Abstract
The genus Antholoba Hertwig, 1882 (Actiniaria, Enthemonae) is characterized by the presence of short and numerous tentacles, a folded oral disc in lobes or cup-shaped with an expanded distal part, transversely wrinkled body wall surface, a very long mesogloeal sphincter, parietobasilar muscles poorly developed, and the absence of acontia. Currently, the genus Antholoba is classified within the family Actinostolidae Carlgren, 1893 (superfamily Actinostoloidea Carlgren, 1932) and comprises two valid species: A. achates (Drayton in Dana, 1846) which have been recorded in Antarctica, the southwestern Atlantic, and the southeastern-western Pacific; and A. perdix (Drayton in Dana, 1846) which is distributed in the northwestern Atlantic, including the Gulf of Mexico. In recent collections along Ubatuba Bay in northern São Paulo, Brazil, we found specimens of a third, unknown species, which exhibits morphological and genetic differences from the only other species recorded from that place, A. achates (Drayton in Dana, 1846). Additionally, we examined five specimens of A. achates collected in Penha, Santa Catarina State, for morphological comparison. Our phylogenetic analyses, using molecular data, affirm the difference between the two species. Furthermore, the resultant phylogenetic trees recover the species of the genus Antholoba as a sister group to the acuticulate clade, within the superfamily Metridioidea, instead of within Actinostoloidea. We describe the material from Ubatuba as a new species, A. fabiani sp. nov., providing information and photographs of its external and internal anatomy, as well as cnidom, along with sequences of mitochondrial (12S, 16S, and COIII) and nuclear (18S and 28S) markers. Additionally, we propose placing the genus Antholoba within Metridioidea, and introduce the new family Antholobidae fam. nov.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A persistent issue in comprehending biodiversity is the ambiguity surrounding the identity and delineation of taxa initially described before the twentieth century. Problems with historical descriptions include species being described without types or voucher specimens, the use of typological species concepts that do not address the potential for variation within the species, and descriptions based on a small number of specimens. These problems are exacerbated in marine species, which may have never been observed alive by the describer and whose biology and ecology might have been unknown.
In his compendium of “zoophytes”, Dana (1846) illustrated and cited the sea anemones Actinia achates and Actinia reticulata from the coast of South America. Dana’s (1846) account serves as the original description for these taxa, but he attributes their authorship to Drayton and Couthouy, respectively. Both species presented a folded oral disc and very numerous tentacles. They were distinguished by the appearance of the body wall (smooth in A. achates and reticulate in A. reticulata). Hertwig (1882) established genus Antholoba for Actinia reticulata by monotypy, in agreement with Stephenson (1921) and Fautin et al. (2007), but contrary to Carlgren (1949), who assigned the type to Actinia achates Dana, 1849 (Fautin 2016). Following studies by McMurrich (1893), and Carlgren (1899), McMurrich (1904), acting as the first reviser, synonymized A. reticulata and A. achates under the name Antholoba achates (Drayton in Dana 1846), arguing that the species A. reticulata was identical to A. achates. Subsequent authors refined the understanding of these relatively common and widespread species, finding, for instance, that McMurrich’s study material was heterogeneous, including at least one specimen of Isotealia (see Carlgren 1934) and that Hertwig (1882) overestimated the number of tentacles (see Fautin 1984).
The current concept of the genus Antholoba comprises two valid species: Antholoba achates (Drayton in Dana 1846) and Antholoba perdix (Verrill, 1882) (Rodríguez et al. 2023). The species A. achates has been reported in Antarctica, the southwest Atlantic (southeast coast of Brazil, the coast of Argentina, and the Falkland Islands (Islas Malvinas), as well as in the southwestern (New Zealand) and southeastern Pacific (Chile, Galapagos Islands, Strait of Magellan, among others) (reviewed by Häussermann and Försterra 2009). In contrast, A. perdix is known from considerably fewer records and sites, with most of these in the northwest Atlantic (mainly in the USA) (see Widersten 1976; Rodríguez et al. 2023).
In recent collections along the coast of Ubatuba, located on the northern coast of the state of São Paulo, Brazil, we found specimens of an Antholoba species that exhibit morphological features that appear to resemble those of A. reticulata, albeit with a different coloration pattern from what has been reported in Chile (Dana 1846). The anatomy and geographical location are inconsistent with those described for A. perdix. Additionally, mitochondrial and nuclear markers indicate that the specimens from Ubatuba are distinct from A. achates. Due to the absence of reference material for A. reticulata, a direct comparison with the original holotype is not feasible (Fautin 2016), but our data suggest that the Ubatuba specimens belong to an undescribed species.
In this study, we describe the new species as Antholoba fabiani sp. nov., and compare it with some Antholoba achates specimens of Penha (Santa Catarina State, Brazil), for which we provide data and photographs of their external and internal morphology, as well as their cnidom. We provide a molecular diagnosis to satisfy recommendations of Rheindt et al. (2023) for diagnostic traits that purport to distinguish each new species.
Our phylogenetic analyses using molecular data also show that the genus Antholoba is situated within the superfamily Metridioidea, in agreement with previous studies (Lauretta et al. 2014; Rodríguez et al. 2012, 2014; Grajales and Rodríguez 2016; Gusmão et al. 2018, 2019, 2020; Gusmão and Rodríguez 2021a; Yoshikawa et al. 2022). Antholoba is the sister group to the members of the acuticulate clade, a group of metridioideans in which some members also lack acontia and have a mesogloeal sphincter muscle. They are very distant from other members of Actinostolidae Carlgren, 1893 included in this analysis. Based on these results, we establish Antholobidae fam. nov. as a new family within Metridioidea. This family includes one genus and three accepted species.
