Abstract
Growth and reproduction of the Thai river sprat Clupeichthys aesarnensis (Teleostei: Clupeidae) in Sirindhorn Reservoir, Thailand, and Nam Ngum Reservoir, Laos, were investigated using age estimates obtained from daily growth increments in otoliths and gonad observations. This species breeds throughout the year in both reservoirs, although water temperature was higher in Sirindhorn than in Nam Ngum. The Sirindhorn population tended to mature at smaller size (length) and younger age than the Nam Ngum population; the Sirindhorn population also tended to grow faster before reaching sexual maturity, growing slowly afterwards, than did the Nam Ngum population. Additionally, the maximum size in the Sirindhorn population was observed to be smaller than that of the Nam Ngum population. These observations indicated that higher water temperature resulted in earlier maturation and smaller maximum size in C. aesarnensis. Meanwhile, the smaller maximum size observed in the Sirindhorn population was possibly caused by an evolutionary down-sizing resulting from the overexploitation of this population, because Sirindhorn reservoir is exposed to more intensive fishing pressure.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Thai river sprat Clupeichthys aesarnensis (locally called Pla kaew in Thailand and Pa keo in Laos) is a small clupeid [maximum standard length (SL) approximately 70 mm] (Baird et al. 1999) that is widely distributed across the Mekong river basin, including Laos (Kottelat 2001), Cambodia (Rainboth 1996) and Thailand (Jutagate et al. 2003; Poungcharean 2006). This species is an important fishery resource for artisanal fishing communities, particularly around large man-made reservoirs, such as Sirindhorn, Pasak Jolasid, and Ubolratana reservoirs in Thailand (Vijverberg et al. 2001; Jutagate et al. 2003; Poungcharean 2006) and Nam Ngum Reservoir in Laos (Mattson et al. 2001), where large populations of C. aesarnensis have developed since the reservoirs were constructed. This species contributes more than 20% of the annual fish catch in Sirindhorn and Nam Ngum reservoirs, but there have been steady increases in fishing effort since the application of fishing gear that exclusively targets C. aesarnensis, i.e. the “lamp-light” scoop net, in such reservoirs (Mattson et al. 2001). This is causing concerns about stock declines, although little evidence has been collected, despite fishermen’s perceptions of decreasing catches (Mattson et al. 2001; Jutagate et al. 2002). In addition to the above concern, the establishment of breeding populations of approximately 20 alien fish species (including carnivorous fish species, e.g. African catfish Clarias gariepinus) in the Mekong basin (Phillips 2002; Welcomme and Vidthayanon 2003; Hung et al. 2011) is another potential cause of declines in native fish diversity and stock levels of indigenous fishes of the region. Accordingly, there is a need for biological research that contributes to the conservation and sustainable exploitation of the indigenous fishes in the region, including C. aesarnensis. Moreover, because of wide geographical distribution of this species, the characteristics of growth and reproduction of remote populations are possibly different, due to variations of environmental conditions, particularly water temperatures. Therefore, biological information on such remote populations of this species and its comparisons are contributory to further considerations on species conservation and sustainable exploitation.
Several previous studies have focused on the efficient exploitation and management of C. aesarnensis. For instance, Costa-Pierce and Soemarwoto (1990) estimated its life span to be approximately 9 months, and Sirimongkonthaworn and Fernando (1994) reported on its zooplanktivorous feeding habit in the Ubolratana Reservoir. Poungcharean (2006) investigated the distribution and early development of C. aesarnensis in Pasak Jolasid Reservoir, and Jutagate et al. (2003) estimated yield/growth/mortality rate and demonstrated probable overfishing of this species in Sirindhorn Reservoir. However, detailed information on the life history of C. aesarnensis is still largely lacking, especially information on growth, gonadal development and reproduction. Such information is essential for stock management of the species and contributory to conservation of fish diversity of the region.
