Abstract
The age, growth, and reproductive biology of the five-lined snapper Lutjanus quinquelineatus was estimated using 709 specimens (44.2–197.7 mm) collected from November 2012 to November 2014 around Okinawa-jima Island, southern Japan. The oldest age estimated by sectioned otoliths and maximum standard length were 24 years and 192.1 mm for females and 27 years and 197.7 mm for males. The relationship between age and standard length was expressed by the following von Bertalanffy growth equations; female: Lt = 172.2 × {1 − exp[−0.55(t + 0.70)]}; male: Lt = 182.5 × {1 − exp[−0.52(t + 0.70)]}. The spawning season estimated by the gonadosomatic index and histological observations was from May to September, with a peak season from June to August. The length (age) at first maturity was 130.3 mm (2 years) for females and 129.5 mm (1 year) for males. Among the small Lutjanus species, L. quinquelineatus has a long lifespan and matures at a small size and young age.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Lutjanus species are an important resource for fisheries in tropical and subtropical regions (Allen 1985). Small lutjanid species are commonly found in fish markets and are a popular food of the people living on Okinawa-jima Island, Japan. The catch of small Lutjanus species on Okinawa-jima Island is at a low level, and the catch trend has been decreasing in the long term and flat in the short term (Ohta et al. 2017). However, previous studies have been conducted only on L. fulviflammus around Okinawa-jima Island (Shimose and Tachihara 2005).
The five-lined snapper Lutjanus quinquelineatus is a small lutjanid species that is widely distributed in tropical and subtropical regions of the Indian Ocean and the western Pacific Ocean (Allen 1985). This species is used as a food in tropical and subtropical regions, and information on its growth and maturity has been reported in several areas (Loubens 1980a, b; Mehanna et al. 2017). On the Great Barrier Reef in Australia, the oldest age was 31 years, and the age structure varies among coral reefs (Newman et al. 1996a, b). In New Caledonia, the oldest age observed was 22 years (Loubens 1980b). The standard length at first maturity of females has been observed to be 112 mm in New Caledonia (Loubens 1980a) and 160 mm in Hurghada, Egypt (Mehanna et al. 2017). Thus, the age and reproductive biology of this species are different dependent on the sea area.
The basic knowledge of life history such as growth and maturity size are essential for stock management. To our knowledge, there is no available information on the life history of L. quinquelineatus on Okinawa-jima Island. The aim of this study was to determine the age, growth, and reproductive biology of L. quinquelineatus around Okinawa-jima Island.
Materials and methods
Fish sampling and processing
Fish samples of 709 individuals were purchased at Nago, Henza, Awase, and Chinen Fishing Ports from November 2012 to November 2014 (Fig. 1). The standard length (SL), fork length (FL), and total length (TL) of the collected samples were measured to the nearest 0.1 mm and body weight (BW) was measured to the nearest 1 g.
The relationships between SL-FL and SL-TL were estimated by linear functions using the least squares method. The SL-BW relationship was estimated using a power function.
The mean monthly water temperature at Okinawa-jima Island (26–27°N, 128–129°E, water depth 30 m) was obtained from the Japan Oceanographic Data Center (JODC: https://jdoss1.jodc.go.jp/vpage/bts_j.html “Accessed 28 Dec 2020”).
Age determination
Sagittal otoliths of 706 individuals (female 293, male 387, sex unknown 26) were removed from the head and used for age determination. As per the general rule, the otolith on the right side was used for age determination, and if it was damaged, the left was used. The sagittal otoliths were embedded in epoxy resin and cut in the minor axis direction of the otolith, including the nucleus, to prepare 0.6 mm-thick sections using Buehler Isomet Low-speed jewelry saw (ISOMET, Microstructural Analysis Div., Buehler, Lake Bluff, IL, USA). The otolith sections were observed under a microscope with a Leica EC3 (Leica Microsystems, Wetzlar, Germany), and the number of opaque zones was counted, and otolith edge conditions (translucent or opaque) were recorded (Fig. 2). The birth month was assumed to be June 1. Specimens with an opaque zone formed on the otolith edge collected in March–May were aged as the number of opaque zones minus 1 year, and those with a translucent zone on the otolith edge collected in June–August were aged as the number of opaque zones plus 1 year. Based on the relationship between age and SL, the von Bertalanffy growth parameters for each sex were estimated using the non-linear least squares method:
where Lt is the standard length at age t; L∞, K, and t0 are the asymptotic length, the growth coefficient, and the hypothetical age at length 0, respectively. The growth equations for females and males were estimated including individuals of sex unknown. The von Bertalanffy growth equations for females and males were compared using an F test.