Material and methods
Collection methods
Four specimens of Antholoba achates and five of A. fabiani sp. nov. were collected in two coastal locations of Brazil (Fig. 1), using trawl nets at depths from 5 to 30 m (Perroca et al. 2022). Antholoba achates specimens were collected by trawling (ICMBio Soloncy Moura Vessel) near Penha, Santa Catarina State (26°43'S 48°32'W) on September 20, 2006. These specimens were preserved and deposited in the collection of São Paulo University (USP). Specimens of A. fabiani sp. nov. were collected in Ubatuba Bay, São Paulo State (23°26'S 45°01'W), between June 27 and July 8, 2022, with the assistance of the staff from the Study Group of the Laboratory of Biology of Marine and Freshwater Shrimps (LABCAM), at the São Paulo State University.
Morphological analysis methods
The collected specimens of A. fabiani sp. nov. were transferred to the laboratory and placed in aquariums for photographing their color while alive. The dimensions of the oral disc, pedal disc, and column were recorded. Small pieces of tissue (~ 25 mg) from the pedal disc were excised from all collected samples and preserved in absolute ethanol for molecular analysis. The specimens were relaxed in 7% MgCl2 seawater solution, then fixed in 10% formalin seawater (for a minimum of two months) and preserved in 70% ethanol.
Squash preparations of small pieces of tissue (~ 1 mm2) from the tentacles, column, actinopharynx, and filaments, were made from two preserved specimens of each species. From each squash preparation, the length and width of at least 30 undischarged capsules of each type of cnidocyst present were randomly measured using a Nikon eclipse E200 light microscope (equipped with an adapted Moticam 5.0MP camera) at 1000 × oil immersion, along with Motic Images Plus 2.0 software. The terminology for cnidocysts follows that of Gusmão et al. (2018). Descriptive statistical parameters, including the mean, minimum (min) and maximum (max) range, proportion, frequency, and size (length and width) abundances, were calculated.
Longitudinal and cross Sects. (7 µm) were prepared from two specimens of each species. The specimens were dissected, dehydrated in ethanol and isobutyl alcohol, and embedded in paraffin for histological sections (Spano and Flores 2013). The sections were stained with hematoxylin and eosin (Estrada-Flores et al. 1982). All specimens were deposited in the Museum of Zoology of the University of São Paulo (Brazil) (registration code: MZSP 8748–8751, 8758–8761). Taxonomy follows Rodríguez et al. (2014).
Molecular data collection
Tissue extracts from one individual of Antholoba fabiani sp. nov. were used for molecular analysis. Genomic DNA was isolated using EasyPure® Genomic DNA Kit (TransGen Biotech). We amplified three mitochondrial (12S rRNA, 16S rRNA, and COIII) and two nuclear markers (the first two regions of 18S rRNA and 28S rRNA) following the primers used by Brugler et al. (2018). Annealing conditions of 55 °C, 51 °C, and 54 °C, were used for 12S rRNA, 16S rRNA, and COIII amplifications, respectively. A temperature of 52 °C was employed for two reactions of 18S rRNA, and 56 °C for the two reactions of the 28S rRNA. Across all thermal protocols, the process included an initial denaturation at 94° C for 2–5 min, followed by a 30 s step at 94 °C, a 45 s annealing step, and 45 s at 72 °C for 40 cycles, concluding with a final extension at 72 °C for 6–7 min. The amplified products were visualized using 2% agarose gel electrophoresis. All amplification products were purified using PCR Products Purification Kit (MEBEP BIOSCIENCE) and subjected to direct Sanger sequencing conducted by CREBIO-UNESP (Jaboticabal-Brazil).
Phylogenetic analysis methods
Forward and reverse sequences were assembled and edited using Geneious Prime version 2023.0.4 (Kearse et al. 2012). These sequences were then blasted against the nucleotide database of GenBank to confirm the successful amplification of the target marker/organism. The new DNA sequences for Antholoba fabiani sp. nov. (OR000444, OR001827, OR014502, OR069378, and OR470688) were combined and analyzed with sequences provided by Rodríguez et al. (2014), Larson and Daly (2016), Daly et al. (2017), Gusmão et al. (2019), Gusmão and Rodríguez (2021a), and Sanamyan et al. (2021) for each of the five molecular markers from selected taxa within of the order Actiniaria. Members of suborder Anenthemonae were used as outgroups (Supplementary material 1).
Sequences for each marker were aligned separately in MAFFT v7.53 with L-INS-I algorithm and “—maxiterate 1000” option (Katoh et al. 2009). Poorly aligned regions were removed using Gblocks v0.91b (Castresana 2000; Dereeper et al. 2008). For a less stringent selection, the following options were applied: allowing smaller final blocks, permitting gap positions within the final blocks, and accepting less strict flanking positions. The concatenated dataset was generated using SequenceMatrix v1.8 (Vaidya et al. 2011). The original dataset of the five markers comprised 10,088 bp, including gaps. However, after running GBlocks, it was filtered to 3,541 bp (35.1% conserved positions) (Supplementary Material 2).