The use of otolith daily growth increments to clarify the life-history traits of small fishes, such as C. aesarnensis, has progressed since the method was developed by Pannella (1971), and has been applied to determine age and to estimate growth models, spawning season and life span in many fish species (Kon and Yoshino 2002; Morales-Nin 2000; Morioka and Sano 2009; Morioka et al. 2014). The combined analysis of daily age and gonadal development can provide further biological insight, and examination of stomach contents can provide information on the species’ feeding habits and food supply at the sampling sites.
Along with the above context, in this study we investigated two populations of C. aesarnensis from Sirindhorn Reservoir, Thailand, and Nam Ngum Reservoir, Laos, the two reservoirs being located far apart and considered to be under different temperature regimes, to determine their growth patterns based on ages estimated from otolith daily growth increments, size and timing of sexual maturity based on the observations of gonads, and feeding habit based on the analysis of stomach contents.
Materials and methods
Characteristics of sampling sites and fish sample collection
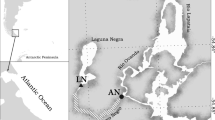
Thai river sprat, Clupeichthys aesarnensis, samples were collected from two man-made reservoirs that were constructed in 1971: the Sirindhorn Reservoir in Thailand and Nam Ngum Reservoir in Laos. The former reservoir was constructed mainly for irrigation, with a surface area of approximately 288 km2 and an average depth of 5 m (Jutagate et al. 2012) and is located about 63 km east of downtown Ubon Ratchathani (Fig. 1a). The latter was constructed for hydroelectric generation and irrigation, with a surface area of approximately 370 km2 and an average depth of 19 m (Mattson et al. 2001), and is located about 61 km north of downtown Vientiane (Fig. 1b). Samples were collected from the eastern shore of the Sirindhorn Reservoir (15°21′12.70″N, 105°45′48.38″E, altitude 153 m) (Fig. 1a) and from the western shore of the Nam Ngum Reservoir (18˚50′74.45″N, 102˚56′47.40″E, altitude 355 m) (Fig. 1b). Fish were collected at night using lamp light and four-armed scoop nets (1–2 mm mesh) from September 2012 to August 2013 in Sirindhorn Reservoir and from November 2016 to October 2017 in Nam Ngum Reservoir. Samples were preserved in 70% ethanol immediately after collection. Water temperatures at the bottom of nearshore waters (< 100 cm depth) were recorded hourly using data loggers (HOBO Water Temperature Pro v2 Data Logger, Onset Computer Corporation, USA) in both reservoirs.
Maps showing two man-made reservoirs in Thailand and Laos for sample collection of the Thai river sprat Clupeichthys aesarnensis. a Sirindhorn Reservoir, Thailand, b Nam Ngum Reservoir, Laos. Open and closed circles in a and b represent sample collection sites and the towns Ubon Ratchathani and Vientiane, respectively
Sex identification and gonadosomatic index
Ethanol-preserved fish were measured for standard length (SL, to the nearest 0.1 mm) using dial callipers, and body weight (BW, to the nearest 0.001 g) using a precision balance. All specimens were dissected under a stereoscopic microscope (× 0–80 magnification) for gonad extraction. The gonads were observed under an optical microscope with transmitted light (100–200 × magnification) for identification of ovaries or testes to determine the sex. The gonad weights of both females and males were measured (to the nearest 0.001 g) for calculation of the gonadosomatic index (GSI = gonad weight/body weight × 100).
