Abstract
Sharpsnout seabream Diplodus puntazzo has been successfully introduced in Mediterranean aquaculture for reasons of species diversification. However, there is limited information related to specific rearing conditions that the species may require for production optimization in land-based aquaculture. The aim of the present study was to investigate the combined effects of rearing density and background colour on growth performance of sharpsnout sea bream D. puntazzo reared in a recirculating water system. Juveniles (4.0 ± 0.03 g) were reared (duplicated groups) under low (D 16 fish tank−1 or 1 kg m−3) and high (3D 48 fish tank−1 or 3 kg m−3) density in white, light blue and black tanks for 88 days. During the first 53 days of rearing, fish under low density presented better growth than fish under high density. However, in the following period (days 53–88) SGR in 3D-reared fish was significantly higher than in D-reared fish, resulting in no mass differences on day 88. Regardless of rearing density effects, black tanks had a negative effect on growth and should be avoided in sharpsnout sea bream rearing. Within the life stage studied, present growth results, along with those of fish groups homogeneity (coefficient of mass variation, frequency distribution of mass), indicate a change in social interactions according to body size. It is suggested that juvenile sharpsnout seabream should be stocked at low density until they reach approximately 16 g. Afterwards, a higher density seems more appropriate for this species while it also ensures a higher productivity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Since 1990s, sharpsnout seabream Diplodus puntazzo has been introduced in Mediterranean aquaculture for reasons of species diversification. This marine species is appreciated by consumers for its nutritional attributes, as well as by producers for its production characteristics and high sales price. Moreover, the species adapted well to rearing techniques and formulated feeds used for gilthead seabream Sparus aurata. Today, it is the third (after gilthead seabream and European seabass Dicentrarchus labrax) most important marine species produced in Mediterranean countries, mainly Italy, Greece and Spain (Basurco et al. 2011). Continuing research has helped to improve many of the key production aspects, e.g. artificial reproduction and larval rearing (Lahnsteiner and Patarnello 2004; Papandroulakis et al. 2004), intensive rearing in cages, nutritional requirements (Hernández et al. 2001; Atienza et al. 2004; Tramati et al. 2005) and pathology (Katharios et al. 2011; Alama-Bermejo et al. 2013).
Sharpsnout seabream is currently reared in cages and reaches commercial size of approximately 400 g within 15–18 months (Basurco et al. 2011). It has, however, been suggested that the species may perform better in land-based tanks since it also feeds on the tank bottom, thus taking advantage of food that might be lost through cage nets (Sarà et al. 1999; Orban et al. 2000; Favaloro et al. 2002; Karakatsouli et al. 2006). Nevertheless, there is limited information related to specific rearing conditions that the species may require for production optimization in land-based aquaculture (Abellan and Basurco 1999).
A basic issue that must be first confronted when stocking a specific species of a certain life stage for aquaculture reasons is how many fish should be put together in a specific space and, in the case of tanks, what colour should the tanks have. Deciding the appropriate density requires that good water quality is maintained and that species-specific needs for space and presence of conspecifics are adequately met. However, this decision should not ignore that density is a dynamic rearing factor since it continuously changes as fish grow (Ellis et al. 2001, 2002). On the other hand, tank colour should be chosen so that it does not distress the fish while it facilitates everyday practices such as feeding, detection of dead fish and cleaning (Karakatsouli et al. 2007; Luchiari and Pirhonen 2008). Both factors are crucial for effective fish rearing in tanks, influencing many aspects of fish performance and welfare, e.g. growth, physiological status, behaviour (e.g. Höglund et al. 2002; Karakatsouli et al. 2007, 2010; Batzina et al. 2014). The aim of the present study was to investigate the effects of density and tank colour on growth performance and several biochemical parameters of juvenile sharpsnout seabream reared in a recirculating water system.