Maximum likelihood (ML) reconstruction was conducted using IQ-Tree2 (Minh et al. 2020). We adopted a concatenation approach with an edge-linked proportional partition model, employing ModelFinder (Kalyaanamoorthy et al. 2017) to ascertain the optimal evolutionary model for each partition (Supplementary Material 3). To assess branch support, we utilized 1000 SH-like approximate likelihood ratio test (SH-aLRT) replicates (Guindon et al. 2010), 1000 ultrafast bootstrap (UFBoot2) replicates (Hoang et al. 2018), as well as parametric aLRT (Anisimova and Gascuel 2006) and approximate Bayes tests (Anisimova et al. 2011). The selection of the best partition and evolutionary model for each dataset was based on the Bayesian Information Criterion (BIC) score using ModelFinder, as implemented in IQ-Tree2 (Supplementary Material 4). The resulting trees were edited and visualized using TreeGraph v2 (Stöver and Müller 2010).
We compared estimates of divergence in sequences of Antholoba achates (from Argentina and Chile) and A. fabiani sp. nov., with that estimated for other pairs of closely related actiniarians: Actinostola chilensis McMurrich, 1904 vs. A. georgiana Carlgren, 1927, Anthothoe chilensis (Lesson, 1830) vs. A. sphyrodeta (Gosse, 1858), Exaiptasia brasiliensis Grajales & Rodríguez, 2016 vs. E. diaphana (Rapp, 1829), Isoparactis fabiani Häussermann & Försterra, 2009 vs. I. ferax (Stuckey, 1909), Metridium senile (Linnaeus, 1761) vs. M. farcimen (Brandt, 1835), and Sagartia lacerata (Dalyell, 1848) vs. S. undata (Müller, 1778). For these analyses, the five markers were first aligned separately using MAFFT and then concatenated using SequenceMatrix (Supplementary Material 5). The Kimura 2-parameter model (K2P) (Kimura 1980) was employed for estimation using MEGA11 (Tamura et al. 2021). The analysis included 1000 bootstrap replicates and considered substitutions (Transitions + transversions). A gamma distribution was used to model the rate variation among sites, with a shape parameter of 1. There final dataset comprised a total of 866 (12S), 532 (16S), 752 (COIII), 1924 (18S), 3449 (28S), 2500 (Mit), 5373 (Nucl), and 7873 (Mit + Nucl) base pairs positions.
For molecular diagnosis, we used DNAdiagnoser as implemented in iTaxoTools (Vences et al. 2021) to identify and tabulate pairwise diagnostic differences between A. achates and A. fabiani sp. nov. for the 12S, 16S, 18S, 28S, and COIII fragments using MAFFT alignment.
Results
Systematics
Phylum Cnidaria Hatschek, 1888
Class Anthozoa Ehrenberg, 1834
Subclass Hexacorallia Haeckel, 1896
Order Actiniaria Hertwig, 1882
Suborder Enthemonae Rodríguez & Daly in Rodríguez et al. (2014)
Superfamily Metridioidea Carlgren, 1893
Family Antholobidae fam. nov.
https://zoobank.org/NomenclaturalActs/768904B0-7AF4-455D-84D2-E1C046945B19
Diagnosis. Enthemonae with well-developed base. Column wall smooth or transversely wrinkled (with “papillae”) with wrinkles varying in size, but without tubercles or verrucae. Margin not distinct. Sphincter mesogloeal, very long and strong, confined to the endodermal side of the mesogloea of column, not separated from the circular endodermal musculature of the column, with muscle meshes being reticular and crowded. Uppermost part of column and oral disc wider than pedal disc, folded (with 5–7 lobes in larger individuals) or cup-shaped with expanded distal part. Tentacles short but numerous (600 to > 750 in number), hexamerously arranged in eight cycles (?), outer shorter than inner. Longitudinal muscles of tentacles ectodermal to meso-ectodermal. Radial muscles of oral disc ecto-mesogloeal. Two well-developed siphonoglyphs, aborally prolonged. Mesenteries numerous, in five (?) to seven cycles at level of actinopharynx; additional mesentery cycle(s) may be present distally. Two pairs of directives. Perfect pairs of mesenteries between 24 or 48 (first two or three cycles), sterile. Retractor muscles fairly weak or well-developed and diffuse, parietobasilar muscles poorly-developed, basilar muscles fairly weak to poorly-developed. No acontia. Cnidom: spirocysts, basitrichs, p- mastigophores A, and p-mastigophores B1. Azooxanthellate. [Notable diagnostic features highlighted in bold].
Type genus. Antholoba Hertwig, 1882
Remarks. This diagnosis incorporates attributes identified and discussed by Hertwig (1882), McMurrich (1904), Fautin (1984), Carlgren (1949), Carter (1965), and Häussermann and Försterra (2009). We consider the number of tentacles and mesenteries reported by the different studies and in this one, we calculate that Antholoba species could have eight tentacle cycles and seven mesentery cycles based on the ranges between 600 to > 750 tentacles. In the material we examined, we found a few pairs of the seventh cycle of mesenteries in the distal part of the actinopharynx in A. fabiani sp. nov. It is possible that the other pairs of mesenteries occur in regions where the ends of the oral disc are lobulated.