Gonadal observation
The gonads of 299 females and 385 males were removed from the abdominal cavity and weighed to the nearest 0.01 g. A chi-square test was used to test whether the sex ratios of all samples or of each SL class differed from the expected 1:1 ratio. The gonadosomatic index (GSI) was calculated with the following equation using gonad weight (GW) and BW:
For histological observation, the gonads of 266 females and 346 males were fixed in Bouin’s solution for 24 h. Fixed gonads were dehydrated with a series of ethanol dilutions and embedded in paraffin wax. The embedded gonads were sectioned to 8–10 µm thickness and stained with Mayer’s hematoxylin and eosin.
Ovarian development was classified into six phases based on the most advanced stage of oocyte, abundance of alpha stage atresia, and presence or absence of postovulatory follicles (Ebisawa 1999; Shimose and Nanami 2014). “Immature” was defined as containing only the peri-nucleolus stage or yolk vesicle stage (Fig. 3a). “Maturing” was defined as yolk globules starting to accumulate in the cytoplasm of oocytes and the most advanced stage of oocyte was at the early yolk globule stage (Fig. 3b). “Maturation” was defined as completed yolk globule accumulation and the most advanced stage of oocyte was the late yolk globule stage (Fig. 3c). “Ripe” was defined where most advanced stage of oocyte with a migratory nucleus stage or mature stage oocyte occurred, but no postovulatory follicles were observed (Fig. 3d). “Spawned” was defined where postovulatory follicles were observed (Fig. 3e). “Atresia” was defined where the stage of atresia accounted for > 50% in the section (Fig. 3f). In this study, the maturation, ripe, and spawned phases were considered to be reproductively active. Ripe and spawned phases were considered as evidence of spawning.
Photographs of histological sections of six ovarian phases of Lutjanus quinquelineatus. a immature phase: only peri-nucleolus stage oocyte (PN) or yolk vesicle stage oocyte (YV); (b) maturing phase: the most advanced stage of early yolk globule stage oocyte (EY); (c) maturation phase: the most advanced stage of late yolk globule stage oocyte (LY); (d) ripe phase: the most advanced stage of migratory nucleus stage or mature stage oocyte (MA); (e) spawned phase: postovulatory follicles (POF) were observed; (f) atresia phase: atresia accounted for > 50%. Each scale bar indicates 100 μm
The testicular development was classified into four phases based on the area of spermatozoa occupied in the section and the frequency of areas showing active spermatogenesis (Ebisawa 1999; Shimose and Nanami 2014). “Immature” indicated mostly spermatogonia and inactive spermatogenesis (Fig. 4a). “Inactive” indicated < 40% of the area being occupied by spermatozoa and the occurrence of spermatogenesis (Fig. 4b). “Ripe” indicated 40–70% of the area occupied by spermatozoa and active spermatogenesis in progress (Fig. 4c). “Spent” indicated a part of the spermatozoa being released and spermatogenesis decreased, and > 70% of the area being occupied by spermatozoa (Fig. 4d). In this study, inactive, ripe, and spent phases indicated sexual maturity. Ripe and spent phases were considered reproductively active.
Photographs of histological sections of four testicular phases of Lutjanus quinquelineatus. a Immature phase: mostly spermatogonia and inactive spermatogenesis; (b) inactive phase: spermatozoa (SZ) accounted for < 40% and inactive spermatogenesis; (c) ripe phase: spermatozoa (SZ) accounted for 40–70% and active spermatogenesis; (d) spent phase: spermatozoa (SZ) accounted for > 70% and active spermatogenesis but decreased. Each scale bar indicates 100 μm
The length and age at first maturity (LM and AM) and 50% maturity (L50 and A50) for each sex were estimated from 124 females and 184 males during the spawning season (May–September). The maturity rate for length-class intervals of 5 mm was fitted to a logistic equation. The parameters a and b were estimated using the maximum likelihood method. The logistic equation was as follows:
where a and b are constants, and PSL is the percentage of maturity in the SL class.