Materials and methods
Sharpsnout seabream D. puntazzo juveniles were offered by a commercial Greek hatchery and acclimated to laboratory conditions for approximately 30 days. Three hundred and eighty-four fish of mean initial body mass (±SEM) 4.04 ± 0.03 g were randomly distributed in six duplicated treatments according to a 3 × 2 factorial design. Fish were reared in white (W), light blue (LB) and black (B) rectangular fibreglass tanks (height 31 cm, length 42 cm, width 48 cm, water volume 62.5 L) at two rearing densities, 16 (D) and 48 (3D) fish per tank (the equivalent of 256 and 768 fish m−3 or 1.04 and 3.08 kg m−3, respectively). All 12 fish groups were homogeneous with initial coefficient of mass variation (±SEM) 16.3 ± 0.2 % (P > 0.05). Fish were maintained to experimental conditions for 88 days. Fish were fed by hand a commercial pelleted diet for gilthead seabream (moisture 5.9 %; crude protein 58.8 %; crude lipid 20.0 %; ash 10.5 %; nitrogen-free extract + crude fibre 4.8 %) four times daily from Monday to Friday (equal meals on 8:30, 10:30, 12:30 and 14:30), thrice on Saturday (equal meals on 8:30, 10:30 and 12:30), while no food was given on Sunday. During the first 63 days of the experiment, feeding level was set at 4 % of body mass, which was thereafter reduced to 3 %. In all treatments, each meal was consumed within 5 min. All fish groups were individually weighed every 2 weeks, and food quantity was adjusted according to fish biomass. Mortality was recorded daily, and dead fish were not replaced. Direct observations on fish behaviour were performed during daily regular group monitoring, feeding and water measurements.
Experimental tanks were part of an indoor recirculating seawater system, provided with mechanical and biological filters, UV sterilization, compressed air supply and cooling water apparatus. Water flow was 1.2 L min−1 (complete water turnover 19.5 times day−1), and all tanks were thoroughly cleaned once per week. Water quality (dissolved oxygen, pH, total ammonia nitrogen, un-ionized ammonia nitrogen and nitrite nitrogen) of each tank was monitored twice daily (before the first meal and 30 min after the last meal). Water temperature and salinity were maintained at 23.5 ± 0.05 °C and 34.1 ± 0.04 g kg−1 throughout the experiment. Tanks were illuminated by room ambient light (12L–12D, approximately 100 L× at water surface, fluorescence lamps).
At the end of the experimental period, all fish were killed by a lethal dose of 2-phenoxyethanol within their tanks and subjected to individual weighing and total length measurement. Afterwards, liver was removed from each fish and used for the determination of water content and total lipids (Folch et al. 1957), as well as for the calculation of hepatosomatic index [HSI = (liver mass, g) × 100 × (body mass, g)−1]. All fish of each group were minced (without viscera) and lyophilized for carcass proximate composition determination, namely crude protein and lipid content according to Kjeldahl and Soxhlet methods, respectively (AOAC 1984), water (105 °C for 24 h) and ash (550 °C for 12 h) content.
Specific growth rate [SGR = (lnM fn−lnM in) × 100 × t −1, M fn: mean final body mass (g), M in: mean initial body mass (g), t: days of rearing], mass gain [MG = 100 × (M fn− M in)/M in], food conversion ratio [FCR = (food consumed, g) × (mass gain, g) −1] and coefficient of mass variation [CV = (100 × SD) × (mean body mass)−1] were calculated for the whole fish group in each tank. To allow for slight differences in initial CV, relative change in CVs was also estimated [CV change, % = (CVfn−CVin) × 100 × CV −1in , CVfn: final CV, CVin: initial CV]. Condition factor [CF = 100 × (body mass, g) × (total length, cm)−3] was calculated for each fish.
Data were analysed by two-way analysis of variance (ANOVA, general linear model) with tank as a random factor nested within treatments to account for tank effects. All data were analysed using tank as the experimental unit. In the case of SGR data, body mass was used as a covariate. Where P values were significant (P < 0.05), multiple comparisons were carried out using the Duncan test. Wherever necessary, data were transformed (logarithm, square root or arcsin) in order to obtain normal distribution and/or homogeneity of variance. All values presented in the text and tables are untransformed mean ± SEM.
Results
Fish body colour was adapted to background colour within 2–3 days. Fish of white tanks became very pale, and transverse stripes were hardly distinguishable. The same occurred in fish of black tanks, although in this case it was due to darkening of body colouration. Fish reared in light blue tanks retained their normal body colour with distinct transverse stripes.
During feeding, fish in white and light blue tanks responded immediately to the presence of food particles, usually by approaching water surface and being eager to feed. Fish of black tanks were sluggish and preferred to feed after food pellets reached tank bottom. No differences in feeding behaviour were observed due to rearing density. However, fish in D were dispersed into their tanks and fish in 3D showed distinct shoaling behaviour no matter tank colour. Aggressive behaviour (mainly chasing and biting) was also observed, but a specific pattern according to treatments could not be distinguished.