Stomach contents and otolith-increment observations
The stomach contents were recorded, and the feeding incidence (number of specimens with stomach contents/observed number of specimens × 100%) was calculated. Subsequently, the otoliths (sagittae) were removed under a dissecting microscope and mounted in epoxy resin on glass slides. The sagitta was identifiable by its large size and flat arrowhead shape and was easily distinguishable from the lapillus and asteriscus. The sagittae were subsequently ground using waterproof sandpapers (#1500, 3 M Japan, Japan) and lapping films (6- and 9-μm mesh, 3 M Japan, Japan) and the ground surfaces were occasionally etched by hydrochloric acid (0.1 N) to emphasize increment contrast. Otolith increments were counted under an optical microscope with transmitted light (200–400 × magnification) to estimate fish daily age and hatch date. For otolith increment analysis, the Otolith Daily Rings Measurement System (Ratoc System Engineering, Co., Ltd., Japan) was used. Although daily periodicity of increment deposition in otoliths has not yet been validated in C. aesarnensis, it has been demonstrated in many other clupeids, e.g. Clupea harengus (Moksness 1992), Spratelloides robustus (Rogers et al. 2003) and Limnothrissa miodon (Meisfjord et al. 2006), as well as numerous other fish species (Campana and Neilson 1985). Hence, the otolith increments in C. aesarnensis were considered to have been formed daily.
Growth model estimation
Comparisons in growth patterns of C. aesarnensis, i.e. relationship between daily age and SL, were made between Sirindhorn and Nam Ngum populations with applying the von Bertalanffy growth model as follows;
where Lt is the estimated length of the fish at daily age t; L∞ is the asymptotic length at which growth is zero; K is growth constant; and t0 is the theoretical age at SL zero. The differences in growth models between the populations were statistically examined by an F-test (Akamine 2010). Additionally, specific daily growth (SDG = Lt − Lt − 1) was calculated based on the von Bertalanffy growth models.
Care and handling of the sample specimens were conducted in accordance with institute regulations and Japanese law (Kurosawa 2008).
Results
Water temperature fluctuations
The water temperatures at Sirindhorn and Nam Ngum reservoirs ranged from 21.8 to 36.1 °C (mean ± SD: 28.8 ± 3.3 °C) and 21.3–34.4 °C (27.3 ± 1.5 °C), respectively, with seasonal fluctuations. Overall, the water temperature was generally higher in Sirindhorn Reservoir than in Nam Ngum Reservoir, particularly between February and August (Fig. 2). At Sirindhorn Reservoir, the water temperatures elevated from January to June and declined from June to January of the following year (Fig. 2), and at Nam Ngum, the temperatures elevated from February to May, were more or less constant from May to October, and declined from October to February of the following year (Fig. 2).
Sex identification
Based on gonad observations, among a total of 378 specimens of C. aesarnensis from Sirindhorn Reservoir (9.3–45.2 mm SL), 193 were females (16.2–45.2 mm SL, 0.03–1.36 g BW), 134 were males (16.6–40.7 mm SL, 0.04–0.88 g BW), and the sex of 51 individuals was unidentifiable (9.3–21.7 mm SL, 0.002–0.09 g BW). Among 486 specimens from Nam Ngum Reservoir (7.2–59.5 mm SL), 247 were females (16.6–49.2 mm SL, 0.03–1.65 g BW), 203 were males (15.6–59.5 mm SL, 0.02–2.32 g BW), and the sex of 36 individuals was unidentifiable (7.2–21.7 mm SL, 0.001–0.10 g BW). These observations indicated that sexual dimorphism in gonads (differentiation of gonads into ovaries or testes) becomes distinctive at approximately 16–22 mm SL in this species.
Feeding incidence and stomach contents
The feeding incidence for the fish from Sirindhorn Reservoir was 24.6% (n = 93 specimens with stomach contents), and the predominant stomach contents were zooplankton (daphniids, copepods and brachionids), found in 73 specimens (19.3%), followed by aquatic/terrestrial insect fragments in 46 specimens (12.2%). Zooplankton were observed in these specimens regardless of fish size, while insects were primarily found in stomachs of fish larger than 25 mm SL (39 of 43 specimens > 25 mm SL).The feeding incidence (%) of fish from Nam Ngum Reservoir was 12.9% (n = 63 specimens with stomach contents), and the predominant stomach contents were also zooplankton, found in 47 specimens (9.7%), followed by fragments of insects in 18 specimens (3.7%). Zooplankton in the stomach was also observed regardless of fish size, while insect fragments were mostly observed in fish larger than 30 mm SL (i.e. 17 of 18 specimens > 30 mm SL). In addition, the stomach of a 37-mm-SL specimen, collected in November 2016, contained nine larval C. aesarnensis (sized 7.6–9.1 mm SL), indicating filial cannibalism. These findings indicate that C. aesarnensis feeds primarily on zooplankton up to a size of about 25 mm SL, whereas larger individuals can feed on larger prey, such as insects.
