Abstract
This article describes the life-history strategy of the blue sprat Spratelloides robustus in South Australia and compares the demographic traits observed with those of other clupeoids. Validation studies that involved marking the sagittae of captive fish with oxy-tetracycline suggested that growth increments are deposited daily. The oldest fish examined was 82 mm caudal fork length and 241 days old, which suggests S. robustus may live for less than 1 year. Growth rates were high during larval stages (0.34 mm day−1) and remained high throughout juvenile (0.33 mm day−1) and adult stages (0.19 mm day−1). S. robustus reached 50% maturity at approximately 60 mm caudal fork length after approximately 135 days. Spawning occurred from October to February (spring to late summer) and larvae were found mainly in Spencer Gulf, Gulf St Vincent, and Investigator Strait. Females spawned multiple batches of demersal eggs every 1–2 days. Batch fecundities were low (mean=756, SD=341) and increased linearly with length and weight. The life history of S. robustus is dissimilar to other small to medium-sized temperate clupeoids, but similar to those of many small sub-tropical and tropical clupeoids, including other Spratelloides species. Gulf St Vincent and Spencer Gulf may be considered to be "seasonally subtropical systems" in an otherwise temperate region that support a suite of species, including S. robustus, that have life-history strategies similar to those of sub-topical and tropical taxa.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Life-history strategies are complex sets of growth and reproductive traits that co-evolve to maximize reproductive success. These strategies are the product of trade-offs in the allocation of resources and energy between growth, maintenance, and reproduction and are defined by the manner in which these processes occur (Stearns 1992). Life history strategies among groups of related taxa are constrained by phylogenetic and physiological limitations and can be differentiated by variation in demographic traits, such as longevity, growth patterns, size and age-at-first maturity, reproductive periodicity, and fecundity. These traits vary in response to environmental conditions including those associated with latitudinal differences in climatic regimes (Conover 1992).
Age information provides a chronological framework in which the life-history patterns of fishes can be described and compared (Campana and Neilson 1985). The most commonly used method for determining the age of fishes involves the analysis of growth increments in otoliths. Daily growth increments are used to determine the ages of the early life-history stages of long-lived species and the age of all life-history stages of short-lived species. Before otoliths can be used for age determination, the periodicity of increment deposition must be validated (Beamish and McFarlane 1983). Daily formation of otolith increments has been validated for many small pelagic fishes including Thryssa aestuaria, Spratelloides delicatulus, and Herengula jaguana, using chemical immersion techniques that rely on metabolic uptake to mark the increments in captive specimens (Hoedt 1992; Milton et al. 1993; Pierce and Mahmoundi 2001).
Significant variation exists in the patterns of growth with age among marine fishes that inhabit regions with different environmental regimes. Clupeoids, which have been extensively studied, occupy a range of tropical and temperate marine environments and exhibit a diverse range of life-history strategies. Small to medium-sized species from temperate environments, including the sardine Sardinops sagax and sandy sprat Hyperlophus vittatus, typically live for approximately 4–8 years, have growth rates of 0.06 to 0.38 mm day−1, and spawn multiple batches of pelagic eggs over several spawning seasons (Butler et al. 1996; Gaughan et al. 1996; Velazquez et al. 2000). Some small tropical clupeoids such as Spratelloides spp. and Encrasicholina purpurea live for less than 1 year, have growth rates of 0.70 to 0.83 mm day−1, and spawn multiple batches of eggs over a single protracted spawning season (Clarke 1987; Milton et al. 1991, 1995).
The genus Spratelloides (Clupeidae: Dussumeriinae) includes a single temperate species that is endemic to Australian waters(S. robustus)and three tropical and sub-tropical species (S. lewisi, S. gracilis, and S. delicatulus) (Whitehead 1988). Like the sub-tropical and tropical co-geners, S. robustus is a small pelagic species that occupies sheltered embayments, estuaries, beaches, and offshore waters and ispreyed upon by pelagic fishes, marine mammals, and seabirds. The only biological data available for S. robustus suggest that it grows to approximately 110 mm total length and spawns between October and February (Blackburn 1941).
This is the first intensive investigation of the life-history strategy of a Spratelloides species in a temperate system. The objective of the study is to determine the key demographic traits of S. robustus in South Australia, including longevity, growth rates, sex ratio, length and age at sexual maturity, the timing and location of spawning, spawning frequency, and batch fecundity. Findings are used to compare the life-history strategy of S. robustus to those of other clupeoids from temperate, sub-tropical, and tropical systems.
Materials and methods
Sample collection
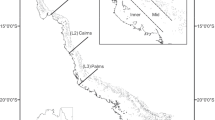
Larvae used for age determination were collected using dab-nets at locations 3, 6, 14, and 15 (Fig. 1; Table 1) during November and December 2000. Samples of juvenile and adult S. robustus were collected between January 1999 and April 2001. Most juvenile and adult samples were obtained after dusk using a multi-panelled, monofilament gillnet and an underwater light that were suspended 3–10 m directly under the research vessel. Other sampling methods, including trawling and beach-seining, were used opportunistically (Table 1). Samples were preserved using three different methods. Larvae for age determination were stored in 70% ethanol. Adult samples for histological analysis were fixed in 5% formalin solution. Juveniles and adults used for age determination and macroscopic gonad staging were frozen.