Results
Length–frequency distribution and sex ratio
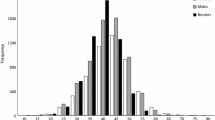
The range of standard length in the collected samples was 112.2–192.1 mm for females, 120.0–197.7 mm for males, and 44.2–124.7 mm for sex unknown (Fig. 5). The sex ratios were biased towards males in size classes of 140–149 mm, 180–189 mm, and 190–199 mm (chi-square test, p < 0.01). The sex ratio of all specimens was biased toward males (Chi square test, p < 0.01).
The relationships between SL-FL, SL-TL, and SL-BW were expressed by the following equations:
Age and growth
The otoliths with opaque edges appeared March–September, with the highest frequency observed in April (95%, Fig. 6). Otoliths with opaque edges were not observed between October and February. The mean monthly water temperature at Okinawa-jima Island was the lowest in March (21.3 °C, Fig. 6), began to rise in April (22.6 °C), and reached the highest in July–September (27.6–28.7 °C).
Observed ages for unknown-sex, females, and males ranged from 0 to 1 years, 0 to 24 years, and 0 to 27 years, respectively (Table 1). The frequency of age was high in the 2- and 3-year-old fish, accounting for 26.5% and 18.0% of the total, respectively. However, the frequency after 4 years of age was less than 8%. Both females and males grew rapidly in the initial stage, reaching 87% and 85% of the maximum length in females and males, respectively, at 3 years (Fig. 7). After 4 years, the annual growth was less than 10 mm. The growth curve parameters were different between females and males (F-test, p < 0.01). The relationship between age and SL was expressed by the following equations:
von Bertalanffy growth curves of males and females of Lutjanus quinquelineatus on Okinawa-jima Island. Solid line and broken line indicate male and female growth curves, respectively. The equations are \({L}_{t}=172.2 \times \{ 1 - \mathrm{exp} \left[ - 0.55 \left( t + 0.70\right)\right]\}\) (n = 319, r2 = 0.87) for females and \({L}_{t}=182.5 \times \{ 1 - \mathrm{exp} \left[ - 0.52 \left( t + 0.70\right)\right]\}\) (n = 413, r2 = 0.89) for males
Spawning season
The mean GSI for females increased from May, peaked in June (GSI = 3.46), and decreased in September (Fig. 8). The mean GSI from October to April was less than 1.0. The mean GSI for males increased moderately from March (GSI = 0.08), remained high in May–July (GSI = 0.17–0.21), and decreased in September (GSI = 0.11, Fig. 8). The mean GSI was low between October and February (GSI < 0.06).
From histological observations of the females, the reproductively active ovaries (mature, ripe, and spawned phases) were observed in April–September and accounted for a high proportion (75–89%) of females in May–September (Fig. 9). The ripe and spawned phases, which show evidence of spawning, were observed in May–September. The atresia phase was observed in May, August, and September. Females with spawned phase ovaries were observed at all moon phases. The immature phase accounted for 95–100% of females in October–April. From histological observations of males, the reproductively active males (ripe and spent phases) were observed in April–October and accounted for a high proportion (59–89%) in May–September (Fig. 9).
Maturation size and age
The LM for females and males was 130.3 mm (GSI = 0.5) and 129.5 mm (GSI = 0.03, Fig. 10a), respectively. The AM females and males were 2 and 1 year old, respectively (Fig. 10b).
The logistic equation showing the maturity rate for each sex was expressed as follows:
The L50 estimated from the above formula was 142 and 131 mm for females and males, respectively (Fig. 11).
Discussion
It was confirmed that the opaque zone in otolith of L. quinquelineatus is an annual ring, which was revealed to be useful for age determination. The opaque zone of some Lutjanus species around Okinawan waters has been confirmed as an annual ring and used for age determination (Shimose and Tachihara 2005; Nanami et al. 2010a; Shimose and Nanami 2014).
The opaque zone of L. quinquelineatus was formed in March–July, which approximately coincided the time when water temperature rises from lowest (21.3 °C, Fig. 6). The opaque zone of L. quinquelineatus estimated by the change in the marginal growth index was formed in August–November in New Caledonia, and seasonal changes in water temperature reach the lowest in September (21.6 °C), then rise from October (Loubens 1978). The opaque zone of L. quinquelineatus was formed May–August in the Great Barrier Reef (Newman et al. 1996a), and the water temperature reaches lowest in May–June (23.8–24.2 °C) and starts to rise in July (https://jdoss1.jodc.go.jp/vpage/bts_j.html “Accessed 28 Dec 2020”). Therefore, it is considered that the opaque zone of L. quinquelineatus is formed periodically once a year at the time when the water temperature starts to rise from the lowest regardless of the sea area.