The higher density used (3D) led to significantly lower dissolved oxygen and pH values, as well as to higher levels of total ammonia nitrogen (Table 1). No significant differences were detected for un-ionized ammonia nitrogen and nitrite nitrogen (Table 1). None of the above parameters were affected by tank colour nor was there any interaction detected between factors.
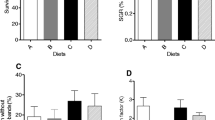
During the experimental rearing, both tank colour and fish density significantly affected D. puntazzo growth, while no interaction was found between the two factors examined (Table 2). Rearing in black tanks resulted in lower final body mass, total length, SGR, MG and CF, while fish growth in white and light blue tanks was equally better (Table 2). Significant differences between white and black tanks were obvious after 25 days and between light blue and black tanks after 53 days of rearing. Fish body mass was significantly higher in D groups after 25 and up to 53 days of rearing (Fig. 1). Thereafter, no differences were detected between D and 3D fish (Fig. 1; Table 2). SGR was higher in D fish for the period 0–25 days (Fig. 1). For the next month (25–39 and 39–53 days), SGR was similar between D and 3D groups, while for the last month (53–67 and 67–88 days) SGR values were inversed and fish reared in 3D grew faster than fish in D conditions (Fig. 1). During the experiment, similar differences were observed for MG and FCR (data not presented). When SGR, MG and FCR are calculated for the total experimental period (0–88 days), no density effects are obvious (Table 2). Besides, final CF was higher in fish at 3D (Table 2).
A significant interaction effect was detected for CV, which was better manifested when CV change (%) was evaluated (Table 2). Tank colour affected CV only when fish were reared at D, white and black tanks resulting in the most and the least homogeneous groups, respectively. Such an effect was not observed for fish in 3D. Or else, rearing at 3D increased size heterogeneity in white tanks, ameliorated it in black tanks and did not differentiate it in light blue tanks (Table 2). The above-mentioned results were also supported by mass frequency distribution data (Fig. 2). For example, fish in white tanks at D condition presented the greater percentage of higher body mass (weight classes 4 and 5; Fig. 2) compared with fish groups of other experimental treatments.
Size frequency distribution for final mass (relative frequency, %) of D. puntazzo reared in white (W), light blue (LB) and black (B) tanks under two different densities (D, 3D) for 88 days. K–Sdis, the goodness of fit to a normally distributed population (Kolmogorov–Smirnov distance). Data on mean, median, skewness and kurtosis for each treatment are provided. Weight classes are as follows: 1 10.0–17.1 g, 2 17.1–24.3 g, 3 24.3–31.4 g, 4 31.4–38.6 g, 5 38.6–45.7 g, 6 45.7–52.9 g, 7 52.9–60.0 g
Cumulative mortality upon completion of experimental period was not differentiated between treatments (Table 2) and was rather sporadic during the experiment (0–4 dead fish per tank). Mortality was firstly observed at the third rearing week and stabilized after the seventh week. Dead fish were not cannibalized although some injuries were seen on the body.
Carcass protein content was higher in groups in 3D and those reared in white and light blue tanks (Table 2). The two experimental factors acted independently, while no other effects were observed on carcass composition. Regarding liver parameters, HSI was not affected by density, while fish in light blue tanks had higher HSI (Table 2). Moreover, rearing at 3D resulted in lower liver water and higher liver total lipid content (Table 2).
Discussion
Present results showed that rearing of sharpsnout seabream juveniles in black tanks resulted in lower growth performance compared with rearing in white or light blue tanks. Similar results have been previously reported for juveniles of another Sparidae species, white seabream Diplodus sargus (Karakatsouli et al. 2007), as well as for other freshwater and marine species (Papoutsoglou et al. 2005; Yamanome et al. 2005; Pérez Sirkin et al. 2012). In previous studies, it has been suggested that reduced growth may be mainly related to lower food intake due to poor contrast of food particles against the dark background. The observation that fish in black tanks showed a certain latency to reach the food may indeed indicate some difficulty in distinguishing food particles. However, once food reached the bottom, it was entirely consumed by the fish and remaining food was never observed. Besides, food utilization was similar among treatments. Consequently, differences in growth are probably not related to food intake or food efficiency.