Otolith increment analysis, hatch month, and gonadosomatic index
Daily growth increments were clearly deposited and countable in the sagittal otoliths (Fig. 3a, b). Around the otolith core, a thin and distinctive check was observed at approximately 4 μm from the otolith core and was thus designated as the hatch check (Fig. 3b). Estimated ages were 15–238 days (9.3–45.2 mm SL; n = 378) for fish from Sirindhorn Reservoir, and 14–243 days (7.2–59.5 mm SL; n = 486) for those from Nam Ngum Reservoir. Hatch months, estimated from the daily ages of each specimen, were distributed throughout the year in both populations (Fig. 4), indicating that C. aesarnensis breeds year-round in both reservoirs.
The gonadosomatic index (GSI) of C. aesarnensis females from Sirindhorn Reservoir started to increase at around 20 mm SL and reached approximately 16, whereas that of females from Nam Ngum Reservoir started to increase at around 26–27 mm SL and reached approximately 14 (Fig. 5a). The GSI of males from Sirindhorn Reservoir tended to increase at around 20 mm SL and reached 8–10, whereas that of males from Nam Ngum Reservoir tended to increase at around 22 mm SL and reached approximately 6 (Fig. 5b). These data demonstrate that C. aesarnensis in Sirindhorn Reservoir were sexually mature at a smaller size (ca. 25 mm SL in females, 23–25 mm in males) than fish in Nam Ngum Reservoir (ca. 28–30 mm SL in females, ca. 25 mm SL in males).
Inter-population comparison of growth patterns
The relationship between the daily age and SL of the Sirindhorn specimens was fitted using the following von Bertalanffy equation:
The SDG was also calculated for specimens from the Sirindhorn Reservoir (Fig. 6d). Similarly, the relationship between the daily age and SL of the Nam Ngum specimens was fitted by the following equation:
The SDG was calculated for the Nam Ngum specimens based on the above equation (Fig. 6d). A significant difference was observed between the growth equations for the two populations (F-test, p < 0.01) (Fig. 6c). A comparison of the estimated SDG for each population (Fig. 6d) showed that fish in Sirindhorn Reservoir had grown faster than those in Nam Ngum Reservoir up to ca. 45 days after hatching (to a size of approximately 18–20 mm SL), whereas beyond this, the growth of Nam Ngum fish was faster than that of fish in Sirindhorn Reservoir.
von Bertalanffy growth models [relationships between age in days and standard length (mm)] and relationship between age in days and specific daily growth (SDG) of the Thai river sprat Clupeichthys aesarnensis. a, b Growth models of specimens collected from Sirindhorn and Nam Ngum reservoirs, respectively. c, d comparisons in growth models and SDGs between Sirindhorn and Nam Ngum reservoirs, respectively
Discussion
There were 4 years of lag time between the surveys at Sirindhorn Reservoir (2012–2013) and at Nam Ngum Reservoir (2016–2017) in the present study. However, the annual average air temperature (the average of over the past 30 years) in the former was generally higher (ca. 27.1 °C) than in the latter (25.4 °C) (Climate.data.org; https://en.climate-data.org/), the former located at lower latitude and altitude (ca. 15°21′N and 153 m) than at the latter (18°51′N and 355 m). Hence, the higher water temperature in the Sirindhorn Reservoir than that in the Nam Ngum Reservoir, as observed in the present study, is considered a phenomenon occurring annually, and thus the biological information of the Thai river sprat Clupeichthys aesarnensis collected from these two reservoirs is considered reasonably comparable. Further, although we used the otolith increments of C. aesarnensis to be daily increments without validation in reference to the previous reports on daily periodicity of otolith increment deposition in other clupeids (Moksness 1992; Rogers et al. 2003; Meisfjord et al. 2006), validation of periodical increment deposition in this species would be eventually necessary.