The oldest age confirmed in this study was 24 years for females and 27 years for males on Okinawa-jima Island (26–27°N, Fig. 7, Table 1). The oldest age in other waters has been confirmed to be 30 years for males and 31 years for females in the Great Barrier Reef (18–19°S, n = 577, Newman et al. 1996a), and 22 years for New Caledonia (22–23°S, n = 191, Loubens 1980b). The differences in the oldest age were considered to be the change in life history characteristics depending on the latitude or difference in fishing pressure. The oldest age of L. fulviflammus changes depending on the water temperature associated with the latitude (Shimose and Nanami 2015). However, for L. quinquelineatus, the relationship between latitude and oldest age is negative. Regarding the impact of the fishery, L. quinquelineatus is not used commercially in Australia (Newman et al. 1996a) and is not considered to be affected by the catch, but it is an important fishery species on Okinawa-jima Island and in New Caledonia (Loubens 1980b). Therefore, the difference in the oldest age in each area may be influenced by the presence or absence of fisheries for this species.
The maximum size, oldest age, LM, and AM of small Lutjanus fish in the Okinawa region were L. fulvus: 279 mm SL, 34 years, 185 mm SL, and 4 years (Shimose and Nanami 2014), L. fulviflammus (Okinawa-jima Island): 304 mm SL, 24 years, 174 mm SL, and 2 years (Shimose and Tachihara 2005), L. fulviflammus (Ishigaki-jima Island): 300 mm SL, 23 years, 176 mm SL, and 2 years (Shimose and Nanami 2015), respectively. The maximum size and oldest age of L. kasmira in the southern hemisphere are 241 mm SL and 8 years (Loubens 1980b), L. vitta: 257 mm FL, and 12 years (Newman et al. 2000). Thus, among the small Lutjanus species, L. quinquelineatus has a small maximum size, lives longer, and has early maturity.
The spawning season was estimated to be from May to September, with the peak season in June–August based on seasonal changes in GSI and histological observations (Figs. 8, 9). The mature phase for females was confirmed to be April (Fig. 8), but it was not included in the spawning season because of the small number (n = 1) and the low mean GSI. The spawning season of Lutjanus species distributed in the northern hemisphere is June–September (4 months) for L. fulvus on Ishigaki-jima Island (Shimose and Nanami 2014), April–August (5 months) for L. fulviflammus on Ishigaki-jima Island (Shimose and Nanami 2015), June–October (4 months) for L. decussatus off Ishigaki-jima Island (Nanami et al. 2010b), May–October (6 months) for L. gibbus off Ishigaki-jima Island (Nanami et al. 2010a), April–July (4 months) for L. fulviflammus on Okinawa-jima Island (Shimose and Tachihara 2005). The length of the spawning period was different, but was mainly in the summer (June–July), which is consistent with the present study.
Sexually mature females were observed between April and September in this study (Fig. 9). In addition, the mean water temperature started to rise in April (22.6 °C, Fig. 6), and a high water temperature was maintained in July–September (27.6–28.7 °C). Therefore, sexually mature females were observed during the period of rising water temperature. Generally, in teleost fish, changes in water temperature greatly affect the start and end of sexual maturity (Sheaves 2006). In New Caledonia, sexually mature L. quinquelineatus females have been observed in October–February (Loubens 1980a); the mean water temperature reached a minimum in July–August (20.7 °C), started to increase in September (22.7 °C), and was the highest in February (26.6 °C, Loubens 1978). In Hurghada, Egypt (26–27°N), the spawning season of L. quinquelineatus is estimated to be March–July based on the changes in GSI (Mehanna et al. 2017). The water temperature was lowest in March (22.1 °C) and highest in August (26.8 °C, https://jdoss1.jodc.go.jp/vpage/bts_j.html “Accessed 28 Dec 2020”). Therefore, the length, start, and end of the spawning season of L. quinquelineatus varies with the changes in water temperature in each area. However, the spawning season typically starts when the water temperature begins to rise and exceeds 22–23 °C and ends when the water temperature begins to fall.