From another point of view, fish reared in the white background may be those that grew better due to the possible involvement of melanin-concentrating hormone (MCH). Although MCH levels were not measured in this study, MCH has been related to the regulation of the synthesis and release of growth hormone in fish (Pérez Sirkin et al. 2012). Moreover, it has been widely reported that its concentration in plasma and several brain areas or MCH gene expression increases when fish are adapted to a white background (e.g. Green et al. 1991; Amiya et al. 2005; Papoutsoglou 2012).
Present density effects on sharpsnout seabream growth showed that the low initial density used favoured fish growth for up to 53 days of rearing. By that time, fish had reached a body mass of 14–16 g. However, thereafter mass differences between D- and 3D-reared fish were reduced mainly because growth at the higher density was accelerated, as the higher SGR values indicate. These results strongly suggest that fish of 4 g should be reared at a density of 1.04 kg m−3 that could be changed to 3.08 kg m−3 after achieving a body mass of 14–16 g. Similarly, Tremblay-Bourgeois et al. (2010) reported a lower optimal density (<40 kg m−3) for smaller size (50–100 g) spotted wolffish Anarhichas minor and a higher optimal density (>40 kg m−3) for larger fish (100–150 g). Additionally, aquaculture practice for the widely reared Sparidae gilthead seabream S. aurata also uses lower densities (5–10 kg m−3) for younger (2.5–150 g) fish and higher densities (10–20 kg m−3) for older (>150 g) fish (EFSA 2008). These data, along with those obtained in the present study, support the findings of van de Nieuwegiessen et al. (2009) that the effects of density are not uniform throughout the growth cycle.
Generally, density effects in farmed fish have been related to alterations in water quality and fish social behaviour (Ellis et al. 2002). In the present study, although water DO and pH were slightly lower in 3D, levels reached were within the recommended limits for intensive rearing of sharpsnout seabream or other Sparidae species (Souto and Villanueva 2003; Poli 2009). Moreover, total ammonia levels were higher in 3D, but un-ionized ammonia and nitrite levels were similar between density treatments. Consequently, present density effects do not seem to be related to water quality. Instead, differences in fish social behaviour are more likely to be involved, especially considering present behavioural observations and results of CV for final mass. Similar to present results, shoaling or schooling behaviour has been previously reported for several fish species at increasing densities and may be related to limitation of available swimming space in combination with reduced energetic costs (Jørgensen et al. 1993; Hensor et al. 2005; Uglem et al. 2009). CV has been suggested as an indirect indicator of social environment within a fish group, with lower values indicating milder social interactions (Jobling 1995; North et al. 2006). Thus, D density (as compared to 3D) is indicated as an environment of higher social stress when sharpsnout seabream is reared in black tanks, but rearing in white tanks rendered the D density an environment of milder social stress. On the other hand, the social environment established in fish reared in light blue tanks was not affected by the density levels examined.
Differences observed for carcass and liver chemical composition probably reflect the metabolic adaptation to rearing conditions imposed. For example, tank colour effects on carcass protein content are in parallel with colour effects on growth, indicating that rearing in black tanks involves higher metabolic cost. Similar results have been previously reported for rainbow trout Oncorhynchus mykiss (Papoutsoglou et al. 2005). Furthermore, density effects in liver moisture and total lipids also reflect density effects on growth. The lower liver lipids in fish reared in D may suggest an increase in energy demand and supports the indicated negative effect of the D density on growth during the last 35 rearing days.
In conclusion, present results indicate that black tanks are not recommended for the intensive rearing of sharpsnout seabream since growth performance was clearly compromised. Fish rearing in white and light blue tanks presented similar benefits for growth. Despite an advantage of white tanks towards more homogeneous fish groups, light blue tanks favour natural body colouration, which may be a more attractive trait from the consumer point of view. Besides, present density effects clearly suggest that juvenile sharpsnout seabream should be stocked at low density until they reach approximately 16 g. Afterwards, a higher density seems more appropriate for this species while it also ensures a higher productivity.
