Abstract
Cyclopiazonic acid (α-CPA) is a tremorgenic mycotoxin that is commonly produced by certain species of the aspergilli, in particular Aspergillus flavus, which is more widely known for production of the aflatoxins. Despite the fact that α-CPA may co-occur with aflatoxins, immunoassay-based methods for monitoring for CPA have not been widely developed. We report the development and evaluation of several monoclonal antibodies (mAbs) for α-CPA. Two mAbs in particular were very sensitive, with IC50s of 1.1 and 1 ng/mL (clones 1418 and 1231, respectively). Tolerances to aqueous methanol or acetonitrile were good, which permitted the development of an antigen-immobilized competitive enzyme-linked immunosorbent assay (CI-ELISA) for detection of CPA in maize. Spiked or naturally contaminated maize, extracted with aqueous methanol, was diluted with buffer for analysis. The working range for the assay (IC20 to IC80) was from 5 to 28 μg/kg. Recoveries from maize spiked over the range from 2 to 50 μg/kg averaged 88.6 ± 12.6%. Twenty-eight samples of maize were tested by both the CI-ELISA and a liquid chromatography-fluorescence (LC-FLD) method. For the five samples above the limits of quantitation of both methods, CI-ELISA tended to overestimate CPA content, a difference that we speculate may be due to related metabolites or perhaps “masked” derivatives of CPA. The antibodies developed and the resulting CI-ELISA will be useful tools for further evaluation of the prevalence of this mycotoxin in maize.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
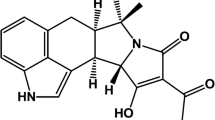
α-Cyclopiazonic acid (α-CPA) is an indole tetramic acid, with a molecular weight of 336.38 (Fig. 1). The toxin was first isolated by Holzapfel (1968) from Penicillium cyclopium Westling cultured on maize (corn) meal. Since that time, many species of Aspergillus and Penicillium have been found to produce CPA (Dorner 2002). These include some of the same fungi that produce the aflatoxins (Trucksess et al. 1987; Goto et al. 1996; Horn and Dorner 1999). Specifically, a high proportion of Aspergillus flavus strains produce CPA (Horn and Dorner 1999; Vinokurova et al. 2007), as do certain strains of Aspergillus fumigatus and Aspergillus phoenicis (Vinokurova et al. 2007). The toxin has been found as a natural contaminant in cheeses, figs, corn, rice, peanuts, millet, and feeds (Lansden and Davidson 1983; Rao and Husain 1985; Urano et al. 1992a; Burdock and Flamm 2000; Hayashi and Yoshizawa 2005; Heperkan et al. 2012). When fed to animals, α-CPA is capable of causing symptoms such as weight loss, diarrhea, pyrexia, dehydration, ataxia, immobility, and finally extensor spasm at death (Richard and Payne 2003). Turkeys and chickens can be affected by α-CPA (Miller et al. 2011), and it has been speculated that α-CPA contributed to the original outbreak of turkey X disease (Cole 1986). Toxicity of α-CPA is believed to be derived from the disruption of calcium homeostasis (Riley et al. 1995). The toxin is available commercially as an inhibitor of the sarcoplasmic reticulum Ca2+-ATPase. An acceptable daily intake for humans was estimated at 10 μg/kg body weight/day (Burdock and Flamm 2000). Because of possible co-occurrence and potential for synergistic toxicity with the aflatoxins, detection of CPA is relevant to both animal and human health (Urano et al. 1992a).
There are a variety of technologies used to detect CPA, including chromatographic and immunochemical methods. Most chromatographic methods involve steps to extract and isolate the toxin before the determinative step. Various combinations of acidified chloroform, chloroform/methanol, or mixtures of non-chlorinated solvents such as aqueous methanol or aqueous acetonitrile have been used to extract CPA from commodities. The latter typically use an aqueous component that is alkaline, in order to extract the toxin in the ionized form, as opposed to the neutral form extractable with acidified chloroform (Fig. 1). Whether the extraction is alkaline or acidic has been based, in part, upon the downstream cleanup. For example, silica solid-phase extraction (SPE) or silica minicolumns have been paired with the use of acidified chloroform (Goto et al. 1996; Norred et al. 1987). More recently, methanol/water or acetonitrile/water (7 + 3 v/v) with bicarbonate added has found more widespread use, likely because they avoid halogenated solvents (Urano et al. 1992b; Yu and Chu 1998; Dorner et al. 2001; Losito et al. 2002; Hayashi and Yoshizawa 2005; Aresta et al. 2003). Once extracted and isolated, samples are often submitted to a chromatographic step such as thin-layer chromatography (TLC) or liquid chromatography (LC). Chromatographic methods for CPA were reviewed by Dorner (2002) and include a variety of normal- and reverse-phase techniques. LC coupled to various forms of mass spectrometry is commonly used to detect mycotoxins, and CPA is no exception (Moldes-Anaya et al. 2009). However, application of the technique to CPA is not without its difficulties (Diaz et al. 2010). Aside from mass spectrometry, LC methods for CPA have typically relied upon detection of the absorbance of the toxin in the ultraviolet (UV) range at circa 279 nm. Several years ago, we noted that CPA, which is non-fluorescent, could be rendered fluorescent upon photolysis (Maragos 2009). The phenomenon has been used to develop an LC-fluorescence detection (LC-FLD) method for the simultaneous detection of aflatoxins and CPA in fungal cultures (Soares et al. 2010).
