Abstract
Purpose
The aim of the study was to establish and propose the utilisation of paediatric National Diagnostic Reference Levels (DRLs) for dose length product (DLP) and volume computed tomography dose index (CTDIvol) for most commonly routine computed tomography (CT) for paediatric examinations.
Method
These were based on the most commonly used routine brain protocol, the chest protocol and the abdominal-Pelvis protocol for head, chest and abdominal scans respectively. 200 images which met the selection criterion were used for the analysis.
Results
The measured median and 75th percentile CTDIVOL of head were 14.9 and 15.5 mGy, chest was 1.4 and 1.7 mGy, abdomen-pelvis were 5.1 and 15.3 mGy respectively. While DLP for head were 457 and 498 mGy-cm, chest was 13 and 14 mGy-cm, abdomen-pelvis were 26 and 29 mGy-cm. Similar trends were observed in aged 6.-10y and 11-16y.
Conclusion
The study recommends the use of the established CTDIVOL and DLP as reference values for clinical application in Ghana.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The International Commission on Radiation Protection (ICRP) first introduced the term ‘diagnostic reference level’ (DRL) in Publication 73 [1]. The concept was subsequently developed further in ICRP Publication 135 and European Commission (EC) Directive on Radiation Protection mainly for the implementation of the principle of optimisation of radiation protection in medical exposure [2, 3]. Additionally, it is used in medical imaging with ionising radiation to indicate whether, in routine clinical practice, the patient dose or administered activity from a specified procedure is unusually high or low for that procedure [1,2,3].
The DRL has proven to be an effective tool that aids in optimisation of protection in the medical exposure of patients for diagnostic and interventional procedures. DRLs are not intended for use in radiation therapy, but they should be considered for imaging for treatment planning, treatment rehearsal, and patient set-up verification in radiotherapy.
In recent times, International Commission on Radiological Protection (ICRP) in ICRP publication 135 recommend the establishment of Diagnostic Reference Levels for medical imaging and interventional procedure. This recommendation has become the basis for the establishment of DRL for optimisation of specific clinical procedure to promote safe use of ionising radiation for the collective benefit of society [3].
Additionally, optimisation of these procedures based on DRL enable dose estimates from different imaging modalities. This is to determine whether in clinical routine practice the variations of dose between healthcare facilities is substantial high or low for the same examination or procedure and similar patient groups. Such observations indicate the need for standardization of dose reduction techniques of each examination or procedure [4,5,6].
Furthermore, the use of DRL has been proven to be an effective tool for optimization and patient protection in diagnostic radiology and interventional procedures. These requires the application of patient-specific protocols tailored to patient age or size, region and clinical indication in order to ensure that patient doses are as low as reasonably achievable for the clinical purpose of the examination [1, 3, 7]. Hence, can provide the stimulus for monitoring practice to promote improvements in radiation protection of patients.
Generally, DRLs are set in terms of a specific practical dose quantities used to monitor practice and promote improvements in patient protection and not for patient specific dose limits. For instance, in computed tomography (CT), DRLs are set using Computed Tomography Dose Index (CTDI) and Dose Length Product (DLP). These two parameters are commonly used to monitor and promote practice in CT examination. In practice, CTDI is defined for axial scanning, however it can also be used in helical protocols with an application of the necessary correction factor. Additionally, CTDI are generally measured as a weighted CTDI (CTDIW) or volumetric CTDI (CTDIV) with respect to dose expressed as weighted average in the scan area or dose within the scan volume respectively [2, 6, 8].
The concept of DRL was initially based on mean values of dose parameters rather than median values. However, the median values have been recommended by ICRP in recent Publication 135 [2]. These median values are recommended to be used to compared with the relevant national, regional, or international benchmarks of similar practice. Clinical protocols for performing a particular examination or procedure should be reviewed if the comparison shows that the local or facility’s typical dose estimates exceeds the national DRL. In addition, if the facility’s typical dose is substantially below the national DRL, and it is evident that the exposures are not producing images of diagnostic usefulness or are not yielding the expected medical benefit to the patient [2, 9].
Additionally, for DRL to be used to determine whether in clinical routine practice the variations of dose between healthcare facilities is substantial low or high for the same examination or procedure. In addition to similar patient group in terms of age or size, a selected numerical value of the DRL quantities is set at the 75th percentile of the median quantity distributions observed at multiple facilities or an institution [1, 3, 10].