A Map of South Australia and locations in Spencer Gulf, Gulf St Vincent, and the far-west coast, sampled for Spratelloides robustus. (Numbers correspond to those for locations in Table 1). Inset (B) shows the location of relative to the Australian coastline
Ichthyoplankton samples were collected from Spencer Gulf, Gulf St Vincent, and Investigator Strait between 1986 and 1989 and in offshore shelf waters during February and March 2000 (Table 2). At shelf stations, plankton nets were deployed vertically to a depth of 10 m from the bottom or 70 m in water depths greater than 80 m and retrieved at 1 m s−1. Mechanical flow-meters were used to measure the distance travelled by the nets. The volume of water passing through the nets during each tow was calculated by multiplying the distance travelled (metres) by the surface area of the net (square metres). Plankton samples were fixed in a 5% formaldehyde and seawater solution. Larvae were identified using keys in Neira et al. (1998). Larval densities were calculated by dividing the number of larvae in a sample by the volume of water sampled.
Environmental data collection
Mean sea-surface temperatures (SSTs) for each month were obtained from the CSIRO website (http://www.marine.csiro.au) in 2000 to determine monthly trends in Spencer Gulf and Gulf St Vincent waters. SSTs were also recorded in situ during sampling for juveniles and adults.
Laboratory processing of samples
Each larval specimen was measured to the nearest 0.01 mm from the snout to the tip of the notochord using electronic vernier callipers. Juveniles and adults were measured to the nearest millimetre (caudal-fork length, CFL) and weighed to the nearest 0.01 g. Gonads from all juvenile and adult fish were removed and weighed to the nearest 0.01 g. The sex was determined for all individuals, except for some fish with very immature gonads.
Age validation
Two tank trials were undertaken in which fish were immersed in liquid oxy-tetracycline (OTC) and seawater for different periods to mark the otoliths. These fish were subsequently killed and the otoliths were analysed using an Olympuscompound microscope and ultraviolet light with WU (purple) and WIB (green) filters to determine the number of increments deposited between the fluorescent OTC mark and the outer edge.
Trial 1
Juveniles (n=4) ranging between 40 and 45 mm CFL were immersed in 0.1 mg ml−1 pfizer liquid OTC solution for 10 h. Fish were transferred into a 400-L fibre-glass tank filled with seawater and maintained under normal light conditions. Seawater was maintained at ambient temperatures (~20–22ºC) for the duration of the experiment (29 days). Fish were fed daily on a combination of fish and marine algal pellets. Following the death of each fish the otoliths were removed and analysed.
Trial 2
Individuals (n=11) were treated using the same method as in Trial 1 except the immersion time was 8 h and surviving individuals were killed 13 days after the OTC treatment. The results from both trials were combined and linear regression analysis was used to examine the relationship between the number of captive days elapsed since OTC treatment and number of increments deposited in sagittae during the captive period. The coefficient of non-determination (1−r 2) was used to determine the level of total variation not explained by the regression and the slope was tested to determine if it varied significantly from 1.0, using a paired t-test (P<0.05).
Otolith preparation and analysis
Sagittae from fish of each life-history stage required different preparation techniques. From each sample of larvae, sub-samples of 10–15 individuals were used for age determination. The head of each individual was removed and re-hydrated in distilled water. Sagittae were located inside the semi-circular canals of each specimen using a cross-polarising filter under a light microscope. Both sagittae were removed with fine needles, cleaned in distilled water, dried, and fixed whole to microscope slides with Supaglue.
Sub-samples of 30–40 juveniles and adults were selected for ageing. A longitudinal incision was made on the ventral side of the head under the gills and both sagittae were removed from the semi-circular canals, using fine forceps. Sagittae were cleaned in 12% H2O2, rinsed in distilled water, and dried in plastic trays. One sagitta from each pair was mounted on a microscope slide with thermoplastic cement. Longitudinal sagittal sections were prepared by grinding and polishing the convex and concave surfaces with a series of 9-μm, 3-μm, and then 1-μm lapping film until the primordium and the edge increments were visible.
A Leitz (Diaplan) compound microscope (250–625× magnification) and Image Capture software was used to analyse and count increments in prepared sagittae. The readability of increments was improved by placing a drop of immersion oil onto each ground section. In otoliths with clearly discernible increments, two to three replicate counts were made from the primordium to the outer edge along the same axis. The mean of the increment counts was used if replicates varied by <5%, otherwise the otolith was rejected.
Age and growth patterns
The general Gompertz growth model was used to describe the relationship between age and length and is represented by Eq. 1:
where L t is the estimated length of the fish at time t, a is the asymptote, b and c are growth constants and x is the age in days. Fitting of the curve was achieved using the Levenberg-Marquardt non-linear curve-fitting routine.
Instantaneous growth rates were calculated from the first derivative of the Gompertz growth equation for all individuals, at 10-day age intervals. The first derivative of Eq. 1 is summarised in Eq. 2:
Sex ratio
The sex ratio was determined for all samples collected throughout the year (n=808). One-way analyses of variance (ANOVA) were used to determine if sex ratios in samples were significantly different (P<0.05) from 1:1.
Macroscopic gonad staging
Each ovary was assigned to one of five developmental stages where stage I was immature, stage II was maturing, stage III was mature, stage IV was ripe (hydrated oocytes present), and stage V was spent (recently spawned) (Table 3). Hydrated oocytes in ovaries were identified by their large size (1–2 times larger than mature yolked oocytes in stage III ovaries) and fluid-filled appearance. Testes were assigned to three stages where stage I was immature, stage II was mature, off-white in colour with no milt present, and stage III was mature, off-white in colour with milt present.