While chromatographic methods for detecting CPA have been widely reported, there have been far fewer reports describing immunochemical methods for this toxin. Several laboratories have previously developed antibodies that recognize CPA (Hahnau and Weiler 1991, 1993; Yu and Chu 1998). Such antibodies have been applied in both enzyme-linked immunosorbent assay (ELISA) and immunoaffinity column (IAC) formats (Dorner et al. 2001; Hahnau and Weiler 1991; Huang and Chu 1993; Hahnau and Weiler 1993; Yu and Chu 1998; Kononenko et al. 2012). Unfortunately, neither the CPA antibodies nor the IAC are available commercially, so most analysts must still resort to the more laborious isolation and detection procedures.
The objective of this work was to develop novel monoclonal antibodies that recognize α-CPA, so that they could be applied to the detection of α-CPA in maize and, later, as components of biosensors for multi-toxin detection. In the process of doing so, we also decided to update some of the isolation, cleanup, and detection procedures for α-CPA. For the latter, we chose to use a mixture of alkaline methanol/water for extraction, cleanup using strong anion exchange (SAX) SPE, and detection of the fluorescence of CPA following photochemical reaction (LC-FLD).
Materials and methods
Materials
Except where noted otherwise, deionized water (Nanopure II, Thermo Scientific) was used in the preparation of all reagents. N,N-Dimethylformamide (DMF) was ACS reagent grade. Α-CPA was obtained from two sources. The CPA used for chemical reactions was previously prepared by Odette Shotwell (USDA-ARS, Peoria, IL, USA) and had been stored for several years at −20 °C (batch 13125-5-c). The CPA used as analytical standard was produced by MP Biomedicals, LLC (Solon, OH, USA). CPA stock solution was prepared at a nominal concentration of 2 mg/mL by dissolving solid toxin in LC-grade acetonitrile. The actual concentration of toxin was determined by obtaining the UV spectrum of 1:200 dilutions of the stock in methanol (Beckman, model DU640 spectrophotometer) and using the extinction coefficient of 20,417 at 284 nm (Holzapfel 1968; Nesheim and Stack 2001). The concentrated stock was used to prepare an intermediate stock solution at 20 μg/mL in acetonitrile/water (4 + 1 v/v). For LC-FLD experiments, standards were prepared daily over the range of 20 to 1000 ng/mL in 1% ammonium hydroxide. For immunoassays, fresh standards were prepared daily over the range of 0.1 to 100 ng/mL. Several buffers were used in the immunoassays. These included 10 mM phosphate-buffered saline (PBS) (0.01 M sodium phosphate and 0.15 M sodium chloride in water, adjusted to pH 7.2) or 0.1 M PBS (0.1 M sodium phosphate and 0.15 M sodium chloride in water, pH 7.2). Citrate phosphate buffer (CPB) was composed of 0.05 M sodium citrate and 0.1 M sodium phosphate, adjusted to pH 5.0. Chicken egg albumin (OVA) and polyvinyl alcohol (PVA) were purchased from Sigma-Aldrich (Milwaukee, WI, USA). Β-Lactoglobulin (BLG) was purchased from MP Biomedicals. Bovine serum albumin (BSA) was obtained from Fisher Scientific (Waltham, MA, USA). All other chemicals were reagent grade or better and purchased from major suppliers.
Maize samples
Samples of control (no detectable CPA at 30 μg/kg, as determined by LC-FLD) and naturally contaminated maize were obtained from a variety of sources. Control maize was purchased from a local health food store (Peoria, IL, USA) as maize meal (ground whole maize). For spiking and recovery studies, α-CPA was added to ground maize prior to extraction and analysis by either LC-FLD or CI-ELISA. Sufficient α-CPA stock solution in acetonitrile was used to spike 25-g aliquots of control maize to levels of 2, 5, 10, 20, 50, 100, 200, 400, 1000, 2000, and 4000 μg/kg. The CI-ELISA method was more sensitive than the LC-FLD method and was used to test samples spiked over the range of 2 to 50 μg/kg. For ELISA tests, five samples were spiked at each level. The LC-FLD method was used to test samples spiked over the range of 100 to 4000 μg/kg. For LC-FLD tests, four samples were spiked at each level. Naturally contaminated samples were obtained by either selecting from the corresponding author’s supply of retained samples previously tested for aflatoxin contamination (21 samples) or by purchasing reference maize meal with established aflatoxin levels (7 samples). The latter were obtained from Trilogy Laboratories (Washington, MO, USA). Selection of the aflatoxin-contaminated reference materials was done with the intention of increasing the chances of observing CPA contamination, as both groups of toxins may be produced by certain aspergilli. The data set should, therefore, not be considered as representative of US maize.