Genus Antholoba Hertwig, 1882
Type species. Actinia reticulata Couthoy in Dana, 1946 (a junior subjective synonym of Antholoba achates).
Included species. Antholoba achates (Drayton in Dana, 1846), A. fabiani sp. nov., and A. perdix (Verrill, 1882).
Antholoba achates (Drayton in Dana, 1846) (Figs. 2–3)
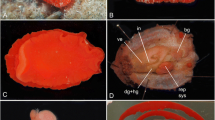
Antholoba achates (Dayton in Dana, 1846). Preserved specimen in; (a) upper; (b) lateral, and (c) below view. Histological sections: longitudinal section through; (d) entire, (d’) upper and (d’’) lower column, showing sphincter mesogloeal; e longitudinal section through pedal disc; (f) cross section through column, at the level of the actinopharynx, showing the five mesentery cycles and (f’) the gametogenic tissue of the last two pairs mesenteries. Abbreviations: 1–5: first to fifth pairs of mesenteries; co = column; di = pairs of directive mesenteries; ep = epidermis; g = gastrodermis; m = mesogloea; s = siphonoglyph; sm = sphincter mesogloeal; spm = spermatic cysts; pd = pedal disc; t = tentacle; rm = retractor muscle. Scales: a–c: 1 cm; d–f: 1 mm, d’-d’’, f’: 0.5 mm
Cnidae from Antholoba achates (Dayton in Dana, 1846). a, c–e: basitrich; b: spirocyst; f: p-mastigophore A I; g: immature p-mastigophore A I; h: p-mastigophore A II; i: immature p-mastigophore A II; j: p-mastigophore B1
Actinia achates Drayton in Dana, 1846, p. 142. Dana, 1849, Plate 3, fig. 28.
Actinia reticulata Couthouy in Dana, 1846, p. 144. Dana, 1849, Plate 4, fig. 31.
Actinia fuegiensis Couthouy in Dana, 1846, p. 145. Dana, 1849, Plate 4, fig. 32.
Sagartia Fuegensis: Gosse, 1855, p. 274.
Sagartia Achates: Gosse, 1855, p. 274.
Sagartia reticulata: Gosse, 1855, p. 274.
Metridium Achates: Milne-Edwards, 1857, p. 254.
Metridium reticulatum: Milne-Edwards, 1857, p. 255.
Discosoma fuegiensis: Milne-Edwards, 1857, p. 257.
Actinoloba reticulata: Gosse, 1860, p. 24.
Actinoloba Achates: Gosse, 1860, p. 24, 39.
Cereus Fuegiensis: Verrill, 1869, p. 480, 567.
Antholoba reticulata: Hertwig, 1882, p. 47, 116.
Actinoloba achates: Andres, 1883, p. 181.
Actinolobopsis reticulata: Verrill, 1899, p. 144.
Actinostola callosa: Rees, 1913, p. 382.
Material examined. MZSP 8758–8760: three adult specimens, 3–22 mm length and 29–32 mm diameter, Penha, Santa Catarina State, Brazil (10–30 m depth − 26°45'S, 48°36'W), Morandini A. coll. (20/x/2006); MZSP 8761: voucher of longitudinal and transversal histological sections from a specimen (male), Penha, Santa Catarina State, Brazil (10–30 m depth − 26°45'S, 48°36'W), Morandini A. coll. (20/x/2006).
External anatomy (Fig. 2a–c). Oral disc to 28–31 mm diameter in preserved specimens, without clear lobes in contraction, light brown in preservation, with mouth atop oral cone. Mouth gapes in preservation, exposing actinopharynx (Fig. 2a). Tentacles 0.4–1.8 mm in length and 0.9–1 mm in diameter, longest tentacles are shorter than oral disc radius, do not cover oral disc even in contraction, numbering 660–710, hexamerously arranged in seven or more cycles (last cycle incomplete). All tentacles contractile, smooth, conical, tapering to blunt distal end, light brown with blue line around base in preserved specimens. Column stout, contractile, light brown, 13–22 mm in height and 29–32 mm in diameter, (Fig. 2b). Margin not distinct. Column body wall surface transversely wrinkled (“reticulate”) (Fig. 2b). Pedal disc firmly attached, translucent light brown in preserved material, 28 to 31 mm in diameter (Fig. 2c).
Internal anatomy (Fig. 2d–f’). Mesenteries regularly arranged in five cycles (6 + 6 + 12 + 24 + 48 = 96 pairs at level of the actinopharynx in the examined specimens): first three cycles perfect, including the two directive pairs, fourth and fifth cycles imperfect (Fig. 2f). Two pairs of directive mesenteries, each associated with a well-developed siphonoglyph (Fig. 2f). Gonochoric: two specimens examined male, spermatic cysts (64 to 213 µm in diameter) in imperfect, fourth and fifth cycle mesenteries (Fig. 2f). Retractor muscles fairly weak and diffuse (Fig. 2f). Basilar muscles poorly-developed (Fig. 2e). Parietobasilar muscles poorly developed (Fig. 2f). Sphincter muscle mesogloeal (SM), very elongate and strong, confined to the endodermal side of the mesogloea of column, not separated from the circular endodermal musculature of the column, extends along the entire column, distinctly reticular, with muscle meshes being large and crowded (Fig. 2d–d’’); in upper column, thickness of SM and mesogloea (M) 260 ± 20 and 265 ± 40 µm (mean ± standard deviation), respectively; at the level of the actinopharynx, 300 ± 98 (SM) and 645 ± 109 (M) µm; in mid column, 170 ± 40 (SM) and 807 ± 150 (M) µm; in lower column near limbus, 150 ± 29 (SM) and 322 ± 84 (M) µm. Azooxanthellate.