Length and age at first maturity
The mean length at which 50% of males and females were sexually mature (L 50) was determined by fitting the logistic curve to the percentage of stage II–IV fish in each 5-mm length class. The logistic equation (3) is
where P L is the proportion of mature fish at fork length class L, and a and b are constants derived from the linear regression of Ln [(1−P)/P] versus caudal fork length (millimetres), where P is the proportion of mature fish in 5.0-mm length classes for fish between 50 and 90 mm CFL. L 50 was derived from L 50=−a/b. Age at first maturity for males and females was derived from the Gompertz growth curve, based on the L 50. ANOVA(P<0.05) was used to determine whether males and females reached 50% maturity at significantly different sizes.
Gonosomatic index
Gonad and gonad-free fish weights from male and female fish above the length of 50% maturity (L 50) were used to establish gonosomatic indices (GSIs). Arbitrary weights of 0.05 g were assigned to some immature (stage I) gonads that could not be weighed accurately. GSIs were calculated from Eq. 4:
where G wt is gonad weight and GF wt is gonad-free fish weight. Univariate general linear models (GLMs) with type III sums of squares were used to determine if GSIs were significantly different (P<0.05) between sexes by month.
Microscopic characteristics of ovaries
During the spawning season (2000–2001), males and females(n=442) were sampled at locations 3, 6, 10, and 15 (Table 1) and ovaries were prepared for histological preparation. Each stage II, III, and V ovary was sectioned at 6–7 μm, fixed to slides, and stained with haematoxylin and eosin dyes. Slides were examined under a compound microscope at 250x magnification. Oocyte developmental stages were identified using criteria established during studies of the reproductive biology of the engraulids Engraulis mordax and Encrasicholina heteroloba (Hunter and Macewicz 1985; Wright 1992).
Spawning fraction and frequency
Histological sections were analysed for the presence of postovulatory follicles (POFs). POFs were classified as day 0 (<24 h old) and day 1+ (>24 h old), based on the assumption that the structural characteristics and degeneration rates were similar to those for Engraulis mordax (Hunter and Macewicz 1985). Fish with day 0 POFs were assumed to have spawned on the night or afternoon of capture, and those with day 1+ POFs, on nights or afternoons before capture. Hydrated oocytes were identified by their large size and fluid-filled appearance. In each sample (N=14) the spawning fraction was estimated from the proportion of mature females (stages II–V) with hydrated oocytes or day 0 POFs. Day 1+ POFs were not used in the calculations. Mean spawning frequency was calculated from the reciprocals of the estimates of spawning fraction.
Batch fecundity
Batch fecundity was estimated from ovaries (n=63) containing hydrated oocytes by the gravimetric method (Hunter et al. 1985). Both whole ovaries were weighed and three 0.0-g to 0.04-g sub-sections were removed from one ovary of each pair. These sections were placed in 5–10 drops of Gilson's solution to soften connective tissue and allow separation of individual oocytes. The hydrated oocytes in each sub-section were counted and batch fecundity was determined by multiplying the mean number of oocytes per unit weight in the three sub-sections by the total weight of the ovaries. Linear regression analysis was used to describe the relationship between batch fecundity, fish weight, and CFL. GLMs with type-III sums of squares were used to determine if batch fecundities were significantly different (P<0.05) between locations and months.
Results
Sea-surface temperature
SSTs in southern Spencer Gulf ranged from 13°C in July and August to 23°C during the January to March period (summer; Fig. 2). In the northern region of Spencer Gulf, SSTs were slightly warmer and ranged from 14°C in mid-winter to 26°C during late summer. Similar seasonal ranges were recorded in Gulf St Vincent, where SSTs varied from 14 to 26°C in the northern region. SSTs remained low (13–18°C) from May to October in both gulfs. During the October to November (spring) period, mean SSTs increased to 24°C and 19°C in upper and lower Spencer Gulf, respectively, and 22°C in upper Gulf St Vincent.
Age validation
A fluorescent OTC mark and corresponding stress check (mark) were present in the sagittae from each juvenile treated with OTC (Fig. 3A). In trial 1, there was a direct relationship between the number of increments deposited in sagittae and the number of days between OTC treatment and death (n=4). In trial 2, nine fish sampled after 13 days deposited approximately 1 increment per day during the period after OTC treatment (mean number of post-treatment increments=12, SD=0.88). Additionally, of the two fish that died after 2 days, the first had deposited 1 and the second, 2 increments. The linear regression describing the relationship between the number of increments deposited and number of days elapsed after OTC treatment in trials 1 and 2 (Fig. 3B) has a slope of 0.949, which does not differ significantly from 1.0 (paired t-test, t=−0.53, df=28). Only 2.11% of the total variation was not explained by the linear regression, suggesting that increment formation occurred at a rate of 1 per day during the OTC trials.
Structure and interpretation of daily increments
Of 306 sagittae examined from larvae, juveniles, and adults, 272 (88%) had clear daily increments that could be interpreted from the primordium to the outer edge (Fig. 4A–D). Daily increments in sagittae of larvae and juveniles were easily interpreted in most cases, however sagittae from adults were more difficult to interpret towards the outer edge due to the increasing number of discontinuities with increasing age.