Even though, the establishment of DRLs relies on standardised indicators of dose parameters for optimizing high dose procedures. It however important to note that these dose parameters reflect the output of the scanners and do not provide estimates of individual patient dose. Hence, the investigation will only reflect the comparison of dose parameters with other facilities using the DRLs values [3, 5, 7].
Several studies have adopted this procedure in establishing and utilising DRL as part of dose optimization procedure. For instance, Salama et al. established paediatric DRL in Egypt [11] using the ICRP Publication 135 recommended protocol [1,2,3, 5].
The aim of the study was to establish and propose the utilisation of paediatric National Diagnostic Reference Levels (NDRLs) using CTDIVOL and DLP for most commonly used paediatric diagnostic imaging examination/procedures in Ghana.
2 Methods
2.1 Materials/equipment
Five radiology centres including 3 Government of Ghana facilities and 2 private facilities across the country, where most paediatric CT imaging are done where use for the study [11, 12]. All the facilities had picture archiving and communication (PAC) system with radiology information system (RIS) for managing the imaging process and the data systems with special focus on paediatric patients.
Initially QC assessments were performed on all the CT scanners using Head and Body Phantom with calibrated ion chamber (PTW DIADOSE Meter, ref. number T11035). This was done using the harmonized International Atomic Energy Agency (IAEA) standardize QC protocol for diagnostic radiology recommendation. As part of the technical cooperation regional project RAF6/053 to improve safety and effectiveness of medical imaging in Africa.
The CT scanners that were used for the data collection in this study include one General Electric (GE-USA), two Philips (Switzerland), one Toshiba (Japan) and one Siemens (Germany) machines brands, details of which are presented in Table 1. The scanners were manufactured and installed in the radiology centres between 2016 and 2020. The number of slices of the Scanners ranged from 16 to 640.
The operating tube voltage of the CT scanners ranged between 80 and 140 kVp, and effective voltage mean value of 60 kVp with Automatic Exposure Control (AEC) activated protocols and fixed tube current settings and an effective mAs of 40.
The slice technology of the detector-row/per slice were 8/16, 32/64, 64/128 320/640 of the survey sample for GE, Philips, Siemens, and Toshiba machines respectively.
The designed form was distributed to and completed by the five facilities in Ghana. The hospitals/facilities included District/Regional Hospitals, Private Diagnostic Imaging Centres and Teaching Hospitals.
3 Methods
The biodata and the dose parameters were retrieved from the registry and the structured dose report from the PAC system respectively. The patient biodata (Age, and Gender) and the imaging input parameters of interest (scan length, slice thickness, pitch factor, kVp, mA and exposure time) and output parameters (CTDIvol and DLP) were extracted into the IAEA structured Form/Questionnaire (Table 2) distributed to the five CT facilities. Data for routine head CT, chest CT and abdominal-Pelvis CT examinations for paediatric patients between the ages of 0–5, 6–10, 11–16 years old were collected.
Furthermore, as part of the inclusive criterion experienced radiologists reviewed the images of the patient data that were collected and those that were accepted were included in the study otherwise rejected. Additionally, inclusion criterion includes children under 16 years old whose images were accepted by experienced radiologists (qualitative assessment). In addition, only patients’ data with complete dose report with no contrast medium administered were also accepted as part of this study. Outpatients who sought CT examination for varied diagnostic requests other than those linked with any pathological problem related to the region of interest made up of the inclusive study's sample population of 200 patients out of a total of 300 patients data collected. Whilst exclusion criterion were patients who had contrast materials with an incomplete dose report together with images which were not accepted by the radiologist and failed the quantitative assessment (signal to noise ratio). That is for dose report to be accepted both the quantitative and qualitative assessment must meet the accepted protocol of the IAEA harmonised protocol.
3.1 Validation of the CT scanners
The equipment specific daily QC mostly referred to as calibration (warming) were performed on each of the five CT equipment. This was to ensure that basic CT components are working properly. Additionally, this was also to ensure that the CT equipment are performing as indicated by the manufacturers and by international standards. Furthermore, the scanner readings were validated using the recommendations by harmonized International Atomic Energy Agency standardize protocol for diagnostic radiology. The available daily scanning procedure and protocol of the various manufacturers were used to complete the calibration check, as illustrated in Fig. 1.