Cnidom (Fig. 3). basitrichs, spirocysts, p-mastigophores A (two forms), p-mastigophores B1. Five stenoteles were found in the filaments (8.6 ± 1.2 µm length × 6.1 ± 1.5 µm width), they are contamination by feeding (possibly of a Hydrozoa). The size ranges and distribution of the cnidae are shown in Table 1.
Distribution and natural history. Antholoba achates has been reported from rocky or biogenic substrates such as shells of gastropods and large decapod crabs (Acuña et al. 2003; Luzzatto and Pastorino 2006; Bigatti et al. 2009; Averbuj et al. 2012). It thrives in areas with medium to strong currents, ranging from the intertidal to depths of 836 m (Stotz 1979; Fautin 1984; Häussermann and Försterra 2009). This species is found in several regions, including Antarctica, the southwest Atlantic (along the southeast coast of Brazil, the coast of Argentina, and the Islas Malvinas/Falkland Islands), as well as in the southwestern (New Zealand) and the southeastern (Chile, Galapagos Islands, Strait of Magellan) Pacific (reviewed by Häussermann and Försterra 2009). Asexual reproduction is unreported and not implied by the condition of these or previous specimens.
Remarks. The presence of “papillae” or of “reticulations” on the column wall of A. achates has been a subject of extensive discussion (Hertwig 1882; McMurrich 1904; Carlgren 1959; Carter 1965; Fautin 1984). These are essentially raised portions of the body wall, comprising both mesoglea and unspecialized ectoderm with depressions between them (see Carter 1965; Fautin 1984). Historically, specimens having reticulations were attributed to A. reticulata because this feature is highlighted in Couthouy’s notes about that species (Dana 1846). However, McMurrich (1904) observed that both the coloration and reticulation of A. achates display variability. More recently, Deserti et al. (2012) reported specimens of A. achates from Uruguay and in their illustrations revealed small reticulations on the column wall.
Dana (1846) and Fautin (1984) identified juveniles brooded within the column of specimens of A. achates. We see no evidence of juveniles in any of the specimens we have examined, suggesting that brooding is a seasonal phenomenon, as in other sea anemones (reviewed in Larson 2017), or that it is restricted to females (by chance, we dissected only males).
Based on the cnidocyst classification proposed by Gusmão et al. (2018), the nematocysts we observed are p-mastigophores A (two forms), and p-mastigophores B1. Fautin’s (1984) account of the cnidae described what we term b-mastigophores as basitrichs, and did not differentiate among the microbasic p-mastigophores. While the capsule morphologies described in the referenced study appear markedly different from ours, we attribute this discrepancy to potential inconsistencies in terminology and prevailing understandings of these structures, rather than signifying actual variations in the cnidome composition of the examined specimens. In the same way, Carter (1965) and Deserti et al. (2012) found microbasic p-mastigophores in the filament, but no p-mastigophores B1 were reported. However, Carter (1965) detected microbasic b- and p-mastigophores in the tentacles. Additionally, we identified a type of hydrozoan nematocyst (stenotele) within the filament; we follow Gusmão and Rodríguez (2021b) and infer that this animal had consumed the hydrozoan.
We did not identify the number and cycles of tentacles more precisely due to the state of preservation of all samples. Of four samples of A. achates, we calculated between 660 to 710 tentacles, which could have 8 cycles (~ 768 in number). Likewise, based on the study of Häussermann and Försterra (2009), they have counted around 700 tentacles. On the other hand, due to the contraction and diameter of the specimens’ oral disc, we were only able to identify five cycles of mesenteries, and possibly the last two are in the distal region.
Antholoba fabiani sp. nov. (Figs. 4–5)
Histological sections of the specimen MZSP 8751: (a) cross section through column, at the level of the actinopharynx, showing the seven mesentery cycles; (b-h); (i-i’) longitudinal section through upper column showing sphincter mesogloeal; (j) longitudinal section through pedal disc showing basilar muscles; (k) cross section of tentacles. Abbreviations: 1–7 = first to seventh pairs of mesenteries; bm = basilar muscles; cn = cnidocystes; co = column; di = pairs of directive mesenteries; ep = epidermis; et = ectodermal longitudinal muscles of tentacles; g = gastrodermis; m = mesogloea; s = siphonoglyph; sm = sphincter mesogloeal; o = oocytes; pd = pedal disc; t = tentacle; rm = retractor muscle. Scales: a: 5 mm; b–f, i-j: 1 mm; i’: 100 μm; g-g’: 250 μm; h: 50 μm; k: 5 μm; l: 20 μm
https://zoobank.org/NomenclaturalActs/B535CD3F-DF89-4E34-9EE1-D8F1CAA74658
Material examined. Holotype: MZSP 8748: adult individual, 18 mm long and 19 mm wide, Ubatuba Bay, São Paulo State, Brazil (5 m depth − 23°26'S, 45°01'W), Durán-Fuentes J. coll. (27/vi/2022). Paratypes: MZSP 8749: adult individual, 22 mm long and 24 mm wide, Ubatuba Bay, São Paulo State, Brazil (5 m depth − 23°26'S, 45°01'W), Durán-Fuentes J. coll. (27/vi/2022); MZSP 8750: adult individual, 29 mm long and 43 mm wide, Ubatuba Bay, São Paulo State, Brazil (15 m depth − 23°26'S, 45°01'W), Durán-Fuentes J. coll. (28/vi/2022); MZSP 8751: voucher of longitudinal and transversal histological sections from a specimen (female), Ubatuba Bay, São Paulo State, Brazil (12 m depth − 23°26'S, 45°01'W), Durán-Fuentes J. coll. (7/vii/2022).