LC-FLD of maize samples for CPA
After exposure to strong UV light in a photoreactor under certain conditions, α-CPA will yield fluorescent products that can be measured by LC-FLD (Maragos 2009, Soares et al. 2010). For LC-FLD experiments, α-CPA was extracted with aqueous methanol and isolated from maize using strong anion exchange solid-phase extraction (SAX SPE). Ground maize (25 g) was extracted with 100 mL of a 7 + 3 (v/v) mixture of methanol and 0.1 M aqueous sodium hydroxide by blending for 3 min. The solution was centrifuged at 3000×g for 10 min at 20 °C. Six milliliters of the supernatant was diluted with 18 mL water and applied to a conditioned 1-g SAX SPE column (Mega Bond Elut SAX, Agilent Technologies, Santa Clara, USA) at a flow rate of approximately 1 drop/s. The column was washed successively with 6 mL each of aqueous solutions of acetonitrile/water in the proportions of 1 + 9, 1 + 2, and 1 + 1. The α-CPA was eluted with 6.3 mL of 1% (v/v) trifluoroacetic acid in acetonitrile. Ammonium hydroxide (0.7 mL of a 1-M solution in acetonitrile) was added to the eluate and mixed. One milliliter of the mixture was filtered through a 0.2-μm PVDF filter into an autosampler vial for analysis. The purified extract was evaluated by LC-FLD. The column was an Atlantis HILIC Silica 3 μm, 4.6 × 150 mm (Waters Corporation, Milford, MA, USA), maintained at 30 °C. The LC system was a Dionex Ultimate 3000 with Chromeleon 7 software (Dionex Corporation, Sunnyvale, CA, USA). The mobile phase was an isocratic mixture of 93% acetonitrile and 7% 0.1 M aqueous ammonium acetate, at a flow rate of 1 mL/min. Injection volume was 100 μL. A photochemical reactor (model UVE, LCTech, GmBH, Dorfen, Germany) was placed in-line between the column and the fluorescence detector. The reactor contained a 1-mL loop through which the LC eluant was exposed to a 254-nm lamp (LCTech, model UVC). Excitation was at 380 nm and emission was at 462 nm. LC run times were 20 min, with α-CPA eluting between 6.13 and 6.17 min. Calibration curves were constructed over the range of 20 to 1000 ng/mL. The CPA content was estimated from calibration curves prepared each day. The summary calibration curve is provided in the supplementary materials (Fig. S1), R 2 = 0.99999958.
Preparation of CPA-protein conjugates
Conjugates of α-CPA with two proteins were prepared. A CPA-ovalbumin (CPA-OVA) conjugate was used for immunization of animals, while a CPA-β-lactoglobulin (CPA-BLG) conjugate was used as an immobilized antigen for competitive ELISA. Α-CPA, which does not contain a free carboxylate, was reacted with O-carboxymethoxylamine according to procedures described previously (Chu et al. 1977; Hahnau and Weiler 1991). The product of the reaction, CPA-carboxymethyloxime (CPA-CMO), was extracted into ethyl acetate, dried by vacuum evaporation, reconstituted with acetone, and examined qualitatively by LC-mass spectrometry (LC-MS). The LC system was comprised of a model AS3000 autosampler, model P4000 pump (both Thermo Scientific, Waltham, MA, USA), and a Synergi Polar-RP analytical column, 4 μ, 80 Å, and 15 cm by 3.00 mm (Phenomenex, Torrance, CA, USA, part number OOF-4336-YO). The volume injected was 100 μL. A binary grade of solvent mixture “A” (methanol/acetonitrile/2-propanol, 10 + 5 + 1, v/v/v) and “B” (2% acetic acid in water, v/v) was applied at a rate of 0.5 mL/min. The gradient began with 65% A, with a linear increase to 100% A over 15 min, followed by a return to 65% A and re-equilibration (minimum of 10 min) before the next injection. The MS used was a model LQQ (Thermo Fisher Scientific, Waltham, MA, USA) equipped with an electrospray ionization source and operated in negative ionization mode (−ESI). Samples were sprayed using a voltage of 4.58 kV. Optimized parameters of the system were inlet capillary voltage −38 V, tube lens voltage −35 V, and capillary temperature 175 °C. All analyses were conducted with a mass scan range of 150–2000 Da. The major product (CPA-CMO) eluted at 4.95 min and was observed under these conditions as a mixture of the monomer, dimer, and trimer (m/z = 408.1, 816.8, and 1225.5, respectively; see Supplemental Materials (Fig. S2)). The free carboxylate group of CPA-CMO was reacted with the primary amines of either OVA or BLG using the mixed anhydride reaction described previously for making similar conjugates (Maragos 2015). Briefly, 28 mg of CPA-CMO in 0.31 mL of N,N-dimethylformamide was reacted with 0.025 mL of triethylamine and 0.025 mL of isobutylchloroformate for 15 min before mixing with 2 mL (30 mg) of ovalbumin. Following the addition of 0.1 mL of 0.1 M sodium hydroxide, the reaction was allowed to proceed for 16 h at 4 °C. The conjugates were dialyzed extensively against 0.1 M PBS, then diluted to 2 mg/mL with PBS and freeze-dried. The dried conjugates were sent to Envigo (Madison, WI, USA) for immunization of mice and collection of antisera.