Based on the hatch months estimated by daily ages of the sampled specimens, C. aesarnensis is considered to breed throughout the year in both the Sirindhorn and Nam Ngum reservoirs (Fig. 4); this result agrees with the findings of previous studies for the species (e.g. Costa-Pierce and Soemarwoto 1990; Jutagate et al. 2003; Poungcharean 2006). The maximum ages of the sampled fish observed in the present study were 238 days (45.2 mm SL) in the Sirindhorn population and 243 days (41.8 mm SL) in the Nam Ngum population. This suggests a life span of at least 8 months for the species, which is close to the 9-month life span reported by Costa-Pierce and Soemarwoto (1990). Considering the previously reported maximum size of the species (70 mm SL; Baird et al. 1999) being larger than that observed in the present study, a 9-month life span seems reasonable.
Stomach content analysis indicated that C. aesarnensis feeds mainly on zooplankton and occasionally on insects, consistent with the findings reported by Sirimongkonthaworn and Fernando (1994). Insects were more frequently observed in the stomachs of larger specimens (> 30 mm SL in the Nam Ngum population, > 25 mm SL in the Sirindhorn population), suggesting that the prey-size selectivity of C. aesarnensis changes with growth. An instance of filial cannibalism was observed in the present study. This type of cannibalism occurs in numerous teleost fishes and is thought to provide an alternative food source for adult fish when this is needed to maximize future reproductive success (Smith and Reay 1991; Manica 2002). Considering the low feeding incidence observed in the present study (12.9% in the Nam Ngum population, 24.6% in the Sirindhorn population), filial cannibalism may also be important in providing supplemental food for adult C. aesarnensis. However, only a single instance was observed among 864 specimens examined in the present study, and it is not clear whether filial cannibalism is a biologically meaningful behaviour in this species.
Gonadal development commenced in smaller specimens in the Sirindhorn population of C. aesarnensis compared with the Nam Ngum population (Fig. 5), and water temperatures at the former reservoir were higher throughout most of the year (Fig. 2). Furthermore, fish in the Sirindhorn population tended to grow faster than those in the Nam Ngum population up to around ca. 45 days of age (ca. 18–20 mm SL), and thereafter the Nam Ngum population showed faster growth (Figs. 6d). In the Sirindhorn population, gonadal development progressed rapidly after reaching 20 mm SL as shown by an increased GSI (Fig. 5). In general, higher water temperature promotes early maturation among fish species, as reported in the Japanese rice fish Oryzias latipes (Yoshioka 1970) and the combtooth blenny Dasson trossulus (Dotsu 1982). Further, in the silver-stripe round herring Spratelloides gracilis, a marine short-lived small clupeid, higher temperature was also considered to promote earlier growth, particularly during the larval and juvenile stages (Durieux et al. 2009). Besides, growth after sexual maturation is often stagnant due to the shift of energy consumption from body somatic growth to gonad development, as reported in the gilthead seabream Sparus aurata (Kadmon et al. 1985) and the white-spotted spinefoot Siganus canaliculatus (Grandcourt et al. 2007). Considering these previous findings, the differences in patterns of growth and timing of maturity between the Sirindhorn and Nam Ngum populations can be attributed to differences in water temperatures between the two reservoirs. Meanwhile, the females with exceedingly low GSI among the largest specimens observed in this study (> 35 mm SL) in both populations (Fig. 5a) are considered post-spawning specimens, suggesting the possibility of multiple spawning in C. aesarnensis, although re-spawned females were not identifiable in the present study. Multiple spawning was reported in several marine small-sized tropical/subtropical clupeids with short life spans, e.g. the silver-stripe round herring Spratelloides gracilis (Weng et al. 2005), the delicate round herring S. delicatulus and Lewis’ round herring S. lewisi (Milton and Blaber 1991; Milton et al. 1995). Considering the previously reported maximum size of the species (ca. 70 mm SL; Baird et al. 1999) that is much larger than those observed in this study, multiple spawning may be more feasible in larger specimens than those obtained in the present study.