References
Abellan E, Basurco B (1999) Finfish species diversification in the context of Mediterranean marine fish farming development. In: Abellan E, Basurco B (eds) Marine finfish diversification: current situation and prospects in Mediterranean aquaculture. Options Mediterranéennes CIHEAM, No. 24, Zaragoza, Spain
Alama-Bermejo G, Šíma R, Raga JA, Holzer AS (2013) Understanding myxozoan infection dynamics in the sea: seasonality and transmission of Ceratomyxa puntazzo. Int J Parasitol 43:771–780
Amiya N, Amano M, Takahashi A, Yamanome T, Kawauchi H, Yamamori K (2005) Effects of tank color on melanin-concentrating hormone levels in the brain, pituitary gland, and plasma of the barfin flounder as revealed by a newly developed time-resolved fluoroimmunoassay. Gen Comp Endocrinol 143:251–256
AOAC (1984) Official methods of analysis, 14th edn. Association of Official Analytical Chemists, Inc., Arlington
Atienza MT, Chatzifotis S, Divanach P (2004) Macronutrient selection by sharp snout seabream (Diplodus puntazzo). Aquaculture 232:481–491
Basurco B, Lovatelli A, García B (2011) Current status of Sparidae aquaculture. In: Pavlidis M, Mylonas C (eds) Sparidae: biology and aquaculture of gilthead sea bream and other species. Blackwell, Oxford
Batzina A, Dalla C, Papadopoulou-Daifoti Z, Karakatsouli N (2014) Effects of environmental enrichment on growth, aggressive behaviour and brain monoamines of gilthead seabream Sparus aurata reared under different social conditions. Comp Biochem Physiol 169A:25–32
EFSA (2008) Scientific report of EFSA prepared by working group on seabass/seabream welfare on animal welfare aspects of husbandry systems for farmed european seabass and gilthead seabream. EFSA J Annex I 844:1–89
Ellis T, Scott S, Bromage N, North B, Porter M (2001) What is stocking density? Trout News 32:35–37
Ellis T, North B, Scott AP, Bromage NR, Porter M, Gadd D (2002) The relationships between stocking density and welfare in farmed rainbow trout. J Fish Biol 61:493–531
Favaloro E, Lopiano L, Mazzola A (2002) Rearing of sharpsnout seabream (Diplodus puntazzo, Cetti 1777) in a Mediterranean fish farm: monoculture versus polyculture. Aquac Res 33:137–140
Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509
Green JA, Baker BI, Kawauchi H (1991) The effect of rearing rainbow trout on black or white backgrounds on their secretion of melanin-concentrating hormone and their sensitivity to stress. J Endocrinol 128:261–274
Hensor E, Couzin ID, James R, Krause J (2005) Modelling density-dependent fish shoal distributions in the laboratory and field. Oikos 110:344–352
Hernández MD, Egea MA, Rueda FM, Aguado F, Martínezb FJ, García B (2001) Effects of commercial diets with different P/E ratios on sharpsnout seabream (Diplodus puntazzo) growth and nutrient utilization. Aquaculture 195:321–329
Höglund E, Balm PHM, Winberg S (2002) Behavioural and neuroendocrine effects of environmental background colour and social interaction in Arctic charr (Salvelinus alpinus). J Exp Biol 205:2535–2543
Jobling M (1995) Simple indices for the assessment of the influences of social environment on growth performance, exemplified by studies on Arctic charr. Aquac Int 3:60–65
Jørgensen EV, Christiansen JS, Jobling M (1993) Effects of stocking density on food intake, growth performance and oxygen consumption in Arctic charr (Salvelinus alpinus). Aquaculture 110:191–204
Karakatsouli N, Papafotiou P, Papoutsoglou SE (2006) Mono- and duoculture of juvenile sharpsnout seabream Diplodus puntazzo (Cetti) and gilthead seabream Sparus aurata L. in a recirculated water system. Aquac Res 37:1654–1661
Karakatsouli N, Papoutsoglou SE, Manolessos G (2007) Combined effects of rearing density and tank colour on the growth and welfare of juvenile white sea bream Diplodus sargus L. in a recirculating water system. Aquac Res 38:1152–1160