S. robustus. A Larval (14.8 mm, total length) sagitta at 625x magnification (scale bar 14 μm). B Sagitta from a post-larva (35 mm caudal-fork length, CFL) at 250x magnification (scale bar 39 μm). C Daily increments (Di) near outer edge of an adult sagitta (76 mm) at 250x magnification (scale bar 10 μm). D Growth check in a sagitta from a juvenile at 250x magnification (between arrows; scale bar 33 μm)
Age and growth patterns
Larvae metamorphosed at approximately 30–40 days old. During juvenile stages most individuals were 40–140 days old. Adults ranged between 140 and 241 days old (n=272). The estimates of size at age show an increase in variation with increasing age (Fig. 5). The Gompertz growth curve is represented by \( L_t = 96.473{\rm{e}}^{ - {\rm{e}}^{0.6121 - 0.0103{\rm{age}}} } \), and accounted for 66.7% of the total variation in length (CFL).
Instantaneous growth rates for larvae (12–30 mm) ranged from 0.32 to 0.36 mm day−1 (mean=0.34, SD=0.02; Fig. 6) and juvenile (30–60 mm, CFL) growth rates ranged between 0.28 and 0.36 mm day−1 (mean=0.33, SD=0.04). Growth rates peaked when juveniles were approximately 60 days old. Adult growth rates ranged between 0.13 and 0.28 mm day−1 (mean=0.19, SD=0.02).
Sex ratio
Sex ratios were significantly different among samples collected from January 1999 to April 2001 (ANOVA, P=0.01, df=47); (Fig. 7). During the spawning season sex ratios in samples were not significantly different from 1:1 (ANOVA, P=0.47, df=31), but samples collected outside the spawning season were dominated by either males or females.
Macroscopic gonad stages
Information on spawning seasonality was evident from macroscopic staging of testes and ovaries (Fig. 8) based on characteristics summarised in Table 3. Between March and September, immature (stage I) and maturing (stage II) gonads were dominant in both sexes. In October, 20% of females had mature stage III ovaries and 7% had stage IV ovaries, indicating that spawning had begun. During October, males were predominantly stage I (47%) and stage II (41%) with 12% stage III. During November, stage III and stage IV ovaries were more numerous in samples than in any other month and in males stage II and stage III testes were most common. In December, mature females were abundant and only 3.2% were stage V. In males 39% of testes were stage II and 47% were stage III. No immature (stage I) gonads of either sex were observed during January when most females were mature (Stages III–V) and most males had testes that were bloodshot and flaccid, indicating recent spawning. In February, most females had stage I gonads and only 18% had stage IV ovaries, yet 40% of males had mature (stage III) gonads.
Length and age at first maturity
Length at first maturity (L 50)for male (n=295) and female (n=296) S. robustus were 61.7 mm and 60.3 mm, respectively (Fig. 9). These lengths coincided with ages of 137 and 132 days, respectively, from the Gompertz growth curve (Fig. 5). There was no significant difference between the size at which 50% of males and females reached maturity (ANOVA, P=0.73, df=11; n=591). During the spawning period, the smallest male sampled with stage II (maturing) gonads was 50 mm CFL and the smallest female was 52 mm CFL.
Gonosomatic index
Gonosomatic indices for S. robustus were high (>4) from October to the end of February (Fig. 10). From March until September, mean GSI values were consistently low (<3) for both sexes and ranged from 0.76 to 3.2 for males and 1.36 to 2.13 for females. Mean GSI values for both sexes increased during October, peaked in November, and remained high during December and February (summer). GSI values for males were significantly higher than those for females during the spawning season (GLM, P <0.001, df=7).
Seasonal abundance and spatial distribution of larvae
From ichthyoplankton samples collected in Spencer Gulf, Gulf St Vincent and Investigator Strait, 243 S. robustus larvae were identified. Peak larval densities occurred during January (mean=0.011 larvae m−3) and March (mean=0.003 larvae m−3). No larvae were found in samples collected in May, June, or September (Fig. 11). Larvae were only found in Spencer Gulf, Gulf St Vincent and Investigator Strait and no larvae were found in samples collected from offshore waters during February and March 2000 (Fig. 12).
Microscopic characteristics of ovaries
Analysis of histological preparations (n=85) showed there were three oocyte developmental stages present within most mature ovaries (Table 3, Fig. 13). Most ovarian sections from fish sampled in September and early October contained un-yolked, partially yolked, and advanced yolked oocytes (Fig. 13A–C). POFs of various stages of degeneration were present within ovaries from late October through to late January (Fig. 13D–F). During the spawning period day 0 (less than 24 h old) and day 1+ (greater than 24 h old) POFs co-occurred with partially hydrated, un-yolked, partially yolked, and advanced yolked oocytes (Fig. 13D). In samples of spawning females collected in late January most oocytes were advanced yolked and most ovaries contained both day 0 and day 1+ POFs.
