesize reference RNA. The sense-strand RNA was synthesized according to manufacturer instructions using a MAXIscript SP6 Kit (Thermo Fisher Scientific).
The ABI Prism 7700 Sequence Detection System and the One-Step RT-PCR Kit (QIAGEN) were used to carry out qRT-PCR. A TaqMan probe and primers were designed using Primer Express (Thermo Fisher Scientific). The primer sequences were 5′-CTTCCAGGGACATACTCACT-3′ (forward) and 5′-GAAATTGAGCTTCATGTCAG-3′ (reverse), and that of the probe was 5′-(Fam)-AACCTAACTCAGATTCCTGCTGATCTC-(Tamra)-3′. The probe was synthesized at Merck KGaA (Darmstadt, Germany). The PCR mixture (10 µL) contained the OneStep RT-PCR buffer and enzyme mix, 4 μmol deoxynucleotide mix, 2.5 pmol each of the forward and reverse primers, 2.5 pmol of the probe, and 5 ng of the hypothalamic total RNA. Each preparation was assayed in duplicate. The reaction was started with reverse transcription at 50 °C for 30 min, followed by amplification at 95 °C for 15 min (activation of Taq), 40 cycles at 95 °C for 20 s (denaturation), and 55 °C for 20 s (annealing and extension). Each assay was repeated three times and included quantified RNA standards and a non-template control. The mRNA levels of CRH in each sample were quantified based on amplitude scaled to a tenfold dilution series of five template points of the reference RNA (6 × 102−6 × 106 copies/reaction). Linear regression parameters (R2) were calculated using the ABI Prism 7000 SDS Software (Thermo Fisher Scientific), and results showed that the PCR was reproducible (R2 > 0.99). Dissociation curves and gene expression analyses were performed using the ABI Prism 7000 SDS Software and data were presented as 103 copies/ng total RNA.
Measurement of plasma cortisol and glucose
Plasma cortisol levels were measured by TR-FIA (Yamada et al. 2002). In brief, 10 μL of plasma sample was diluted with 200 μL of the assay buffer (0.05 M Tris, 0.9% NaCl, 0.5% BSA, 0.05% NaN3, 20 µM diethylenetriamine-N,N,N′,N″,N‴-pentaacetic acid, 0.01% Tween-40, pH 7.75). Each sample was extracted twice with 1 mL of diethyl ether. For each extraction, tubes were vigorously mixed for 15 min and the bottom layer (aqueous phase) was frozen at –80 °C for 20 min. The top layer (ether phase) was poured into a new tube and air-dried (40 °C). Then, 200 μL of the assay buffer was added, mixed vigorously, and used for TR-FIA. For TR-FIA, 200 μL of cortisol-BSA conjugate (0.5 μg/mL) was immobilized to the wells of a 96-well microtiter plate (Nunc, Denmark) at 4 °C overnight. After three washes with 0.9% saline, the wells were blocked with 300 μL of 0.1% BSA at room temperature (RT) for 1 h, followed by three washes for immunoassays. Fifty microliters of standard or extracted plasma samples and 150 μL of anti-cortisol serum (× 96,000) were dispensed to the wells. After the immunoreaction at 4 °C overnight and three washes, 200 μL of europium (Eu)-labeled anti-rabbit IgG goat IgG (Eu-IgG) (50 ng/mL) was added to the wells, and the plate was shaken at RT for 1 h. Eu was dissociated from the complex of steroid, primary antibody, and Eu-IgG by addition of 100 μL of enhancement solution, and the intensity of Eu was measured with a time-resolved fluorometer (Infinite F500, Tecan Austria GmbH, Austria). Samples were measured in duplicate.
Plasma glucose levels were measured by the Autokit Glucose (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan), according to the manufacturer’s instructions. Samples were measured in duplicate.
Statistics
In Experiment 1, a Mann–Whitney U test was used to compare groups at each sampling day. Time-course changes in each group with different background colors (Experiment 1) and differences among the three density groups (Experiment 2) were evaluated by a Kruskal-Wallis test followed by a Scheffé test. Data were visualized as box plots.