Immunization of animals and isolation of mAb-producing clones
All animal procedures reported herein were approved by the Institutional Animal Care and Use Committee of Envigo. Work was performed under protocol 421-09—Rodent Immunization for Antibody Production (initial review December 2015, first annual review December 2016, second annual review scheduled for December 2017) in accordance with guidelines established by the National Institutes of Health-Office of Laboratory Animal Welfare. Ten female Balb/C mice were initially immunized by injection of 100 μg CPA-OVA per animal using the same procedures as described previously for production of paxilline antibodies (Maragos 2015). A competitive indirect (CI) ELISA was developed and used for screening of mouse sera and culture supernatants for the presence of antibodies. Each hybridoma culture supernatant solution was tested in single replicates at a minimum of three dilutions, and positive cultures were re-assayed. For screening assays, 0.1 mL of CPA-BLG (1 μg/mL in 0.05 M sodium phosphate buffer, pH 7) was added to wells of polystyrene microtiter plates and allowed to attach overnight at 4 °C. After washing the coated plate twice with 0.32 mL Tween-PBS (0.02% v/v Tween-20 in 0.01 M PBS, pH 7), 0.32 mL of PVA-PBS (1% w/v PVA in 0.01 M PBS) was added and allowed to incubate at ambient temperature for 2 h. During this incubation, test solutions were prepared. The test solutions consisted of 0.1 mL of toxin standard solution (or PBS control) mixed with 0.1 mL of serum (or culture fluid) diluted in BSA-PBS (1% w/v BSA in 0.01 M PBS) in the wells of a polypropylene microwell plate (Corning Inc., Corning, NY, USA). The wells of the polystyrene (CPA-BSA immobilized) plate were washed twice with Tween-PBS, and 0.1 mL of test solution was transferred into each well. After incubation at ambient temperature for 30 min, the plate was washed three times and 0.1 mL of goat antimouse peroxidase conjugate (diluted 1:2000 in BSA-PBS) was added. The plate was incubated for 30 min at ambient temperature then washed four times before addition of 0.1 mL of the o-phenylenediamine (OPD) substrate. The OPD solution was prepared by combining 0.02 mL of 30% H2O2 and 20 mg OPD in 50 mL of citrate-phosphate buffer (0.05 M citrate, 0.1 M phosphate, pH 5.0). After 5 min at ambient temperature, the reaction was stopped by the addition of 0.1 mL of 1 N hydrochloric acid. Color development was determined by measuring the absorbance at 490 nm using a Synergy Neo microplate reader (Bio-Tek, Winooski, VT, USA).
Two mice having sera with antibodies reactive to α-CPA were sacrificed and aseptically splenectomized at Envigo. Splenocytes were chemically fused with Balb/C non-immunoglobulin-secreting (NS-1) myeloma cells using polyethylene glycol. Fused cells were plated in HAT selection media. After 11 days, HAT-resistant cultures were isolated and screened for anti-CPA activity by CI-ELISA. The two fusions yielded a total of 12 positive cultures. From these, five cultures were subsequently cloned, expanded, and frozen. The cultures were designated 1-1.2.9 (herein “1129”), 1-2.3.1 (herein “1231”), 1-4.1.8 (herein “1418”), 2-1.2.4.1 (herein “21241”), and 2-8.1.3.13.21.9 (herein “281313219”). With this nomenclature, the first digit describes the fusion and the second digit describes the hybridoma cell line derived from that fusion. These four cell lines were expanded to produce ascites fluid in mice using established procedures (Hoogenraad et al. 1983). The ascites fluid was partially purified by ammonium sulfate precipitation using procedures described previously (Maragos and McCormick 2000), then lyophilized. The protein content of each of the preparations was determined using the BCA Protein Assay according to the protocols provided by the manufacturer (Thermo Fisher).
Effects of methanol and acetonitrile on two CPA mAbs
To determine the effects of solvents, mAbs 1418 and 1231 were evaluated using standards prepared in mixtures of 0.01 M PBS and solvent. Standards were prepared in either methanol (10 to 50% by volume) or acetonitrile (5 to 30% by volume) over the range of 0.05 to 100 ng/mL. Because the standards were added to an equal volume of antibody solution during the assay, the effective concentration of solvent was one half of the concentration in which the standard was prepared. For comparison, each ELISA plate contained two rows of standards in PBS. All data were normalized to the color development of controls lacking added α-CPA (Eq. 1).
where B represents the color from the test sample and B0 represents the color from the CPA-free control. The transformed data were fit using a four-parameter logistic dose-response equation (TableCurve, Systat Software, Inc., San Jose, CA, USA). Fitted curves were used to determine the concentration required for 50% inhibition (IC50s). The IC50s were used to estimate the response of standards in solvent relative to standards in PBS using Eq. 2.