S. robustus. Histologically prepared ovarian sectionsshowing developing oocytes in macroscopic stages I–III and V. A Immature, macroscopic stage I ovary with developing oocytes in un-yolked (Uyo) and partially yolked (Pyo) stages; Nu nucleus. B, C Macroscopic stage-II and stage-III ovarian sections with partially yolked oocyte stages present and yolk granules (Yg) in advanced yolked oocytes; G granulosa layer; Z zona radiata. D Mature, macroscopic stage-IV ovary, with collapsed partially hydrated (H), un-yolked, partially yolked, and advanced yolked oocytes co-existing with day 0 and day 1+ post-ovulatory follicles (POFs 0 and 1+). E Partially hydrated oocytes with day 0 POFs, indicating recent spawning (less than 24 h before capture). F Day 1+ POFs, showing more advanced breakdown of the POF structure (Scale bar=150 μm)
Spawning fraction and frequency
In 14 samples collected between September and February, 71 fish had ovaries containing hydrated oocytes, 27 had day 0 POFs and 30 had day 1+ POFs (Table 4). The samples collected in September and early October contained no actively spawning fish. Two samples collected in late October from Outer Harbour had spawning fraction values of 11.1% and 22.2%. In November in Boston Bay spawning fraction estimates varied between 37.5 and 80% and at Outer Harbour estimates varied between 50 and 100%. The spawning fraction for a single sample (n=23) collected at Fitzgerald Bay in late January was 91%, indicating that during this period each female was spawning almost every day. The mean spawning fraction calculated from all samples with hydrated oocytes and day 0 POFs was 56.6%. The mean spawning frequency equated to one spawning approximately every 1.8 days.
Batch fecundity
Batch fecundities ranged from 209 to 1,797 hydrated oocytes per batch (mean=756, SD=341) for individuals ranging between 67 and 96 mm (CFL) and between 2.60 g and 9.07 g in weight. Batch fecundities for fish of similar lengths, which were collected at Outer Harbour (NGSV) and Cape Donnington (SSG) were significantly different (GLM, P=0.023, df=4). Fish (70–85 mm CFL) sampled at Outer Harbour had lower batch fecundities (mean=555 hydrated oocytes, SD=182) than those from Cape Donnington (72–88 mm CFL; mean=947, SD=253). The linear relationships between batch fecundity and gonad-free weight provided a slightly better fit than batch fecundity and CFL at both locations (Fig. 14A–D).
Discussion
S. robustus is a small short-lived, fast-growing temperate clupeoid that reaches sexual maturity after 4–5 months and spawns small batches of eggs every 1–2 days over an extended period during spring and summer. These traits are similar to those that distinguish the life-history strategies of tropical Spratelloides and stolephorid anchovies Stolephorus heterolobus and S. devisi from other relatively short-lived species of tropical clupeoids (Lewis et al. 1983). The life-history strategy of Spratelloides robustus differs from that of most other temperate clupeoids, such as S. sagax and H. vittatus, which live longer, grow more slowly during adult stages, and spawn over several seasons. S. robustus also appears to spawn mainly in Gulf St Vincent and Spencer Gulf where summer sea-surface temperatures reach levels similar to those in sub-tropical and tropical regions, and where several other species with tropical life histories, such as sygnathids (pipefish), blue crabs Portunicus pelagicus, and yellowfin whiting Sillago schomburgkii, are also found (Edyvane and Shepherd 1999).
The strong correlation between the number of increments deposited and the number of days elapsed since the OTC treatment provides evidence that the growth increments in the sagittae of juvenile Spratelloides robustus are deposited daily. This pattern of increment deposition in sagittae has also been validated for other short-lived and long-lived species, including T. aestauria, S. delicatulus, Herklotsichthys quadrimacultus, and Sardinops sagax (Re 1984; Hayashi et al. 1989; Hoedt 1992; Milton et al. 1993).
S. robustus appears to live for less than 1 year, as no sagittae examined contained more than 241 daily increments or any evidence of annuli. However, this interpretation should be viewed cautiously until the otoliths from fish larger than 100 mm CFL are analysed. Life-spans of less than 1 year are common among small sub-tropical and tropical clupeoids but are rare in small to medium-sized temperate clupeoids, such as Hyperlophus vittatus and Sardinops sagax, which usually reach maximum ages of approximately 4 and 6–8 years, respectively (Table 5). No other essentially temperate clupeoid is known to live for less than 1 year. The scaled herring Herengula jaguana has a similar life history and a distribution that extends into temperate waters; however this species predominantly inhabits the sub-tropical and tropical waters of the Gulf of Mexico and Brazil and is slightly longer lived (approximately 1 year); (Whitehead 1988; Pierce and Mahmoundi 2001).
The growth rates of juvenile S. robustus (0.36 mm day−1), are similar to those of juveniles of other essentially temperate clupeoids, such as Sardinops sagax, which grow at approximately 0.38 mm day−1. However, adult Spratelloides robustus maintain moderate growth rates of 0.13–0.28 mm day−1 after reaching sexual maturity, whereas Sardinops sagax grow more slowly at rates of approximately 0.06 mm day−1 during adulthood(Table 5). Short-lived sub-tropical and tropical clupeoids, including T. aestauria, Spratelloides lewisi, S. delicatulus, and H. jaguana, grow quickly throughout life and exhibit similar growth patterns to S. robustus (Milton et al. 1991; Hoedt 1992; Pierce and Mahmoundi 2001). Like S. robustus these species lack the slower, "asymptotic" adult growth phase observed in longer-lived clupeoids upon reaching sexual maturity, for example, Sardinops sagax and Hyperlophus vittatus (Butler et al. 1996; Fletcher and Blight 1996; Gaughan et al. 1996).