Results
Experiment 1
TL and BW of the eels are shown in Table 1. There were no significant differences in TL between the groups on both days 21 (Mann–Whitney U test, U = 10.5, p = 0.0717) and 35 (Mann–Whitney U test, U = 15.5, p = 0.2491). Moreover, no significant differences in BW were seen between the groups on both days 21 (Mann–Whitney U test, U = 24, p = 0.9491) and 35 (Mann–Whitney U test, U = 19, p = 0.4822). Brightness of the body was significantly higher in white-acclimated fish [56.1 ± 12.7 (mean ± standard deviation), n = 5] than that of black-acclimated fish (39.2 ± 9.7, n = 7) on day 35 (Mann–Whitney U test, U = 4, p = 0.0284) (Fig. 1).
Hypothalamic CRH mRNA levels (103 copies/ng total RNA) were not significantly different between the groups on both days 21 (Mann–Whitney U test, U = 11, p = 0.0845) and 35 (Mann–Whitney U test, U = 23, p = 0.8478) (Fig. 2). Hypothalamic CRH mRNA levels did not significantly change throughout the experiment in both the white background group (Kruskal-Wallis test, p = 0.0795) and black background group (Kruskal-Wallis test, p = 0.1945).
Box plots of hypothalamic CRH mRNA expression levels (103 copies/ng total RNA) in Experiment 1. Line inside the box indicates median. Lower and upper box boundaries indicate 25th and 75th percentiles, respectively. Lower and upper error lines indicate the minimum value and the maximum value, respectively. Numbers indicate the number of fish employed
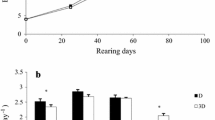
Plasma cortisol levels (ng/mL) were not significantly different between the groups on day 21 (Mann–Whitney U test, U = 17, p = 0.3379) and were significantly higher in the white-acclimated fish than in the black-acclimated fish on day 35 (Mann–Whitney U test, U = 8.5, p = 0.0333) (Fig. 3a). Plasma cortisol levels did not significantly change throughout the experiment in both the white background group (Kruskal-Wallis test, p = 0.4641) and black background group (Kruskal-Wallis test, p = 0.2139).
Box plots of a plasma cortisol levels (ng/mL) and b plasma glucose levels (mg/dL) in Experiment 1. Line inside the box indicates median. Lower and upper box boundaries indicate 25th and 75th percentiles, respectively. Lower and upper error lines indicate the minimum value and the maximum value, respectively. Numbers indicate the number of fish employed. *(p < 0.05) and ††(p < 0.01) indicate the level of statistical difference
Plasma glucose levels (mg/dL) were not significantly different between the groups on day 21 (Mann–Whitney U test, U = 21, p = 0.6547) but were significantly higher in the black-acclimated fish than in the white-acclimated fish on day 35 (Mann–Whitney U test, U = 6, p = 0.0181) (Fig. 3b). Plasma glucose levels did not significantly change throughout the experiment in the white background group (Kruskal-Wallis test, p = 0.0797). In the black background group, compared to the initial levels, plasma glucose levels were significantly higher on day 35 (Scheffé test, p = 0.0023).
Experiment 2
TL and BW of the eels on the sampling day are shown in Table 2. There were no significant differences in TL (Kruskal-Wallis test, p = 0.1659) and BW (Kruskal-Wallis test, p = 0.2105) among the three density groups.
No significant differences were observed in hypothalamic CRH mRNA levels (103 copies/ng total RNA) among the three density groups (Kruskal-Wallis test, p = 0.2823) (Fig. 4).
Box plots of hypothalamic CRH mRNA expression levels (103 copies/ng total RNA) in Experiment 2. Line inside the box indicates median. Lower and upper box boundaries indicate 25th and 75th percentiles, respectively. Lower and upper error lines indicate the minimum value and the maximum value, respectively. Numbers indicate the number of fish employed
Plasma cortisol levels (ng/mL) were significantly lower in the low-density-acclimated fish than in the medium-density-acclimated fish (Scheffé test, p = 0.0486) and high-density-acclimated fish (Scheffé test, p = 0.0152) (Fig. 5a).
Box plots of a plasma cortisol levels (ng/mL) and b plasma glucose levels (mg/dL) in Experiment 2. Line inside the box indicates median. Lower and upper box boundaries indicate 25th and 75th percentiles, respectively. Lower and upper error lines indicate the minimum value and the maximum value, respectively. Numbers indicate the number of fish employed. *(p < 0.05) indicates the level of statistical difference
However, there were no significant differences in plasma glucose levels (mg/dL) among the three density groups (Kruskal-Wallis test, p = 0.6552) (Fig. 5b).