CI-ELISA of maize samples
Maize samples (25 g) were mixed with 5 g of sodium chloride and 125 mL of methanol/water (4 + 1 v/v). The mixture was shaken for 2 h at ambient temperature then filtered (Whatman 2V, Maidstone, England). The filtrate was diluted 1 + 3 (v/v) with 10 mM PBS. For quantification, this 1:4 dilution was diluted an additional 10-, 40-, and 100-fold (overall dilutions, 1:40, 1:160, and 1:400) using 1:4 diluted extract from control maize. Diluted extracts were tested by CI-ELISA, data were transformed using Eq. 1, and the α-CPA content was determined relative to standards prepared in extract from control maize.
Results and discussion
Ten mice were immunized with CPA-OVA and sera were evaluated by competitive indirect ELISA (CI-ELISA). All ten antisera bound to immobilized CPA-BLG and showed a response to free CPA. The two mice with the highest titers were selected for splenocyte fusions, and a total of 12 CPA-responsive cultures were obtained. From these, five antibody-producing monoclonal cell lines were isolated. These were designated 1129 (isotype IgMκ), 1231 (isotype IgG1κ), 1418 (isotype IgG1κ), 21241 (isotype IgG1κ), and 281313219 (isotype IgG2bκ). Preliminary experiments (data not shown) indicated that 1129 no longer bound to CPA-BLG and that clones 1231 and 1418 were more sensitive toward α-CPA than were clones 21241 and 281313219. To determine which of these would be best suited for further method development, both mAbs were evaluated for their tolerance to methanol and acetonitrile. Data on the effects of each solvent on the CI-ELISAs for CPA are listed in Table 1. During these experiments, it was noted that, at high acetonitrile levels (30–50%), there was a difference in color development that seemed to depend upon the timing of when the standards were added to the plate. At equal acetonitrile levels, standards that were added to the plate earlier gave better results (lower IC50) than standards added to the plate later. This was attributed to the evaporation of acetonitrile from the wells during preparation of the plates. This, combined with the sharp decrease in relative response at 30% acetonitrile or 50% methanol (Table 1), put a limit on the solvent concentration that could be used effectively. For mAb 1418, excellent responses were seen with methanol at up to 20% or acetonitrile up to 10%. At 30% methanol, or 20% acetonitrile, the IC50s were roughly doubled relative to those in PBS. For mAb 1231, excellent responses were seen with methanol at up to 20% or with acetonitrile at up to 5%. At 30% methanol, or 10% acetonitrile, the IC50s were roughly doubled relative to α-CPA in PBS. Thus, mAb 1232 appeared to be more sensitive to acetonitrile than did mAb 1418. However, for either mAb, 20% methanol was shown to give acceptable results.
As noted previously, the mAbs reported here are not the first antibodies developed against α-CPA. Hahnau and Weiler (1991) prepared two CPA-BSA conjugates, one synthesized using an O-carboxymethyloxime (CMO) and another using the Mannich reaction. Polyclonal antibodies (pAb) were produced in rabbits and used to develop an assay with an IC50 of 0.6 pmol/assay. Reported in the same manner, the IC50s for our mAbs 1418 and 1231 were 0.16 and 0.15 pmol/assay, respectively. Hahnau and Weiler used the ELISA, which was a competitive direct format, to measure CPA production from fungal cultures. Recoveries from agar medium averaged 99% over the range from 42 to 420 ng/mL, with an LOD of 20 ng/mL. Solvent tolerance was not reported. A later publication from the same authors described the development of mAb from mice immunized with CPA-BSA and a CPA-keyhole limpet hemocyanin (KLH) conjugate (Hahnau and Weiler 1993). The assay reportedly could measure from 7 to 300 ng of CPA, with a midpoint of 50 ng, which equates to a concentration of 250 ng/mL, significantly poorer than the results with their rabbit pAb or the results reported here (Table 1). Another group, at the University of Wisconsin-Madison, also produced several mouse mAb (Huang and Chu 1993). Four of the mAb were used to produce assays. The IC50s observed ranged from 0.014 to 0.17 ng/mL, making them more sensitive than the mAbs reported here. One of the clones (5C8D1) was used to develop a CD-ELISA for CPA in corn, mixed feed, and peanuts (Yu and Chu 1998). Interestingly, while increasing methanol concentration caused the standard curves to “flatten,” the IC50 of the assay remained constant. It was reported that an effective standard curve was obtained even in the presence of 30% methanol. So, while the assays reported by Yu and Chu and those reported here both exhibit similar tolerance to methanol, the mechanism by which methanol is affecting the assays may be different. Based on solvent tolerance, mAb 1418 was selected for further development for a CI-ELISA to use with corn samples.
Recovery of CPA from spiked maize
The CPA content of spiked maize was determined by two techniques: LC-FLD and CI-ELISA. For LC-FLD experiments, the maize was extracted with alkaline methanol/water and the CPA was isolated by SAX-SPE. Purified extracts were subjected to LC, and the eluate was passed through a photoreactor to generate fluorescent products for detection. The limit of detection (LOD), defined as a signal to noise ratio of 5, was estimated to be 30 μg/kg. The limit of quantification (LOQ), defined as a signal to noise ratio of 15, was estimated to be 100 μg/kg. The LC-FLD calibration curve could be fit to a simple line with R 2 = 0.99954, but the fit was much better to a polynomial line (R 2 = 0.99999958, Fig. S1). This suggested that the development of the fluorescent response may be pseudolinear. Based upon this, it was established that samples above 4500 μg/kg should be diluted so that they might remain within the optimal range for detection. To determine recoveries, maize was spiked over the range from 100 to 4000 μg/kg, which was close to the quantitative range of the method (Table 2). Results demonstrated adequate recoveries over this range, with an average recovery of 83.3% and an RSD of 5.9% (n = 24).