A recent analysis of clupeoid life-history strategies suggested that metabolic energy is allocated sequentially to the processes of growth and then reproduction (Rochet 2000). However, this is not consistent with findings for Spratelloides robustus, as only 50–60% of growth occurred before the onset of sexual maturity.Similarly, tropical Spratelloides species reach maturity at approximately 50–60% of their maximum size, at ages of 1–2 months, and growth rates remain relatively high throughout adulthood (Table 5). In contrast, the long-lived temperate clupeoids H. vittatus and Sardinops sagax reach maturity at ages of 12 and 18 months, respectively, after approximately 70% of growth has occurred ,and growth rates decrease significantly after sexual maturity. Therefore, the life-history strategies of long-lived temperate clupeoids appear to conform to the hypothesis of Rochet (2000), whereas short-lived tropical species and Spratelloides robustus have strategies that diverge from this hypothesis.
The monthly patterns in the sex ratios of S. robustus suggest that schooling behaviour changes during the spawning season and mature individuals aggregate in schools comprising similar numbers of each sex. This may be a tactic to optimise fertilisation rates during periods when SSTand food availability are suitable for successful spawning. These findings contrast with those from other studies that found that schools of spawning fish are mostly dominated by males, for example, E. mordax and Seriphus politus; however few detailed studies of the reproductive biology of male clupeoids have been undertaken (DeMartini and Fountain 1980; Hunter and Goldberg 1980).
Macroscopic analysis of gonads and gonosomatic indices suggest the peak spawning season for Spratelloides robustus in South Australian waters occurred between October and February, which is consistent with findings of Blackburn (1941). Similarly, the presence of advanced yolked and hydrated oocytes and post-ovulatory follicles in ovaries suggest S. robustus spawns during this spring and summer period. Furthermore, S. robustus larvae and post-larvae were only found in Spencer Gulf, Gulf St Vincent, and Investigator Strait during spring and summer. Hence, the spawning season begins earlier than for other temperate clupeoids, such as Sardinops sagax and H. vittatus, which mostly spawn from December until March (Ward et al. 2001b).
The spawning season of S. robustus occurred when SSTs in Spencer Gulf and Gulf St Vincent were between 19 and 26°C. The lower limit of this SST range is significantly higher than the minimum critical temperature for spawning by Sardinops sagax, which spawns when SSTs fall as low as 13.8°C (Lo et al. 1996; Ward et al. 2001b). Spratelloides robustus mainly spawns when SSTs in the northern parts of the gulfs are similar to those in sub-tropical and tropical regions of northern Australia and the Indo-Pacific (18.5–26°C; Kailola et al. 1993). H. vittatus also spawn during spring and summer when zooplankton densities in Spencer Gulf and Gulf St Vincent are high. Spawning when food availability is high may allow these clupeoids to acquire the necessary energy to spawn at high frequencies throughout protracted 3 to 5 month reproductive periods.
S. robustus larger than the length at maturity (L 50)were collected from inshore and offshore areas of the Great Australian Bight during the spawning season, yet no larvae were found in this region during February and March. This suggests spawning is confined to warmer, semi-protected South Australian gulf waters. During summer, hydrographic conditions in the shallow embayments of the eastern Great Australian Bight are similar to those in the two gulfs. Hence, S. robustus may also spawn in these systems; however, no extensive ichthyoplankton surveys have been undertaken in these embayments.
The organisation of developing oocytes in ovaries suggests S. robustus spawn multiple batches of eggs, a trait that is common in other temperate and tropical clupeoids, including Sardinops sagax, H. vittatus, Herklotsichthys quadrimaculatus, and E. mordax (Williams and Clarke 1983; Hunter and Macewicz 1985; Butler et al. 1996; Gaughan et al. 1996). Post-ovulatory follicles of S. robustus are similar morphologically to those described in other temperate and tropical clupeoids, including E. mordax, S. sagax and Encrasicholina heteroloba (Hunter and Goldberg 1980; Wright 1992). However, the post-ovulatory follicles of sub-tropical and tropical Spratelloides species have not been described.
The co-occurrence of several different oocyte developmental stages and day 0 and day 1+ POFs within ovaries suggests S. robustus spawn batches of eggs in close succession (Hunter and Macewicz 1985). During the peak reproductive period, S. robustus spawn approximately every 1–2 days, which is consistent with the patterns observed for small sub-tropical and tropical clupeoids, but contrasts those of many temperate clupeoids (e.g. Sardinops sagax, Engraulis mordax) that spawn at frequencies of approximately 7 days over several seasons (Table 5). The high spawning frequencies observed for Spratelloides robustus and some short-lived tropical clupeoids may be a strategy to compensate for low batch fecundities and a single, protracted spawning season. Other studies suggest that spawning frequencies for clupeoids are limited by energy reserves and food availability, which may partially explain why S. robustus spawn in the gulfs where zooplankton densities are high during summer (Hunter and Goldberg 1980).
The rates of degeneration of POFs in marine fishes vary between species and are affected by water temperature (Hunter and Macewicz 1985; Fitzhugh and Hetler 1995). For example, clupeoids such as Sardinops sagax and E. mordax spawn at SSTs of 13–19ºC and have POFs that last for 48 h, whereas Atlantic menhaden Brevooritia tyrannus spawn at similar temperatures and have POFs that remain in the ovary for up to 60 h after spawning (Fitzhugh and Hetler 1995). The effects of temperature on the degeneration rates of POFs have not been determined for Spratelloides robustus. Spawning frequencies were estimated on the basis of day 0 POFs only, because they were the only POFs that could be staged with confidence.