Additionally, as part of the QC procedure, the CTDI and the DLP were measured using the head and body Polymethyl methacrylate (PMMA) phantom. The dosimeter was serially inserted into the center and periphery holes, and the results estimated using Eq. 1 to obtained weighted CTDI. The volume CTDIVOL was then estimated using Eq. 2. A deviation of less than 20% of the commissioning test results and the manufacturers recommendation were accepted. Finally, the DLP was estimated by multiplying the CTDIvol by the scan length as shown in Eq. 3.
where,
\({\mathrm{CTDI}}_{{~}^{100}\!\left/ \!{~}_{\mathrm{centre}}\right.}\) represent measurement at the centre.
\({\mathrm{CTDI}}_{{~}^{100}\!\left/ \!{~}_{\mathrm{periphery}}\right.}\) represent measure at the periphery.
The procedure of how the head and body phantom was placed, and the readings taken are shown in Fig. 2.
The product of the \({CTDI}_{Vol}\) and the length of the CT scan along the \(Z-axis\) of the patient, \(L\) is the Dose Length Product (\(DLP\)).
Dose descriptors (CTDIVOL and DLP) from a sample of 20 typical paediatric patients, were recorded from the structured dose report as part of the PAC system of the RIS at all the five diagnostic facilities. The CTDI was recorded from each patient image data together with the DLP on all the 200 patient who form part of the sample population.
The proposed DRLs were determined based on the recommended method as published in ICRP publication 135. Which recommend the use of the distribution of median doses from each facility and to determine the 75th percentile of the median distribution to determine the proposed DRL.
The proposed DRLs was based on the median values of the head, chest, and abdominopelvic CT examination at the participating facilities.
4 Results
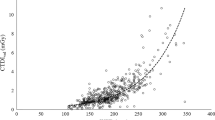
The QC assessment of the CTDIVOL and DLP shows a consistent median distribution for both the head and chest protocol as shown in Figs. 3 and 4 respectively. These results agreed with the commissioning test data with a percentage variation of 6.5% and 3.2% for CTDIvol and DLP respectively. This is less than 10% as recommended by the IAEA Harmonised diagnostic radiology CT QC protocol for Africa, hence the QC results passed the recommended test. Details of these are presented in the appendix Tables 5, 6, 7 and 8.
4.1 Gender distribution
The patient data comprise; 93 females and 107 males, which represent 46.5% female and 53.5% males as shown in Fig. 5. Presentation of the data was summarized as the mean, median, the upper quartile (3rd quartile), maximum and minimum values in terms of age and gender variation of the measured parameters.
The data shows that CT examination of the head was the most frequently requested examination and constitute 50% of the total number of the sample used, followed by abdomen-pelvis examination 30% and then chest scan 20% as shown in Fig. 6.
4.2 Age and gender distribution
The study shows that the attendance of paediatric patients for CT examination increases from 0–5, 6–10 and to 11–16 years with and increase rate of 23%, 34% and 43% respectively as shown in Fig. 7 for female. With 25%, 37%, and 38% distribution of male participants of age 0–5, 6–10 and to 11–16 years respectively. Eventhough the study did not find any specific guideline for paediatric referrals for CT imaging, the outcome come the relactant of referring physicians to referred much younger paediatric patients (0–5 years) to older patients (11–16 years).
4.3 Dose protocol distribution
The protocol distribution of the dose parameters in terms of age variation are shown in Figs. 8, 9 and 10 of ages 0–5, 6–10 and 11–16 for CTDIvol respectively. The figures represent the distribution of CTDIvol values for Head, Chest and Abdominal-Pelvis (A-P) protocols for ages 0–5, 6–10 and 11–16 respectivelly.
Additionally, similar protocol distribution of age 0–5, 6–10 and 11–16 for DLP are shown in Figs. 11, 12 and 13 respectively. The figures represent the distribution of DLP values for Head, Chest and Abdominal-Pelvis (A-P) protocols for ages 0–5, 6–10 and 11–16 respectivelly.
4.4 Dose parameter
The summary of the dose report as part of the image data collected from the DICOM Header are presented in Table 3. This shows the estimated dose parameters of CTDI and DLP with respect to the head, chest and abdominal-pelvis regions, in terms of their minimum (min), median and 75th percentile values based on age specific variation.