Discussion
In this study, we first developed a qRT-PCR protocol for the measurement of eel CRH mRNA. Results and validation show that this protocol is highly sensitive and reproducible, and can be used to detect eel CRH mRNA.
In Experiment 1, plasma cortisol levels were significantly higher in the white-acclimated fish on day 35, indicating that rearing under white background conditions stimulates cortisol secretion. Considering that hypothalamic CRH promotes ACTH secretion and then ACTH stimulates cortisol release from the interrenal tissue (Pankhurst, 2011), it is expected that CRH levels also increase under white background conditions. However, hypothalamic CRH mRNA levels were not significantly different between the groups, although those in the white-acclimated fish tended to be higher than those in the black-acclimated fish on day 21 (Mann–Whitney U test, U = 11, p = 0.0845). Thus, more precise studies are needed to clarify that rearing under white background conditions stimulate hypothalamic CRH mRNA synthesis, followed by ACTH secretion and a rise in plasma cortisol levels.
Judging by the levels of plasma cortisol, juvenile eels may experience higher stress in white background conditions than against a black background. Our preliminary experiment suggested that glass eels and juvenile eels prefer a black background over a white background when, during a light phase, they were reared in a tank where the bottom surface was colored black and white (data not shown). More precise self-referent color preference tests are required to clarify this, as reported in other teleost fish such as juvenile turbot (Li et al. 2016). Furthermore, it is well known that eels in the wild are solitary and territorial (Wilson 2014), live in shallow ocean waters, burrow into sand, mud, or amongst rocks, and hide in shelter during a light phase under rearing conditions (Dou and Tsukamoto 2003). These behaviors of eels in their proper ecological context may help explain the present results.
In contrast to cortisol, plasma glucose levels were significantly lower in the white-acclimated fish than in the black-acclimated fish on day 35. In general, energy metabolism increases in fish subjected to stress, with glucose being the main energy resource (Wendelaar Bonga 1997; Fabbri et al. 1998). Thus, it is possible that prolonged rearing stress in white background resulted in a sustained consumption of energy resources.
The relationship between cortisol and melanin-concentrating hormone (MCH), which pales skin color by concentrating melanin granules, has been reported in rainbow trout Oncorhynchus mykiss (Baker 1994; Green et al. 1991). In this species, increased MCH levels in the pituitary of white-acclimated fish decreased cortisol levels indirectly by depressing CRH release and therefore, its downstream processes (Baker 1994). In the present study, the body color of white-acclimated juvenile eels was significantly brighter than that of black-acclimated fish. Thus, it is possible that pituitary MCH levels were higher in white-acclimated eel, as reported in the rainbow trout (Green et al. 1991). However, plasma cortisol levels were not lower in the white-acclimated eel in this study. Although this difference may be on a specific level, it is interesting to examine the reciprocal relationships of CRH and MCH levels in the brain of teleost fish, including eels.
In Experiment 2, plasma cortisol levels were significantly higher in the high- and medium-density-acclimated fish than in the low-density-acclimated fish, possibly as a result of the stressful conditions of high rearing density. Similar results have been reported from rearing density experiments in the Senegalese sole (Costas et al. 2008; Wunderink et al. 2011) and the sole (Palermo et al. 2008). However, results regarding CRH mRNA levels were inconsistent; no significant differences were observed in hypothalamic CRH mRNA levels among three density groups in this study, whereas both brain CRH mRNA levels and plasma cortisol levels were higher in the high-density grouped fish in the study on Senegalese sole (Wunderink et al. 2011). As for plasma glucose levels, no significant differences were observed among different rearing density groups both in the Japanese eel (present study) and the Senegalese sole (Wunderink et al. 2011). The different patterns of changes in CRH mRNA levels against rearing density between the two fish species may also be a due to species-level differences. Therefore, it is essential to examine the effects of rearing density on CRH mRNA levels in various teleost fish species in order to obtain a general understanding. The negative feedback of cortisol on CRH mRNA levels should also be considered.