Some marine small-sized tropical/subtropical clupeids (e.g. Spratelloides delicatulus, S. gracilis, S. lewisi) are known to be important prey for carnivorous fishes, and predation is probably the major cause of their natural mortality (Milton et al. 1991). Likewise, for C. aesarnensis, predation may be the most common cause of natural mortality (Mattson et al. 2001; Jutagate et al. 2002). If so, the early maturation and year-round spawning of C. aesarnensis observed in this study would be advantageous for maintaining the population by maximizing the number of individuals spawning, as well as by reducing the generation time, as discussed for the above-mentioned marine clupeids (Milton et al. 1991). In addition to natural mortality, fishing is another important source of mortality, and overexploitation can often result in decreased fish sizes with maturation at younger ages, as previously reported for many other species (Hutchings 2005; Jørgensen et al. 2007; Conover et al. 2009; Sharpe and Hendry 2009; Sakai 2009; Ngor et al. 2018). The estimated maximum size in the Nam Ngum population based on the growth models (around 45 mm SL) in the present study was greater than that in the Sirindhorn population (around 37 mm SL) (Fig. 6). Considering this size difference of fish between the reservoirs and the larger fishing effort in Sirindhorn Reservoir than in Nam Ngum Reservoir (inferred on the basis of a larger population of consumers surrounding the former reservoir), the smaller size observed at Sirindhorn Reservoir than at Nam Ngum Reservoir may have been caused by an evolutionary down-sizing due to overexploitation (Sharpe and Hendry 2009), as noted by Jutagate et al. (2003).
References
Akamine T (2010) Introduction to data analyses of fishery resources. Koseisha Koseikaku, Tokyo (in Japanese)
Baird IG, Inthaphaisy V, Kisouvannalath P, Phylavanh B, Mounsouphom B (1999) The fishes of southern Lao. Lao community fisheries and dolphin protection project. Ministry of Agriculture and Forestry, Vientiane
Campana SE, Neilson JD (1985) Microstructure of fish otoliths. Can J Fish Aquat Sci 42:1014–1032
Conover DO, Munch SB, Arnott SA (2009) Reversal of evolutionary downsizing caused by selective harvest of large fish. Proc R Soc B. https://doi.org/10.1098/rspb.2009.0003
Costa-Pierce BA, Soemarwoto O (1990) Biotechnical feasibility studies on the importation of Clupeichthys aesarnensis (Wongratana, 1983) from Northeastern Thailand to the Saguling Reservoir, West Java, Indonesia. In: Costa-Pierce BA, Soemarwaoto O (eds) Reservoir fisheries and aquaculture development for resettlement in Indonesia. ICLARM Technical Paper No. 23, Manila, pp 329–363
Dotsu Y (1982) The early life history of the combtooth blenny Dasson trossulus and spawnings of the laboratory-reared fish about three months after hatching. Bull Facul Fish Nagasaki Univ 52:19–27 (in Japanese with English abstract)
Durieux EDH, Meekan MG, Ponton D, Vigliola L (2009) Temperature, selective mortality and early growth in the short-lived clupeid Spratelloides gracilis. J Fish Biol 74:921–938
Grandcourt E, Al Abdessalaam T, Francis F, Al Shamsi A (2007) Population biology and assessment of the white-spotted spinefoot, Siganus canaliculatus (Park, 1797), in the southern Arabian Gulf. J Appl Ichthyol 23:53–59
Hung LT, Luong VC, Hoa NP, Diana J (2011) Impacts of the introduction of alien tilapias (Oreochromis spp.) on the fisheries and biodiversity of indigenous species in Tri An reservoir, Vietnam. In: Liping L, Fitzsimmons K (eds) Proceedings of the ninth international symposium on tilapia in aquaculture. AquaFish Collaborative Research Support Program, Shangai, pp 88–100