Diagnosis. Tentacles short, very numerous (> 750 in number), arranged hexamerously in eight cycles. Mesenteries irregularly arranged in seven cycles (199 pairs); fourth to seventh cycles imperfect; mesenteries of seventh cycle few in number and underdeveloped in musculature. Gametogenic tissue in fourth and sixth mesenteric cycles.
Etymology. the epithet “fabiani” (Fă.bĭā.ni) pl. masc. noun II decl. honors Dr. Fabián H. Acuña, acknowledging his trajectory as a cnidarian researcher with a focus on South American sea anemones. His contributions include a series of publications covering ecology, taxonomy, and population studies of actiniarians.
External anatomy (Fig. 4). Fully expanded tentacles and oral disc to 32–41 mm in diameter in live specimens. Oral disc cup-shaped with an expanded distal part, cream white with radial brown lines in life (Fig. 4a–b and c). Disc flat (not folded) without prominent lips or an oral cone; tentacles cover outer half to third of oral disc, leaving disc visible except when wide distal margin flops over and obscures it. Tentacles 0.5–3.3 mm in length and 0.4–1.1 mm in diameter, hexamerously arranged in eight cycles (expect 768 if eighth cycle is perfect at distal margin), longest tentacles are shorter than oral disc radius, do not cover oral disc even in contraction. Outermost tentacles notably shorter and more slender than inner tentacles. All tentacles contractile, smooth, conical, bluntly tapering distally, translucent white or beige with dark line around base (Fig. 4a and d). Contractility of tentacles means that they may bulge along their length.
Column stout, contractile, 18–29 mm in height and 19–43 mm in diameter, darker brown in life (Fig. 4a–b), without a distinct distal margin (Fig. 3b and d). Body-wall surface transversely wrinkled (“reticulate”), with reticulations forming ridges irregular in spacing and orientation, typically transverse rather than longitudinal to oral-aboral axis. Each ridge of reticulation tapers slightly towards its distal surface. In contraction, body wall between reticulations may bubble up, resembling inflated papillae, but a well-expanded column has neither papillae, tubercles, nor verrucae (Fig. 4b–c and e–f). Pedal disc 16 to 53 mm in diameter, firmly attached, beige and translucent in living animals (Fig. 4e). In highly contracted specimens, proximal margin of column may extend inwards, with pedal disc appearing sunken inside it (Fig. 4e).
Internal anatomy (Fig. 5). Mesenteries irregularly arranged in seven cycles (6 + 6 + 12 + 24 + 48 + 96 + 7 = 199 pairs at level of actinopharynx in examined specimens): first three cycles perfect, including two directive pairs (Fig. 5a–h); fourth to seventh cycles imperfect. Same number of distally and proximally; seventh cycle incomplete and with underdeveloped musculature. Two pairs of directive mesenteries, each associated with a well-developed siphonoglyph (Fig. 5a-b). Inferred to be gonochoric or protogynous hermaphrodites because specimens were only female, with oocytes (166 to 257 µm in diameter) in fourth and sixth cycle mesenteries (Figs. 5e, g–g’). Retractor muscles well-developed and diffuse (Fig. 5a–g’). Parietobasilar muscles poorly developed. Basilar muscles fairly weak (Fig. 5j). Sphincter muscle mesogloeal (SM), very elongate and strong, confined to the endodermal side of the mesogloea of column, not separated from the circular endodermal musculature of the column, extends along the entire column, with muscle meshes being small, reticular, and crowded (Fig. 5i–i’); in upper column, the thickness of the SM and mesoglea (M) 190 ± 25 and 470 ± 100 µm (mean ± standard deviation), respectively; at level of actinopharynx of 210 ± 30 and 560 ± 100 (M) µm; mid column of 77 ± 9 (SM) and 1011 ± 264 (M) µm; lower column next to limbus, 93 ± 30 (SM) and 487 ± 105 (M) µm. Longitudinal muscles of tentacles ectodermal (Fig. 5k). Azooxanthellate.
Cnidom (Fig. 6). Basitrichs, spirocysts, p-mastigophores A, and p-mastigophores B1. The size ranges and distribution of the cnidae are shown in Table 2.
Distribution and natural history. This species inhabits the shallow coast of southeastern Brazil. Specimens of Antholoba fabiani sp. nov. were collected in Ubatuba Bay, São Paulo State (Brazil), at depths ranging from 5 to 30 m. They are found attached to rocks, trunks, branches, and shells. We found evidence of the epibiosis of Olivancillaria sp. (Gastropoda) with A. fabiani sp. nov.