Conflicting results have been reported regarding the relationship between rearing density and somatic growth in teleost fish. It has been reported that high rearing densities negatively affect somatic growth in various fish species (Irwin et al. 1999; Kristiansen et al. 2004; Palermo et al. 2008; Schram et al. 2006; Salas-Leiton et al. 2010; Azaza et al. 2013; Ribas et al. 2017). These negative effects of high rearing densities have been ascribed to increased stress. Indeed, high plasma cortisol levels have been reported in high rearing density groups of the sole (Palermo et al. 2008) and the Senegalese sole (Salas-Leiton et al. 2010). In contrast, high rearing density has a positive effect on feeding activity and growth rates in Arctic char Salvelinus alpinus, possibly because agonistic behavior decreases and shoaling behavior increases under high-density conditions in this species (Wallace et al. 1988; Brown et al. 1992). It should be noted that these results depend on various conditions, e.g., the amount of food delivered, rearing density, and rearing period. In the Japanese eel (present study), there were no significant differences in final TL and BW among the three density groups, possibly because of a short rearing period. Although the relationship between rearing density and somatic growth in teleost fish is complicated, it would be interesting to examine the relationship between rearing density/period and CRH/cortisol levels in future studies.
In summary, our evaluation of plasma cortisol levels indicates that rearing against a white background and in high-density conditions is stressful for the juvenile Japanese eel. Since no significant differences were observed in hypothalamic CRH mRNA levels, more precise study is required to clarify the relationship between stress and the HPI axis in teleost fish.
References
Amano M, Mizusawa N, Okubo K, Amiya N, Mizusawa K, Chiba H, Yamamoto N, Takahashi A (2014) Cloning of corticotropin-releasing hormone (CRH) precursor cDNA and immunohistochemical detection of CRH peptide in the brain of the Japanese eel paying special attention to gonadotropin-releasing hormone. Cell Tissue Res 356:243–251
Azaza MS, Assad A, Maghrbi W, El-Cafsi M (2013) The effects of rearing density on growth, size heterogeneity and inter-individual variation of feed intake in monosex male Nile tilapia Oreochromis niloticus L. Animal 7:1865–1874
Baker BI (1994) Melanin-concentrating hormone updated functional considerations. Trends Endocrinol Metab 5:120–126
Barton BA (2002) Stress in fishes: a diversity of responses with particular reference to changes in circulating corticosteroids. Integ Comp Biol 42:517–525
Brown GE, Brown JA, Srivastava RK (1992) The effect of stocking density on the behaviour of Arctic charr (Salvelinus alpinus L.). J Fish Biol 41:955–963
Chiba H, Hattori T, Yamada H, Iwata M (2006) Comparison of the effects of chemical anesthesia and electroanesthesia on plasma cortisol levels in the Japanese eel Anguilla japonica. Fish Sci 72:693–695
Costa DC, Mattioli CC, Silva WS, Takata R, Leme FOP, Oliveira AL, Luz RK (2017) The effect of environmental colour on the growth, metabolism, physiology and skin pigmentation of the carnivorous freshwater catfish Lophiosilurus alexandri. J Fish Biol 90:922–935
Costas B, Aragāo C, Mancera JM, Dinis MT, Conceiҫāo LEC (2008) High stocking density induces crowding stress and affects amino acid metabolism in Senegalese sole Solea senegalensis (Kaup 1858) juveniles. Aquacult Res 39:1–9
Cultured Aquatic Species Information Programme (2005) Anguilla japonica (Temmink & Schlegel, 1847). In: FAO Fisheries Division (on line). Rome, Italy. Updated 13 January 2005. www.fao.org/fishery/culturedspecies/Anguilla_japonica/en
Dou S-Z, Tsukamoto K (2003) Observations on the nocturnal activity and feeding behavior of Anguilla japonica glass eels under laboratory conditions. Environ Biol Fishes 67:389–395
Eslamloo K, Akhavan SR, Eslamifar A, Henry MA (2015) Effects of background colour on growth performance, skin pigmentation, physiological condition and innate immune responses of goldfish, Carassius auratus. Aqua Res 46:202–215