The LM of females and males was 130.3 mm and 129.5 mm on Okinawa-jima Island (26–27°N, 128–129°E), respectively (Fig. 10a). In New Caledonia (22–23°S, 166–167°E), LM was confirmed at 113 mm for females and 112 mm for males (Loubens 1980a), which is smaller than that in this study. In Hurghada (26–27°N, 34–35°E), Egypt, the female LM is reported to be 160.5 mm (Mehanna et al. 2017). The ratio of the LM to the maximum length was 67% for females and 65% for males on Okinawa-jima Island, 64% for females in Hurghada (Mehanna et al. 2017), and 57% for females and 59% for males in New Caledonia (Loubens 1980a). Okinawa-jima Island and Hurghada, which have LM, are located in the marginal area of the distribution of L. quinquelineatus (Allen 1985). Maturity size of tropical marine fish increases with the distance from the center of the distribution (Cappo et al. 2013; Trip et al. 2014). Therefore, the large LM of L. quinquelineatus on Okinawa-jima is due to the population being in the marginal area of distribution.
The female LM (AM) of L. quinquelineatus observed in this study was 130.3 mm (2 years) and 85% matured at 3 years. The female LM (AM) of L. fulviflammus is 175 mm SL (2 years) and 75% matured at 3 years (Shimose and Tachihara 2005). The female LM (AM) of L. vitta is 138 mm SL (1 year) and 50% matured at 2 years (Loubens 1980a). Therefore, most females of the small Lutjanus species attain sexual maturity by 3 years. In contrast, in large Lutjanus species, the female LM (AM) of L. argentimaculatus is 314 mm SL (5 years) and 50% matured at 436 mm SL (8 years, Russell et al. 2003). The female LM (AM) of L. bohar is 386 mm FL (3 years), with an L50 (A50) of 428 mm FL (9 years, Marriott et al. 2007). Therefore, large Lutjanus species reach sexual maturity much later than the small Lutjanus species. The catch trends of small Lutjanus species on Okinawa-jima Island decreased in the long term and were flat in the short term, whereas the catch trends of large Lutjanus species decreased in the long term and short term (Ohta et al. 2017). Large Lutjanus species are caught by bottom longlines on Okinawa-jima Island (Shimose and Nanami 2013). The bottom longline mainly targets large fish, and it is thought that a greater number of juveniles of the large Lutjanus fish are caught compared to the small Lutjanus fish. Therefore, the catch trends between large and small Lutjanus species may be influenced by differences in reproductive traits. In addition, the small Lutjanus species mature at a small size and young age, and the maximum body length is also small; therefore, they are unlikely to be growth overfishing (Fig. 11).
This study shows that L. quinquelineatus on Okinawa-jima Island live longer than other small Lutjanus species and reach maturity at a smaller size and younger age. Such traits of life history are unlikely to cause the growth overfishing. However, long-lived, older fish may contribute significantly to reproduction (Beamish 2006). In this study area, the age structure is younger than in non-fishing areas, and long-term catch trends have decreased, which can lead to worse stock conditions. If the stock condition deteriorates further in the future, it is necessary to implement appropriate management strategies based on the baseline information obtained in this study.
References
Allen GR (1985) FAO species catalogue. Snapper of the world
Beamish RJ, McFarlane GA, Benson A (2006) Longevity overfishing. Prog Oceanogr 68:289–302
Cappo M, Marriott RJ, Newman SJ (2013) James’s rule and causes and consequences of a latitudinal cline in the demography of John’s Snapper (Lutjanus johnii) in coastal waters of Australia. Fish Bull 111:309–324
Ebisawa A (1999) Reproductive and sexual characteristics in the Pacific yellowtail emperor, Lethrinus atkinsoni, in waters off the Ryukyu Islands. Ichthyol Res 46:341–358
Loubens G (1978) Biology of some fish species from the new caledonian lagoon. I. Age determination (otolithometry). Cah ORSTOM Sèr Ocèanoger 16:263–283 ((in French with English abstract))
Loubens G (1980a) Biology of some fish species from the new caledonian lagoon II sexuality and reproduction. Cah Indo-Pac 2:41–72 ((in French with English abstract))
Loubens G (1980b) Biology of some fish species from the New Caledonian lagoon. III Growth Cah Indo-Pac 2:101–153 ((in French with English abstract))