H utchings JA (2005) Life history consequences of overexploitation to population recovery in Northwest Atlantic cod (Gadus morhua). Can J Fish Aquat Sci 62:824–832
Jørgensen C, Enberg K, Dunlop ES, Arlinghaus R, Boukal DS, Brander K, Ernande B, Gårdmark AG, Johnston F, Matsumura S, Pardoe H, Raab K, Silva A, Vainikka A, Dieckmann U, Heino M, Rijnsdorp AD (2007) Managing evolving fish stocks. Science 318:1247–1248
Jutagate T, Mattson NS, Moreau J, Srichareondham B, Kumsri M (2002) Ecosystem of Sirindhorn and Nam Ngum reservoirs: a comparison. Fish Res Bull Kasetsart Univ 24:1–14
Jutagate T, De Silva SS, Mattson NS (2003) Yield, growth and mortality rate of the Thai river sprat, Clupeichthys aesarnensis, in Sirinthorn Reservoir, Thailand. Fish Mgmt Ecol 10:221–231
Jutagate T, Srichareondham B, Lek S, Amarasinghe US, De Silva SS (2012) Variations, trends and patterns of fish landings in large tropical reservoirs. Lakes Reservoirs 17:35–53
Kadmon G, Gordin H, Yaron Z (1985) Breeding-related growth of captive Sparus aurata (Teleostei, Perciformes). Aquaculture 46:299–305
Kon T, Yoshino T (2002) Extremely early maturity found in Okinawan gobioid fishes. Ichthyol Res 49:224–228
Kottelat M (2001) Fishes of Laos. Wildlife Heritage Trust, Colombo
Kurosawa TM (2008) Japanese regulation of laboratory animal care with 3Rs. In: Yoshimura I (ed) Proceedings of 6th world congress on alternatives & animal use in the life sciences. Special issue of Japanese Society for Alternatives to Animal Experiments, Tokyo, pp 317–321
Manica A (2002) Filial cannibalism in teleost fish. Biol Rev 77:261–277
Mattson NS, Balavong V, Nilsson H, Phounsavath S, Hartmann WD (2001) Changes in fisheries yield and catch composition at the Nam Ngum Reservoir Lao PDR. In: De Silva SS (ed) Proceedings of an international workshop reservoir and culture-based fisheries: biology and management. Australian Centre for International Agricultural Research, Bangkok, pp 47–55
Meisfjord J, Midtøy F, Forkvord A (2006) Validation of daily increment deposition in otoliths of juvenile Limnothrissa miodon (Clupeidae). J Fish Biol 69:1845–1848
Milton DA, Blaber SJM (1991) Maturation, spawning seasonality and proximate spawning stimuli of six species of tuna baitfish in the Solomon Islands. Fish Bull US 89:221–237
Milton DA, Blaber SJM, Rawlinson NF (1991) Age and growth of three species of tuna baitfish (genus: Spratelloides) in the tropical Indo-Pacific. J Fish Biol 39:849–866
Milton DA, Blaber SJM, Rawlinson NJF (1995) Fecundity and egg production of four species of short-lived clupeoid from Solomon Islands, tropical South Pacific. ICES J Mar Sci 52:111–125
Moksness E (1992) Validation of daily increments in the otolith microstructure of Norwegian spring-spawning herring (Clupea harengus L.). ICES J Mar Sci 49:231–235
Morales-Nin B (2000) Review of the growth regulation processes of otolith daily increment formation. Fish Res 46:53–67
Morioka S, Sano K (2009) Growth and maturation of the bumble-bee goby Brachygobius mekongensis (Perciformes: Gobiidae) occurring in the Mekong basin, in Vientiane Province, Central Laos. Ichthyol Explor Freshwaters 20:267–275
Morioka S, Koizumi N, Vongvichith B (2014) Seasonal growth and reproduction of Rasbora rubrodorsalis, a small-sized cyprinid fish from central Laos (Teleostei: Cyprinidae). Ichthyol Explor Freshwaters 25:277–287