Fabbri E, Capuzzo A, Moon TW (1998) The role of circulating catecholamines in the regulation of fish metabolism: an overview. Comp Biochem Physiol C 120:177–192
Green JA, Baker BI, Kawauchi H (1991) The effect of rearing rainbow trout on black or white backgrounds on their secretion of melanin-concentrating hormones and their sensitivity to stress. J Endocrinol 128:267–274
Irwin S, O’Halloran J, FitzGerald RD (1999) Stocking density, growth and growth variation in juvenile turbot, Scophthalmus maximus (Rafinesque). Aquaculture 178:77–88
Kagawa H, Tanaka H, Ohta H, Unuma T, Nomura K (2005) The first success of glass eel production in the world: basic biology on fish reproduction advances new applied technology in aquaculture. Fish Physiol Biochem 31:193–199
Kristiansen TS, Fernö A, Holm JC, Privitera L, Bakke S, Fosseidengen JE (2004) Swimming behaviour as an indicator of low growth rate and impaired welfare in Atlantic halibut (Hippoglossus hippoglossus L.) reared at three stocking densities. Aquaculture 230:137–151
Li X, Chi L, Tian H, Meng L, Zheng J, Gao X, Liu Y (2016) Colour preferences of juvenile turbot (Scophthalamus maximus). Physiol Behav 156:64–70
Palermo F, Nabissi M, Cardinaletti G, Tibaldi E, Mosconi G, Polzonetti-Magni AM (2008) Cloning of sole proopiomelanocortin (POMC) cDNA and the effects of stocking density on POMC mRNA and growth rate in sole, Solea solea. Gen Comp Endocrinol 155:227–233
Pankhurst NW (2011) The endocrinology of stress in fish: an environmental perspective. Gen Comp Endocrinol 170:265–275
Ribas L, Valdivieso A, Díaz N, Piferrer F (2017) Appropriate rearing density in domesticated zebrafish to avoid masculinization: links with the stress response. J Exp Biol 220:1056–1064
Salas-Leiton E, Anguis V, Martín-Antonio B, Crespo D, Planas JV, Infante C, Cañavate JP, Manchado M (2010) Effects of stocking density and feed ration on growth and gene expression in the Senegalese sole (Solea senegalensis): potential effects on the immune response. Fish Shellfish Immunol 28:296–302
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675
Schram E, van der Heul JW, Kamstra A, Verdegem MCJ (2006) Stocking density-dependent growth of Dover sole (Solea solea). Aquaculture 252:339–347
Tanaka H, Kagawa H, Ohta H, Unuma T, Nomura K (2003) The first production of glass eel in captivity: fish reproductive physiology facilitates great progress in aquaculture. Fish Physiol Biochem 28:493–497
Wallace JC, Kolbeinshavn AG, Reinsnes TG (1988) The effects of stocking density on early growth in Arctic charr, Salvelinus alpinus (L.). Aquaculture 73:101–110
Wendelaar Bonga SE (1997) The stress response in fish. Physiol Rev 77:591–625
Wilson JM (2014) Stress physiology. In: Trischitta F, Takei Y, Sébert P (eds) Eel physiology. CRC Press, Boca Roton, Florida, USA, pp 318–358
Wunderink YS, Engels S, Halm S, Yúfera M, Martínez-Rodríguez G, Flik G, Klaren PHM, Mancera JM (2011) Chronic and acute stress responses in Senegalese sole (Solea senegalensis): the involvement of cortisol, CRH and CRH-BP. Gen Comp Endocrinol 171:203–210
Yamada H, Satoh R, Ogoh M, Takaji K, Fujimoto Y, Hakuba T, Chiba H, Kambegawa A, Iwata M (2002) Circadian changes in serum concentrations of steroids in Japanese char Salvelinus leucomaenis at the stage of final maturation. Zool Sci 19:891–898
Acknowledgements
This study was supported in part by JSPS KAKENHI Grant number JP23380115 to M. Amano, N. Amiya, K. Mizusawa and H. Chiba. We thank Mr. Masahiro Sugimoto and Mr. Hikaru Tsuburaya, School of Marine Biosciences, Kitasato University, for assisting in this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Amano, M., Amiya, N., Mizusawa, K. et al. Effects of background color and rearing density on stress-related hormones in the juvenile Japanese eel Anguilla japonica. Fish Sci 87, 521–528 (2021). https://doi.org/10.1007/s12562-021-01527-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-021-01527-4