Remarks. We identified eight mesentery cycles of A. fabiani sp. nov. through study of live and preserved samples, and photos. The youngest (last) cycles were difficult to observe. However, we counted between 750 to 770 tentacles (~ 768 in number). We observed between five to seven mesentery pairs of the seventh cycle on the histological sections (Fig. 5a and h). We expect that the last cycle of mesenteries could be in the distal region. In addition, we only found the fourth and sixth cycles fertile in two samples, with reproductive tissues at different column heights, these observations are rare within of the actiniarians.
Differential diagnosis (Table 3). Antholoba fabiani sp. nov. has 199 pairs of mesenteries in seven cycles at the level of the actinopharynx, with the first three cycles perfect (24 pairs). In contrast, both A. achates and A. perdix possess 96 pairs of mesenteries arranged in five cycles on the level of actinopharynx. Antholoba achates also has perfect mesenteries only in the first three cycles (24 pairs), whereas A. perdix has perfect mesenteries in the first four cycles (48 pairs) (McMurrich 1904; Carlgren 1949; Widersten 1976; Zamponi and Excoffon 1995; Häussermann and Försterra 2009) (Table 3). The gametogenic tissue in A. fabiani sp. nov. was observed in the fourth and sixth mesenteric cycles, whereas in A. achates, it is present in the last two cycles (fourth and fifth cycles). It is worth highlighting those histological sections from only two specimens of A. fabiani sp. nov. were examined. In both cases, however, no gametogenic tissue was found in the mesenteries of the fifth cycle.
Adult specimens of Antholoba achates feature a folded oral disc with 5–7 lobes and a body wall with transverse reticulations that vary in size (see McMurrich 1904; Carter 1965; Fautin 1984), but young specimens have a round oral disc (Häussermann and Försterra 2009). In contrast, adult specimens of A. fabiani sp. nov. have a cup-shaped oral disc with an expanded distal part and a column wall with transverse wrinkles or a reticulated pattern more pronounced than in A. achates. Based on histological sections along the column, the width of the sphincter muscle in specimens of A. achates is thicker than in A. fabiani sp. nov. (260 ± 20 and 190 ± 25 µm, respectively). It is possible that the formation of lobes in the oral disc of A. achates is due to the development of the sphincter, which might exert stronger constriction on the distal column. Additionally, A. fabiani sp. nov. has a greater thickness of the mesoglea along the column than A. achates (581 ± 261 and 427 ± 202 µm). This feature may explain the formation of a reticulate pattern in both species, with the reticulation being more pronounced in A. fabiani sp. nov.
Regarding tentacles, all three species (A. achates, A. fabiani sp. nov., and A. perdix) possess numerous short tentacles, although the number and arrangement vary among them: Antholoba achates has between 660 to 710 tentacles, hexamerously arranged in 7 or more cycles (with the last cycle incomplete). A. fabiani sp. nov. has > 750 tentacles arranged in eight cycles, while A. perdix has approximately 600 tentacles arranged in five cycles.
Molecular analysis (Fig. 7, Supplementary materials 6–7).
Phylogeny reconstruction resulting from maximum likelihood analysis of Antholoba fabiani sp. nov. within the order Actiniaria using concatenated dataset (12S, 16S, 18S, 28S, and COIII). Antholobidae fam. nov. indicated by a red diamond. Support values with SH-aLRT support (%) / parametric aLRT support / aBayes support / ultrafast bootstrap support (%). Filled-in circles indicate nodes with support of ~ 100% for all inferences. Red circles indicate taxa within Metridioidea that lack acontia based by Lauretta et al. (2014). ‘*’ Although these species fall within superfamily Metridiodea in most recent phylogenetic studies (see Rodríguez et al. 2014); ‘**’ These species were including in the most recent phylogenetic studies (see Sanamyan et al. 2021), they do not form a monophyletic group in these analyses
The comparison of the aligned sequences and maximum likelihood (ML) phylogeny reconstruction of the order Actiniaria utilized conserved positions from concatenated markers, including 12S rDNA (665 bp), 16S rDNA (423 bp), Cytochrome c oxidase subunit 3 (437 bp), 18S rDNA (1383 bp), and 28S rDNA (631 bp). The analysis indicated that Antholobidae fam. nov. is closely related to the acuticulate clade within the superfamily Metridioidea, with strong support from SH-aLRT (99.5%), parametric aLRT (1), aBayes test (1), and ultrafast bootstrap (61%).
In the phylogenetic tree, the sampled species within Antholoba form two distinct clades (100% support): one consisting of A. achates from Argentina and Chile, the other comprising A. fabiani sp. nov. The K2P distance between the sequences of A. achates from Argentina and Chile is 0%, whereas the distance between samples of A. achates and A. fabiani sp. nov. ranges from 0.2 to 0.7% (± 0.001) in concatenated sequences (Supplementary material 6). Those distances were similar in comparison with other species pairs considered: Actinothoe chilensis vs. A. georgiana (0.21 ± 0.06%), Actinothoe chilensis vs. A. sphyrodeta (0.35 ± 0.1%), Exaiptasia brasiliensis vs. E. diaphana (0.2 ± 0.09%), Isoparactis fabiani vs. I. ferax (1.1 ± 0.2%), Metridium senile vs. M. farcimen (0.3 ± 0.12%), and Sagartia lacerata vs. S. undata (0.3 ± 0.07%) (Supplementary material 6).
Diagnostic positions in the 12S, 16S, COIII, 18S, and 28S markers.