Batch fecundities of S. robustus were lower than those of other temperate clupeoids, such as Sardinops sagax, but were similar to those of small short-lived tropical clupeoids, including Spratelloides spp. and Encrasicholina spp. (Table 5). This may reflect the volumetric constraints associated with small body sizes and small visceral cavities. Larger clupeoids, such as sardines and herring, have the visceral capacity to produce larger batches of hydrated oocytes, whereas smaller species like S. robustus, which produce relatively large eggs (~1.00 mm diameter) are limited in batch size. S. robustus may compensate for low fecundity and the constraints of small body size by spawning more frequently and having a protracted spawning season. Batch fecundities of similar-sized fish from southern Spencer Gulf and northern Gulf St Vincent varied significantly, which suggests that the number of eggs spawned is not strictly determined by allometrics and may also be influenced by local variation in hydrographic conditions and food availability (Macewicz et al. 1996).
Hydrographic conditions in Spencer Gulf and Gulf St Vincent may explain why the life history of S. robustus is similar to that of small, short-lived clupeoids that inhabit sub-tropical and tropical regions. SSTs remain high in the gulfs for 4 –5 months of the year, thus these systems are effectively "seasonally sub-tropical" in an otherwise temperate region. The high summer SSTs in the gulf systems may also explain the occurrence of a suite of other species that have life-history strategies similar to those of sub-tropical and tropical taxa.
References
Beamish RJ, McFarlane GA (1983) The forgotten requirement for age validation in fisheries biology. Trans Am Fish Soc 112(6):735–743
Blackburn M (1941) The economic biology of some Australian clupeoid fish. Aust Comm Ind Res Organ Bull138(6):55–63
Butler JL, Granado ML, Barnes JT, Yaremenko M, Macewicz BJ (1996) Age composition, growth, and maturation of the Pacific sardine Sardinops sagax during 1994. Cal COFI Rep 37:152–159
Campana SE, Neilson JD (1985) Microstructure of fish otoliths. Can J Fish Aquat Res42:1014–1032
Clarke TA (1987) Fecundity and spawning frequency of the Hawaiian anchovy or Nehu, Encrasicholina purpurea. Fish Bull U S 85:127–137
Conover DO (1992) Seasonality and the scheduling of life history at different latitudes. J Fish Biol41 (Suppl B):161–178
Dalzell P (1985) Some aspects of the reproductive biology of Spratelloides gracilis (Schlegel) in the Ysabel Passage, Papua New Guinea. J Fish Biol 27:229–237
Dalzell P (1987) Notes on the biology of Spratelloides lewisi (Wongratana, 1983), a recently described species of sprat from Papua New Guinea waters. J Fish Biol30:691–700
DeMartini EE, Fountain RK (1980) Ovarian cycling frequency and batch fecundity in the queenfish, Seriphus politus: attributes representative of serial spawning fishes. Fish Bull U S 79(3):547–560
Edyvane KS, Shepherd SA (1999) The ecology of Australia's rock reefs. South Australia. In: Andrew N (ed) Under the southern seas. University of New South Wales Press, Sydney, pp 40–49
Fitzhugh GR, Hetler WF (1995) Temperature influence on postovulatory follicle degeneration in Atlantic menhaden, Brevoortia tyrannus. Fish BullU S 93:568–572
Fletcher WJ, Blight SJ (1996) Validity of using translucent otoliths to age the pilchard, Sardinops sagax from Albany, Western Australia. Mar Freshw Res 47:617–624
Gaughan DJ, Fletcher RJ, Tregonning RJ, Goh J (1996) Aspects of the biology and stock assessment of the whitebait, Hyperlophus vittatus, in south Western Australia. (Fisheries research report no. 108) Fisheries Department of Western Australia, Perth
Hayashi A, Yamshita Y, Kawaguchi K, Ishii T (1989) Rearing methods and daily otolith ring of Japanese sardine larvae. Nippon Suis Gakkai 55(6):997–1000
Hoedt FE (1992) Validation of daily growth increments in otoliths from Thyryssa aestuaria (Ogiliby), a tropical anchovy from Northern Australia. Aust J Mar Freshw Res 43:1043–1050
Hunter JR, Goldberg SR (1980) Spawning incidence and batch fecundity in northern anchovy, Engraulis mordax. Fish Bull U S 77:641–652
Hunter JR, Macewicz BJ (1985) Measurement of spawning frequency in multiple spawning fishes. In: Lasker R (ed) An egg production method for estimating spawning biomass of pelagic fish: application to the northern anchovy, Engraulis mordax. (National Oceanic and Atmospheric Administration technical report no. 36) National Marine Fisheries Service USA, Seattle, Wash., pp 79–94
Hunter JR, Lo NCH, Leong RJH (1985) Batch fecundity in multiple spawning fishes. In: Lasker R (ed) An egg production method for estimating spawning biomass of pelagic fish: application to the northern anchovy, Engraulis mordax. (National Oceanic and Atmospheric Administrationtechnical report no. 36)National Marine Fisheries Service USA, Seattle, Wash., pp 67–79
Hutchins B, Swainston R (1986) Sea fishes of southern Australia. Swainston Publishing, Perth