The CPA content of spiked maize was also estimated by CI-ELISA using mAb 1418 with standards prepared in control maize matrix. The IC50 was 0.58 ng/mL, which was lower than the IC50 for CPA in PBS (Fig. 2). The working range was defined by the concentrations between the IC20 (the LOQ, 0.24 ng/mL) and the IC80 (1.39 ng/mL). When sample extraction and dilution are accounted for, these correspond to an IC50 of 12 μg/kg and a working range of 5 to 28 μg/kg. For spiking and recovery studies, the spiking levels ranged from 2 to 50 μg/kg. Where necessary, sample extracts were diluted with control extract to keep the responses within the working range. We decided to use diluted control extract, rather than 20% methanol-PBS, because the matrix itself exhibited an effect upon the assay. As depicted in Fig. 2, the ELISA was actually more sensitive in diluted matrix (containing 20% methanol) than in 20% methanol-PBS. From the error estimates in Fig. 2, it is apparent that this enhancement would have been significant enough to affect quantification.
Calibration curves for α-CPA in maize matrix (white circles) and 20% methanol-PBS (blue circles). Data are the average ± 1 standard deviation of six replicate plates in matrix or four replicate plates in methanol-PBS. A sample containing 20 μg/kg CPA and diluted as described in the text would contain the equivalent of 1 ng/mL CPA
Recoveries from individual samples ranged from 70 to 116%. When averaged by concentration, recoveries ranged from 81.6 to 102.4%. The overall average (all recoveries, all levels) was 88.6%, with an RSD of 14.3% (Table 3). In the study by Yu and Chu (1998), detection limits in corn, mixed feed, and peanuts were reported to be 100, 300, and 600 μg/kg, respectively. Recoveries from CPA added to these matrices over the range from 50 to 5000 μg/kg averaged 97.6, 92, and 93%, respectively. Compared to the earlier work in maize, the CI-ELISA reported here had slightly lower recoveries but was of significantly better sensitivity. The recoveries, while lower than the previous work, remain adequate for measuring CPA in maize. Because of the steep dose-response curve (Fig. 2), the working range for the assay was relatively narrow (i.e., 5 to 28 μg/kg). This made quantification less convenient because, to ensure that responses fell within the working range, samples typically needed to be analyzed at multiple dilutions. The steepness of the dose-response curve suggests that mAb 1418 would be an excellent candidate for use in a qualitative (positive/negative) format, where such a response could be very beneficial.
We tested 28 samples of maize by CI-ELISA and LC-FLD. The sample set was biased to contain samples known to be contaminated with AFB1, under the assumption this might increase the chances for observing naturally occurring CPA. Of the 28 samples, 11 were above the LOQ of the CI-ELISA and 5 were above the (higher) LOQ of the LC-FLD method (Table 4). Six of the samples (numbers 2, 3, 5, 9, 11, and 21) were detected as positive by ELISA but negative by LC-FLD, which was not surprising, given the substantially lower LOQ of the ELISA method. Of the six, four were below the LOQ of the LC-FLD method and two (numbers 9 and 21) were slightly above it yet were not detected by LC-FLD. Five of the samples (numbers 1, 14, 20, 25, and 27) were above the LOQ of both methods. For four of these samples, the ELISA gave a higher result than the LC-FLD method. Therefore, it appears that the CI-ELISA based upon mAb 1418 tends to overestimate α-CPA in naturally contaminated maize. In the study of Yu and Chu (1998), a competitive direct (CD)-ELISA was compared to an LC-UV method for 13 samples naturally contaminated with aflatoxins, yielding an r value of 0.79 and a regression slope of 0.62. They also observed that the ELISA results were considerably higher than results with their LC method. A similar overestimation has been observed with immunoassays for other mycotoxins, such as the fumonisins, and deoxynivalenol (Zachariasova et al. 2014; Righetti et al. 2017). In certain cases, this has been hypothesized to result from the cross-reactivity of related fungal secondary metabolites with the antibody. Such metabolites might include precursors in the biosynthetic pathway, modified derivatives, or plant metabolites of the toxins (i.e., the “masked” mycotoxins) (Righetti et al. 2017). Because such metabolites of CPA have not been identified, their contribution to the overestimation observed here remains speculative. The qualitative agreement between results observed with the ELISA and the LC-FLD was good. The better sensitivity of the ELISA and its ease of use relative to LC-FLD suggest that this may make a good screening method for α-CPA in maize.