Marriott RL, Mapstone BD, Begg GA (2007) Age-specific demographic parameters, and their implications for management of the red bass, Lutjanus bohor (Forsskal 1775): Alarge, long-lived reef fish. Fish Res 83:204–215
Mehanna SF, Baker TS, Soliman FM, Soliman HA (2017) Some biological aspects and population dynamics of the five-lined snapper, Lutjanus quinquelineatus (Family: Lutjanidae) from Red Sea off Hurghada, Egypt. Int J Fish Aquat Stud 5:321–326
Nanami A, Kurihara T, Kurita Y, Aonuma Y, Suzuki N, Yamada H (2010a) Age, growth and reproduction of the humpback red snapper Lutjanus gibbus off Ishigaki Island, Okinawa. Ichthyol Res 57:240–244
Nanami A, Okuzawa K, Yamada H, Suzuki N, Aonuma Y (2010b) Reproductive activity in the checkered snapper, Lutjanus decussatus, off Ishigaki Island, Okinawa. Ichthyol Res 57:314–318
Newman SJ, Williams DM, Russ GR (1996a) Age validation, growth and mortality rates of the tropical snappers (Pisces: Lutjanidae) Lutjanus adetii (Castelnau, 1873) and L. quinquelineatus (Bloch, 1790) from the central Great Barrier Reef. Austr Mar Freshw Res 47:575–584
Newman SJ, Williams DM, Russ GR (1996b) Variability in the population structure of Lutjanus adetii (Castelnau, 1873) and L. quinquelineatus (Bloch, 1790) among reefs in the central Great Barrier Reef. Austr Fish Bull 94:313–329
Newman SL, Cappo M, Williams DM (2000) Age, growth and mortality of the stripey, Lutjanus carponotatus (Richardson) and the brown-stripe snapper, L. vitta (Quoy and Gaimard) from the central Great Barrier Reef. Australia Fish Res 48:263–275
Ohta I, Akita Y, Uehara M, Ebisawa A (2017) Current status of the coastal fisheries stocks in the Okinawa Islands waters, based on the historical catch data from 1989 to 2015. Ann Rep Okinawa Prefect Fish Ocean Res Center 77:35–60 ((in Japanese))
Russell DJ, McDougall AJ, Fletcher AS, Ovenden JR, Street R (2003) Biology, management and genetic stock structure of mangrove jack, (Lutjanus argentimaculatus) in Australia. Queensland Department of Primary Industries
Sheaves M (2006) Is the timing of spawning in sparid fishes a response to sea temperature regimes? Coral Reefs 25:655–669
Shimose T, Nanami A (2013) Quantitative analysis of distribution of Lutjanus Fishes (Perciformes: Lutjanidae) by market survey in the Ryukyu Islands, Okinawa, Japan. Pac Sci 67:15–22
Shimose T, Nanami A (2014) Age, growth, and reproductive biology of blacktail snapper, Lutjanus fulvus, around the Yaeyama Island, Okinawa, Japan. Ichthyol Res 61:322–331
Shimose T, Nanami A (2015) Age, growth, and reproduction of blackspot snapper Lutjanus fulviflammus (Forsskål 1775) around Yaeyama Islands, southern Japan, between 2010 and 2014. J Appl Ichthyol 31:1056–1063
Shimose T, Tachihara K (2005) Age, shio growth and maturation of the blackspot snapper Lutjanus fulviflammus around Okinawa Island, Japan. Fish Sci 71:48–55
Trip EDL, Clements KD, Raubenheimer D, Choat JH (2014) Temperature-related variation in growth rate, size, maturation and life span in a marine herbivorous fish over a latitudinal gradient. J Anim Ecol 83:866–875
Acknowledgments
The authors are grateful to Dr. A. Ebisawa (Okinawa Prefectural Fisheries Research and Extension Center), Dr. I. Ohta, and Dr. M. Uehara (Fisheries Division of Okinawa Prefectural Government) for useful advice and information about the landing of lutjanid fish in the market, and Dr. T. Shimose (Japan Fisheries Research and Education Agency) for providing useful advice and information on this manuscript. The authors also thank Ms. H. Shindate and Mr. H. Nakata for collecting fish specimens, and staff of the Fisheries Cooperative of Yonashiro and Nago for allowing the surveys at the fish markets, and the members of the Laboratory of Fisheries Biology and Coral Reef Studies (University of the Ryukyus) for their helpful support. This study was supported by the Okinawa prefectural government.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Araki, K., Tachihara, K. Age, growth, and reproductive biology of the five-lined snapper Lutjanus quinquelineatus around Okinawa-jima Island, southern Japan. Fish Sci 87, 503–512 (2021). https://doi.org/10.1007/s12562-021-01520-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-021-01520-x