Kailola PJ, Williams MJ, Stewart PC, Reichelt RE, McNee A, Grieve C (1993) Australian fisheries resources. Bureau of Resource Sciences, Department of Primary Industries and Energy, Fisheries Research and Development Corporation, Canberra, Australia
Lewis AD, Sharma S, Prakash J, Tikomainiusi-ladi B (1983) The Fiji bait fishery 1981–2, with notes on the biology of the gold-spot herring Herklotsichthys quadrimaculatus (Clupeidae) and the blue sprat Spratelloides delicatulus (Dussumieriidae). (Technical report no. 6) Fisheries Division, Ministry of Agriculture and Fisheries, Suva, Fiji
Lo NCH, Green Ruiz YAA, Cerventes MJ, Moser HG, Lynn RJ (1996) Egg production and spawning biomass of Pacific sardine, Sardinops sagax in 1994, determined by the daily egg production method. Cal COFI Rep37:160–174
Macewicz BJ, Castro-Gonzalez JJ, Cotero-Altamirano CE, Hunter JR (1996) Adult reproductive parameters of Pacific sardine (Sardinops sagax) during 1994. Cal COFI Rep 37:140–151
Milton DA, Blaber SJM, Rawlinson NJF (1991) Age and growth of three species of tuna baitfish (genus: Spratelloides) in the tropical Indo-Pacific. J Fish Biol 39:849–866
MiltonDA, Baber SJM, Rawlinson NJF(1993) Age and growth of three species of clupeids from Kiribiti, tropical central South Pacific. J Fish Biol43:89–108
Milton DA, Blaber SJM, Rawlinson NJF (1995) Fecundity and egg production of four species of short-lived clupeoid from Solomon Islands, tropical South Pacific. ICES J Mar Sci52:111–125
Neira FJ, Miskiewicz AG, Trnski T (1998) Larvae of temperate Australian fishes. Laboratory guide for larval fish identification. University of Western Australia Press, Perth, Western Australia
Pierce DJ, Mahmoundi B (2001) Age and growth of the scaled herring, Herengula jaguana from Florida waters, as indicated by microstructure of the sagittae. Fish Bull U S 99:202–209
Re P (1984) Evidence of daily and hourly growth in pilchard larvae based on otolith growth increments, Sardina pilchardus (Walbaum, 1792). Cybium 8(1):33–38
Rochet MJ (2000) A comparative approach to life-history strategies and tactics among four orders of teleost fish. ICES J Mar Sci57:228–239
Stearns SC (1992) The evolution of life histories. Oxford University Press, New York
Velazquez CQ, Nevarez-Martinez MO, Gluyas-Millan MG(2000) Growth and hatching dates of juvenile Pacific sardine Sardinops caeruleus in the Gulf of California. Fish Res48:99–106
Ward TM, Hoedt FE, McLeay LJ, Dimmlich WF, Kinloch MW, Jackson G, McGarvey R, Rogers PJ, Jones K (2001a) Effects of the 1995 and 1998 mass mortalities on the spawning biomass of Sardinops sagax in South Australia. ICES J Mar Sci 58(4):830–841
Ward TM, Staunton Smith J, Hoedt F, McLeay LJ, Dimmlich WF, Jackson G, Kinloch M, Rogers PJ, Jones K (2001b) Have the recent mass mortalities of Sardinops sagax in Australia facilitated an expansion of the distribution and abundance of Engraulis australis. Mar Ecol Prog Ser220:241–251
Whitehead JP (1988) FAO species catalogue, Part 1, Vol 7. Clupeoid fishes of the world. British Museum of Natural History, London
Whitehead JP, Nelson GJ, Wongratana T (1988) FAO species catalogue, Part 2, Vol 7. Clupeoid fishes of the world. British Museum of Natural History, London
Williams VR, Clarke TA (1983) Reproduction, growth and other aspects of the biology of the gold spot herring, Herklotsichthys quadrimaculatus (Clupeidae), a recent introduction to Hawaii. Fish Bull U S 82(3):587–597
Wright PJ (1992) Ovarian development, spawning frequency and batch fecundity in Encrasicholina heteroloba. J Fish Biol 40:833–844
Acknowledgements
We thank Lachlan McLeay, Wetjens Dimmlich, Paul Jennings, David Fleer, and the crew of the R.V. "Ngerin" (Neil Chigwidden, Dave Kerr, Chris Small, and Ralph Putz) for helping collect fish samples. Craig Noel and David Short provided equipment and advice on larval age determination and tetracycline validation techniques. Dr. Yongshun Xiao helped with derivation of the Gompertz growth model. Dr. William Breed and Sandra Leigh from the Adelaide University Anatomy Department prepared histological sections of ovaries. Drs. Tony Fowler, Scoresby Shepherd, and Keith Jones (South Australian Research and Development Institute) commented on drafts of the manuscript. This project was made possible by logistic support provided by SARDI Aquatic Sciences and Adelaide University, Environmental Biology Department. This study was carried out in accordance with the required legal and ethical standards.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G.F. Humphrey, Sydney
Rights and permissions
About this article
Cite this article
Rogers, P.J., Geddes, M. & Ward, T.M. Blue sprat Spratelloides robustus (Clupeidae: Dussumieriinae): a temperate clupeoid with a tropical life history strategy?. Marine Biology 142, 809–824 (2003). https://doi.org/10.1007/s00227-002-0973-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-002-0973-8