Conclusions
Several mAbs were developed that bind to α-CPA, and two were isolated with excellent sensitivity toward α-CPA, with IC50s near 1 ng/mL. Of these, clone 1418 was used to develop a sensitive CI-ELISA for use with maize. The assay demonstrated good solvent tolerance, with up to 20% methanol or 10% acetonitrile having minimal impact upon sensitivity. The ELISA based upon this mAb was used to measure α-CPA over the range of 5 to 28 μg/kg, with good recoveries from spiked maize. Screening of a set of 28 maize samples revealed a good qualitative relationship between the ELISA results and those by LC-FLD, with the ELISA more likely to overestimate CPA content than the LC-FLD method. The quantitative range of the ELISA was narrow. While this may be a disadvantage when attempting to use this mAb in quantitative assays, it may be a significant advantage for a screening assay where a rapid transition between a “negative” and a “positive” threshold is desired. This combined with the good solvent tolerance of clones 1231 and 1418 suggests that these antibodies may also find use in formats such as lateral flow tests or immunoaffinity columns.
References
Aresta A, Cioffi N, Palmisano F, Zambonin CG (2003) Simultaneous determination of ochratoxin A and cyclopiazonic, mycophenolic, and tenuazonic acids in cornflakes by solid-phase microextraction coupled to high-performance liquid chromatography. J Agric Food Chem 51:5232–5237. doi:10.1021/jf034385r
Burdock GA, Flamm WG (2000) Review article:safety assessment of the mycotoxin cyclopiazonic acid. Intl J Toxicol 19:195–218. doi:10.1080/10915810050074964
Chu FS, Hsia S, Sun PS (1977) Preparation and characterization of aflatoxin B1-(O-carboxymethyl)oxime. J AOAC 60:791–794
Cole RJ (1986) Etiology of turkey X disease in retrospect: a case for the involvement of cyclopiazonic acid. Mycotoxin Res 2:3–7. doi:10.1007/BF03191956
Diaz GJ, Thompson W, Martos PA (2010) Stability of cyclopiazonic acid in solution. World Mycotoxin J 3:25–33. doi:10.3920/WMJ2009.1170
Dorner JW (2002) Recent advances in analytical methodology for cyclopiazonic acid. In: DeVries JW, Trucksess MW, Jackson LS (eds) Mycotoxins and food safety. Kluwer Academic/Plenum Publishers, New York, pp 107–116
Dorner JW, Sobolev VS, Yu W, Chu FS (2001) Immunochemical method for cyclopiazonic acid. In: Trucksess MW, Pohland AE (eds) Methods in molecular biology, Mycotoxin protocols, vol 157. Humana Press Inc., Totowa, pp 71–80
Goto T, Wicklow DT, Ito Y (1996) Aflatoxin and cyclopiazonic acid production by a sclerotium-producing Aspergillus tamarii strain. Appl Environ Microbiol 62:4036–4038
Hahnau S, Weiler EW (1991) Determination of the mycotoxin cyclopiazonic acid by enzyme immunoassay. J Agric Food Chem 39:1887–1891. doi:10.1021/jf00010a041
Hahnau S, Weiler EW (1993) Monoclonal antibodies for the enzyme immunoassay of the mycotoxin cyclopiazonic acid. J Agric Food Chem 41:1076–1080. doi:10.1021/jf00031a012
Hayashi Y, Yoshizawa T (2005) Analysis of cyclopiazonic acid in corn and rice by a newly developed method. Food Chem 93:215–221. doi:10.1016/j.foodchem.2004.09.017
Heperkan D, Somuncuoglu S, Karbancioglu-Güler F, Mecik N (2012) Natural contamination of cyclopiazonic acid in dried figs and co-occurrence of aflatoxin. Food Control 23:82–86. doi:10.1016/j.foodcont.2011.06.015
Holzapfel CW (1968) The isolation and structure of cyclopiazonic acid, a toxic metabolite of Penicillium cyclopium Westling. Tetrahedron 24:2101–2119. doi:10.1016/0040-4020(68)88113-X
Hoogenraad N, Helman T, Hoogenraad J (1983) The effect of pre-injection of mice with pristine on ascites tumour formation and monoclonal antibody production. J Immunol Meth 61:317–320. doi:10.1016/0022-1759(83)90225-9
Horn BW, Dorner JW (1999) Regional differences in production of aflatoxin B1 and cyclopiazonic acid by soil isolates of Aspergillus flavus along a transect within the United States. Appl Environ Microbiol 65:1444–1449
Huang X, Chu FS (1993) Production and characterization of monoclonal and polyclonal antibodies against the mycotoxin cyclopiazonic acid. J Agric Food Chem 41:329–333. doi:10.1021/jf00026a038
Kononenko GP, Burkin AA, Tolpysheva TY (2012) Enzyme immunoassay of the secondary metabolites of micromycetes as components of lichen substances. Appl Biochem Microbiol 48:71–76. doi:10.1134/S0003683812010085
Lansden JA, Davidson JI (1983) Occurrence of cyclopiazonic acid in peanuts. Appl Environ Microbiol 45:766–769