5 Analysis and discussions
The selected paediatric CT examination of the head, chest, and Abdominal-Pelvis from five centres which met the selection criterion are presented in Table 3. The dose parameters of interest were volumetric-CTDI (CTDIVOL) and the DLP. The distribution of the estimated Head protocol of the CTDIVOL for ages 0–5 years old were 14.7 to 19.1 mGy. Additionally, the Chest CTDIVOL for ages 0–5 years old ranges from 1.2 to 1.7 mGy while the Abdominal-Pelvis CTDIVOL range from 3.1 to 15.5 mGy for ages 0–5 years.
Furthermore, the estimated Head DLP estimate ranges from 295 to 635 mGy-cm, Chest DLP range from 8 to 16 mGy-cm, while the Abdominal-Pelvis DLP range from 693.3 to 920.0 mGy-cm for ages 0–5 years. There were similar variation age 6–10 years and 11–16 years. A summary of these parameters is presented in Table 3 below. The estimated median distribution of the CTDIvol for head were higher than chest and Abdominal-Pelvis estimates. Analysis of the data shows that age influence the selection of the scan input parameters as both CTDI and DLP increases from ages 0–5, 6–10 to 11–16 years.
These variations of the minimum, maximum, median distribution and upper quartile were observed in the head, chest and abdomen-pelvis estimates for ages 6–10 and 11–16 as presented in Table 3, which increases with increasing age. Furthermore, Table 4 shows CTDIvol and DLP that are published in other countries are comparable to those established by this study.
There was no significant difference of the measured CTDI and the DLP with the same age bracket of the three varied age groups. However, there were observable increase along varied age groups as shown in Table 3.
Based on the established data, the proposed DRL for paediatric chest CT, abdomen CT and chest CT, for both CTDIvol and DLP are comparable to international published data as shown in Table 3 [13,14,15,16]. The third quartile of the median distribution will be used as the proposed DRL, However, the distribution of the data in terms of minimum and maximum range of values as presented in Table 2. This is to enable the range of the distribution of the estimated values. The median distribution or the 50th percentile is described as the ‘achievable dose’ level introduced by ICRP. However, the upper quartile of the 50th percentile distribution is recommended for institutional comparison.
Furthermore, despite the strong ICRP recommendation in publication 135 for Government to ensure that various diagnostic facilities set up DRL on specific imaging protocols, especially for paediatric patients, there is currently no paediatric patients referral guideline in addition to specific universal agreed procedure and protocol in any of the forty-eight (75) CT imaging facilities in Ghana [13, 17, 18].
Analysis of the 200-dose report shows that, 81% of all the CTDIVOL and DLP were within the accepted range of the recommendations while 19% were above these recommendations in terms of CTDIVOL and DLP.
6 Conclusion
In conclusion, current trend establishing DRL in paediatric CT imaging relies on the measured upper quartile values of CTDIvol and DLP in terms of the various regions of the most common clinical practice of Head, Chest, and Abdominal-Pelvis. These were determined found to be comparable to international accepted recommendations. This led to the proposed established paediatric National diagnostic Reference levels for CTDI vol and DLP values for head, chest and abdominal-pelvis examinations. This is intended to contribute to the ongoing efforts to promote radiation safety in medical imaging and align with international best practices for patients dose optimisation.
Data availability
The data and materials for publication are all available upon request at any time.
Code availability
Not applicable.
References
International Commission on Radiological Protection, Radiological protection in medicine. ICRP Publication. 2017; 135(19):1–234.
Satharasinghe D, Jeyasugiththan J, Wanninayake WMNMB, Pallewatte AS, Samarasinghe RANKK. Patient size as a parameter for determining diagnostic reference levels for paediatric computed tomography (CT) procedures. Physica Medica. 2022;102:55–65.
Blackwell CD, Gorelick M, Holmes JF, Bandyopadhyay S, Kuppermann N. Paediatric head trauma: changes in use of computed tomography in emergency departments in the United States over time. Ann Emerg Med. 2007;49:320–4.
Brady Z, Cain TM, Johnston PN. Paediatric CT imaging trends in Australia. J Med Imaging Radiat Oncol. 2011;55:132–42.
Brady Z, Cain TM, Johnston PN. Differences in using the International Commission on Radiological Protection’s publications 60 and 103 for determining effective dose in paediatric CT examinations. Radiat Meas. 2011;46:2031–4.