Ngor PB, McCann KS, Grenouillet G, So N, McMeans BC, Fraser E, Lek S (2018) Evidence of indiscriminate fishing effects in one of the world’s largest inland fisheries. Sci Rep 8:8947
Pannella G (1971) Fish otoliths: daily growth layers and periodical patterns. Science 173:1124–1127
Phillips MJ (2002) Freshwater aquaculture in the Lower Mekong Basin. MRC Technical Paper No. 7, Mekong River Commission, Phnom Penh
Poungcharean S (2006) Distribution and early-life development of Thai river sprat Clupeichthys aesarnensis Wongratana, larvae, in Pasak Jolasid Reservoir, Lop Buri Province, Thailand. Kasetsart J Nat Sci 40:188–195
Rainboth WJ (1996) Fishes of the Cambodian Mekong. FAO species identification field guide for fishery purposes. FAO, Rome
Rogers PJ, Geddes M, Ward TM (2003) Blue sprat Spratelloides robustus (Clupeidae: Dussumieriinae): a temperate clupeoid with a tropical life history strategy? Mar Biol 142:809–824
Sakai T (2009) Study on the fisheries biology of lizardfishes, Saurida umeyoshii in the East China Sea and Saurida elongata in Tsushima/Korea Strait. Bull Fish Res Agency 28:1–45
Sharpe DMT, Hendry AP (2009) Life history change in commercially exploited fish stocks: an analysis of trends across studies. Evol Appl 2:260–275
Sirimongkonthaworn R, Fernando CH (1994) Biology of Clupeichthys aesarnensis (Clupeidae) in Ubolratana reservoir, Thailand, with special reference to food and feeding Habits. Int Rev Hydrobiol 79:95–112
Smith C, Reay P (1991) Cannibalism in teleost fish. Rev Fish Biol Fish 1:41–64
Vijverberg J, Amarasinghe PB, Ariyaratna MG, van Densen WLT (2001) Carrying capacity for small pelagic fish in three Asian reservoirs. In: De Silva SS (ed) Proceedings of an international workshop “Reservoir and culture-based fisheries: biology and management. Australian Centre for International Agricultural Research, Bangkok, pp 153–166
Welcomme RL, Vidthayanon C (2003) The impact of introduction sand stocking of exotic species in the Mekong basin and policies for their control. MRC Technical Paper No. 9, Mekong River Commission, Phnom Penh
Weng J-S, Liu K-M, Lee S-C, Tsai W-S (2005) Reproductive biology of the blue sprat Spratelloides gracilis in the waters around Penghu, Central Taiwan Strait. Zool Studies 44:475–486
Yoshioka H (1970) On the effects of environmental factors upon the reproduction of fishes: IV. Effects of long photoperiod on the development of ovaries of adult medaka, Oryzias latipes, at low temperatures. J Hokkaido Univ 21:14–20
Acknowledgements
We thank the technical staff of the Living Aquatic Resources Research Center, Laos, for their assistance with collecting the samples. We are also grateful to Yukiyo Yamamoto, Program Director of the Japan International Research Center for Agricultural Sciences (JIRCAS), Japan, for her helpful coordination of the research activity, and to Kensuke Kawamura, Researcher of the JIRCAS for his constructive suggestions. The research activities described in this article were funded by the JIRCAS and the Ministry of Environment, Japan, with the Environment Research and Technology Development Fund (no. 4D-1202).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Morioka, S., Vongvichith, B., Marui, J. et al. Characteristics of two populations of Thai river sprat Clupeichthys aesarnensis from man-made reservoirs in Thailand and Laos, with aspects of gonad development. Fish Sci 85, 667–675 (2019). https://doi.org/10.1007/s12562-019-01319-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-019-01319-x