Losito I, Monaci L, Aresta A, Zambonin CG (2002) LC-ion trap electrospray MS-MS for the determination of cyclopiazonic acid in milk samples. Analyst 127:499–502. doi:10.1039/b200394p
Maragos CM (2009) Photolysis of cyclopiazonic acid to fluorescent products. World Mycotoxin J 2:77–84. doi:10.3920/WMJ2008.1088
Maragos CM (2015) Development and evaluation of monoclonal antibodies for paxilline toxins 7:3903-3915. doi:10.3390/toxins7103903
Maragos CM, McCormick SP (2000) Monoclonal antibodies for the mycotoxins deoxynivalenol and 3-acetyl-deoxynivalenol. Food Agric Immunol 12:181–192. doi:10.1080/09540100050140722
Miller CD, Richard JL, Osweiler GD (2011) Cyclopiazonic acid toxicosis in young turkeys: clinical, physiological, and serological observations. Toxin Rev 30:42–46. doi:10.3109/15569543.2011.589360
Moldes-Anaya AS, Asp TN, Eriksen GS, Skaar I, Rundberget T (2009) Determination of cyclopiazonic acid in food and feeds by liquid chromatography-tandem mass spectrometry. J Chrom A 1216:3812–3818. doi:10.1016/j.chroma.2009.02.061
Nesheim S, Stack ME (2001) Preparation of mycotoxin standards. In: Trucksess MW, Pohland AE (eds) Mycotoxin protocols. Humana Press, Totowa, pp 31–36
Norred WP, Cole RJ, Dorner JW, Lansden JA (1987) Liquid chromatographic determination of cyclopiazonic acid in poultry meat. J AOAC Intl. 70:121–123
Rao BL, Husain A (1985) Presence of cyclopiazonic acid in kodo millet (Paspalum scrobiculatum) causing ‘kodua poisoning’ in man and its production by associated fungi. Mycopathologia 89:177–180. doi:10.1007/BF00447028
Richard JL, Payne GA (eds) (2003) Mycotoxins risks in plant, animal, and human systems. Task Force Report 139. Council for Agricultural Science and Technology (CAST), Ames 65 pp
Righetti L, Galaverna G, Dall’Asta C (2017) Group detection of DON and its modified forms by an ELISA kit. Food Addit Contam A 34:248–254. doi:10.1080/19440049.2016.1265671
Riley RT, Goerger DE, Norred WP (1995) Disruption of calcium homeostasis: the cellular mechanism of cyclopiazonic acid toxicity in laboratory animals. In: Eklund M, Richard JL, Mise K (eds) Molecular approaches to food safety issues involving toxic microorganisms. Alaken, Inc., Fort Collins, pp 461–480
Soares C, Rodrigues P, Freitas-Silva O, Abrunhosa L, Venancio A (2010) HPLC method for simultaneous detection of aflatoxins and cyclopiazonic acid. World Mycotoxin J 3:225–231. doi:10.3920/WMJ2010.1216
Trucksess MW, Mislivec PB, Young K, Bruce VR, Page SW (1987) Cyclopiazonic acid production by cultures of Aspergillus and Penicillium species isolated from dried beans, corn meal, macaroni, and pecans. J AOAC 70:123–126
Urano T, Trucksess MW, Beaver RW, Wilson DM, Dorner JW, Dowell FE (1992a) Co-occurrence of cyclopiazonic acid and aflatoxins in corn and peanuts. J AOAC Intl 75:838–841
Urano T, Trucksess MW, Matusik J, Dorner JW (1992b) Liquid chromatographic determination of cyclopiazonic acid in corn and peanuts. J AOAC Intl 75:319–322
Vinokurova NG, Ivanushkina NE, Khmel’nitskaya II, Arinbasarov MU (2007) Synthesis of α-cyclopiazonic acid by fungi of the genus Aspergillus. Appl Biochem Microbiol 43:435–438. doi:10.1134/S0003683807040138
Yu W, Chu FS (1998) Improved competitive enzyme-linked immunosorbent assay for cyclopiazonic acid in corn, peanuts, and mixed feed. J Agric Food Chem 46:1012–1017. doi:10.1021/jf9707243
Zachariasova M, Cuhra P, Hajslova J (2014) Cross-reactivity of rapid immunochemical methods for mycotoxins detection towards metabolites and masked mycotoxins: the current state of knowledge. World Mycotoxin J 7:449–464. doi:10.3920/wmj2014.1701
Acknowledgements
We thank Candace Lindgren, Paula Dodge, Deanna Morrison, Nahla Elmahdy, Jenny Wiersma, Julie Havlicek, and Shannon Snodgrass of Envigo, for the exceptional quality of the technical assistance provided throughout this project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None
Disclaimer
The mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the US Department of Agriculture. The USDA is an equal opportunity provider and employer.
Source of funding
This research was supported by the US Department of Agriculture, Agricultural Research Service, Current Research Information System project 5010-42000-049-00D.
Electronic supplementary material
ESM 1
(PDF 66 kb)
Rights and permissions
About this article
Cite this article
Maragos, C.M., Sieve, K.K. & Bobell, J. Detection of cyclopiazonic acid (CPA) in maize by immunoassay. Mycotoxin Res 33, 157–165 (2017). https://doi.org/10.1007/s12550-017-0275-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12550-017-0275-0