Brenner D, Elliston C, Hall E, Berdon W. Estimated risks of radiation-induced fatal cancer from pediatric CT. AJR Am J Roentgenol. 2001;176:289–96.
United Nations Scientific Committee on the Effects of Atomic Radiation, Sources and effects of ionizing radiation. Vol. I. UNSCEAR 2008 report to the General Assembly. Annex A: medical radiation exposures. New York, NY: United Nations; 2010.
Wiest PW, Locken JA, Heintz PH, Mettler FA. CT scanning: a major source of radiation exposure. Semin Ultrasound CT MRI. 2002;23:402–10.
Pages J, Buls N, Osteaux M. CT doses in children: a multicentre study. Br J Radiol. 2003;76:803–11.
Moss M, McLean D. Paediatric and adult computed tomography practice and patient dose in Australia. Australas Radiol. 2006;50:33–40.
Shrimpton PC, Hillier MC, Lewis MA, Dunn M. National survey of doses from CT in the UK: 2003. Br J Radiol. 2006;79:968–80.
Smith-Bindman R, Lipson J, Marcus R, Kim KP, Mahesh M, Gould R, et al. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch Intern Med. 2009;169:2078–86.
Optimization program. J Am Coll Radiol. 2010;7:614–24.
International Commission on Radiological Protection, 1990 Recommendations of the International Commission on Radiological Protection. ICRP Publication 60. Ann ICRP. 1991;21:1–201.
International Commission on Radiological Protection, Radiological protection and safety in medicine. ICRP Publication 73. Ann ICRP. 1996;26:1–47.
International Commission on Radiological Protection, Diagnostic reference levels in medical imaging: review and additional advice. ICRP Supporting Guidance 2. Ann ICRP. 2001;31:33–5.
Donnelly LF, Emery KH, Brody AS, Laor T, Gylys-Morin VM, Anton CG, Thomas SR, Frush DP. Minimizing radiation dose for pediatric body applications of single-detector helical CT: strategies at a large children’s hospital. Am J Roentgenol. 2001;176(2):303–6.
International Commission on Radiological Protection, Radiological protection in medicine. ICRP Publication 105. Ann ICRP. 2007;37:1–64.
Acknowledgements
I would like to acknowledge Ghana Atomic Energy Commission and the ICTP through the Associate program (2020–2025) for the support I received from the two institutions. Additionally, I acknowledge the participating facilities for allowing me to carry out the research in their facilities.
Funding
I personally funded this study with my own resources (no external funding was secured and used for this study).
Author information
Authors and Affiliations
Contributions
All the Authors were involved pre and post data collection and analysis during the study. The following specific activities were done by the Authors.
Corresponding author
Ethics declarations
Ethics approval
Approval was given for the research by the participating facilities and the ethical and Protocol Review Committee of the College of Basic and Applied Science, University of Ghana and the Participating Health Facilities. Additionally, the protocol and the application of same for this study was granted ethical clearance by the various Committees. The methods were performed in accordance with the guidelines and regulations as outline by the ethical and Protocol Review Committee of the College of Basic and Applied Science, University of Ghana and the Participating Health Facilities. All participants were assured of confidentiality and anonymity throughout the study.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
Not applicable.
Concept Note
Issahaku Shirazu, Raymond Edziah, Y. B Mensah, Elvis K. Tiburu; C. Schandorf, Theophilus. Sackey.
Pre-data collection activities
Including application for ethical clearance: Issahaku Shirazu, Theophilus Akumea Sackey. Raymond Edziah, Y. B Mensah, Elvis K. Tiburu; C. Schandorf.
Data collection and analysis
All Authors (Issahaku Shirazu, Raymond Edziah, Y. B Mensah, Elvis K. Tiburu; C. Schandorf, Theophilus Sackey.
Drafting of text
Issahaku Shirazu, Theophilus A. Sackey.
Review of text
Issahaku Shirazu, Raymond Edziah, Y. B Mensah, Elvis K. Tiburu; C. Schandorf, Theophilus A. Sackey.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shirazu, I., Sackey, T.A., Edziah, R. et al. Establishing national paediatric computed tomography diagnostic reference levels in Ghana. Health Technol. (2024). https://doi.org/10.1007/s12553-024-00903-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12553-024-00903-1