Karakatsouli N, Papoutsoglou ES, Sotiropoulos N, Mourtikas D, Stigen-Martinsen T, Papoutsoglou SE (2010) Effects of light spectrum, rearing density and light intensity on growth performance of scaled and mirror common carp Cyprinus carpio reared under recirculating system conditions. Aquac Eng 42:121–127
Katharios P, Papadaki M, Ternengo S, Kantham PK, Zeri C, Petraki PE, Divanach P (2011) Chronic ulcerative dermatopathy in cultured marine fishes. Comparative study in sharpsnout sea bream, Diplodus puntazzo (Walbaum). J Fish Dis 34:459–474
Lahnsteiner F, Patarnello P (2004) Biochemical egg quality determination in the gilthead seabream, Sparus aurata: reproducibility of the method and its application for sharpsnout seabream, Puntazzo puntazzo. Aquaculture 237:433–442
Luchiari AC, Pirhonen J (2008) Effects of ambient colour on colour preference and growth of juvenile rainbow trout Oncorhynchus mykiss (Walbaum). J Fish Biol 72:1504–1514
North BP, Turnbull JF, Ellis T, Porter MJ, Migaud H, Bron J, Bromage NR (2006) The impact of stocking density on the welfare of rainbow trout (Oncorhynchus mykiss). Aquaculture 255:466–479
Orban E, Di Lena G, Ricelli A, Paoletti F, Casini I, Gambelli L, Caproni R (2000) Quality characteristics of sharpsnout sea bream (Diplodus puntazzo) from different intensive rearing systems. Food Chem 70:27–32
Papandroulakis N, Kentouri M, Maingot E, Divanach P (2004) Mesocosm: a reliable technology for larval rearing of Diplodus puntazzo and Diplodus sargus sargus. Aquac Int 12:345–355
Papoutsoglou SE (ed) (2012) Textbook of fish endocrinology. Nova Science, New York
Papoutsoglou SE, Karakatsouli N, Chiras G (2005) Dietary l-tryptophan and tank colour effects on growth performance of rainbow trout (Oncorhynchus mykiss) juveniles reared in a recirculating water system. Aquac Eng 32:277–284
Pérez Sirkin DI, Cánepa MM, Fossati M, Fernandino JI, Delgadin T, Canosa LF, Somoza GM, Vissio PG (2012) Melanin concentrating hormone (MCH) is involved in the regulation of growth hormone in Cichlasoma dimerus (Cichlidae, Teleostei). Gen Comp Endocrinal 176:102–111
Poli BM (2009) Farmed fish welfare-suffering assessment and impact on product quality. Ital J Anim Sci 8:137–160
Sarà M, Favaloro E, Mazzola A (1999) Comparative morphometrics of sharpsnout seabream (Diplodus puntazzo Cetti, 1777), reared in different conditions. Aquac Eng 19:195–209
Souto FB, Villanueva XLR (2003) European fish Farming guide. Instituto Galego de Formacion en Aquacultura (IGAFA), Pontevedra
Tramati C, Savona B, Mazzola A (2005) A study of the pattern of digestive enzymes in Diplodus puntazzo (Cetti, 1777) (Osteichthyes, Sparidae): evidence for the definition of nutritional protocols. Aquac Int 13:89–95
Tremblay-Bourgeois S, Le François NR, Roy RL, Benfey TJ, Imsland AK (2010) Effect of rearing density on the growth and welfare indices of juvenile spotted wolffish, Anarhichas minor (Olafsen). Aquac Res 41:1179–1189
Uglem I, Kjørsvik E, Gruven K, Lamberg A (2009) Behavioural variation in cultivated juvenile Atlantic cod (Gadus morhua L.) in relation to stocking density and size disparity. Appl Anim Behav Sci 117:201–209
van de Nieuwegiessen PG, Olwo J, Khong S, Verreth JAJ, Schrama JW (2009) Effects of age and stocking density on the welfare of African catfish, Clarias gariepinus Burchell. Aquaculture 288:69–75
Yamanome T, Amano M, Takahashi A (2005) White background reduces the occurrence of staining, activates melanin-concentrating hormone and promotes somatic growth in barfin flounder. Aquaculture 244:323–329
Acknowledgments
We would like to thank Mr. Giorgos Konstantinou and Mr. Xenophon Vrettos for their technical and laboratory assistance and to Mr. J. Stefanis for providing the fish.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karakatsouli, N., Kassianos, N. & Papoutsoglou, S.E. Effects of rearing density and tank colour on juvenile sharpsnout seabream (Diplodus puntazzo) growth performance. Aquacult Int 23, 943–953 (2015). https://doi.org/10.1007/s10499-014-9853-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-014-9853-8