Antholoba achates vs. Antholoba fabiani sp. nov. 12S rRNA: 421 (C vs. A), 813 (G vs. A); 16S rRNA: no difference; COIII: 286 (T vs. A), 448 (T vs. A); 18S rRNA: 202 (C vs. -), 206 (C vs. T), 259 (T vs. C); 28S rRNA: 7 (- vs. A), 170 (A vs. G), 182 (G vs. A), 189 (C vs. G), 190 (T vs. C), 459 (C vs. T), 490 + 4 (G vs. A), 536 (C vs. T), 613 (A vs. G), 629 (T vs. C), 643 (T vs. C), 643 + 2 (A vs. C), 643 + 7 (A vs. G), 659 (A vs. T), 679 (C vs. T), 680 (Y vs. G), 739 (- vs. G), 745 (T vs. A), 798 (T vs. -), 799 (G vs. -), 800 (A vs. -), 801 (A vs. -), 802 (A vs. -), 803 (C vs. -), 804 (A vs. -), 805 (C vs. -), 806 (G vs. -), 881 (T vs. C), 910 (C vs. T), 911 (T vs. -).
Discussion
The most widespread and well-known species of Antholoba is A. achates. It has a broad geographical and bathymetric distribution (Fautin 1984; Häussermann and Försterra 2009; GBIF 2023) and exhibits variability in its external-morphology presentation, influenced by factors such as preservation methods and the observer’s perception (see McMurrich 1904; Fautin 1984), as well as natural variation among specimens and populations. As an example, the number of tentacles varies between specimens, but reports that cannot be verified with specimens exceed those by almost a factor of two (Fautin 1984; commenting on Hertwig 1882). These issues likely contribute to the high number of synonyms for A. achates, making it difficult to determine new species within the group.
Specimens of A. fabiani sp. nov. from Ubatuba share a similar shape to those described for A. reticulata by Dana (1846), although they exhibit a distinct coloration pattern. Antholoba reticulata from Chile were described as having a yellow-greenish column and oral disc, along with dark green tentacles (Dana 1846). In contrast, A. fabiani sp. nov. features a brown column, white and beige tentacles, and a translucent oral disc (Fig. 3a–b). No holotype of A. reticulata is available for comparison (Fautin 2016) and the species is currently considered a junior synonym of A. achates. The reticulations that characterize A. fabiani are present in some specimens of A. achates, being less pronounced or absent in others, with at least some variation corresponding to preservation (Couthoy in Dana 1846; McMurrich 1904; Fautin 1984). In A. fabiani sp.nov., the reticulations remain discernable in all degrees of expansion or contraction we have observed.
The number of tentacles in A. fabiani sp. nov. exceeds what is predicted based on the number of mesenteries: the tentacles suggest seven cycles of mesenteries, but we observe only six complete cycles and some mesenteries of the seventh cycle. Based on accounts of A. achates, we suspect that there are additional, extremely small mesenteries at the distal margin that complete the seventh cycle. Fautin (1984) confirms that each tentacle corresponds to a single mesenteric space in A. achates, and describes those at the distal margin as thin and small. Given the flaring oral disc of A. fabiani, these are difficult to observe in dissection or section, but are expected to be present given the number and arrangement of tentacles.
In addition to the anatomical and coloration differences between A. fabiani sp. nov. and its congeners, A. achates and A. perdix, we have identified genetic differences that support our interpretation of it as a distinct species. While conventional “barcoding” markers are challenging to apply to actiniarians due to the relatively slow rate of molecular evolution in Anthozoa (see Shearer et al. 2002; Reimer et al. 2006; Daly et al. 2010; Stampar et al. 2012; Pereira et al. 2014; Glon et al. 2021), the K2P distances we observe between A. fabiani sp. nov. and A. achates from Argentina (0.7 (± 0.1) %: Supplementary material 6) or A. achates from Chile (0.2 (± 0.1) %: Supplementary material 6) align with those reported in other closely related species, which exhibit K2P distances ranging from 0.2 (± 0.09) to 1.1 (± 0.2) % (Supplementary material 6). These distances are consistent with reported divergences in mitochondrial and/or nuclear sequences in other anemone genera (e.g., Beneti et al. 2015; González-Muñoz et al. 2015; Grajales and Rodríguez 2016; Durán-Fuentes et al. unpublished results).
Some previous records of A. achates could possibly represent records of A. fabiani sp. nov. For example, Zabala (2012) and Zabala et al. (2013) conducted a study on the trophic ecology, growth, and reproduction of the gastropod Adelomelon ancilla ([Lightfoot], 1786) at Paraná Beach, Argentina Patagonia, which included a discussion of the external morphology of Antholoba achates. This morphology more closely matches the coloration, column shape, and tentacle shape that we report for A. fabiani sp. nov. than what has been previously reported for A. achates. Additionally, at Montemar in Valparaíso Chile, Carter (1965) documented two forms of Antholoba. Some of these had reticulate columns and a purple-brown coloration on the column with reticulations, as we report for A. fabiani sp. nov., raising the possibility of a sympatric distribution of A. achates and A. fabiani sp. nov. in South America. However, it would be necessary to examine reference materials from these studies of specimens from these studied locations to confirm whether these previous records correspond to A. fabiani sp. nov.
The taxonomic classification of the genus Antholoba within the order Actiniaria has been a subject of ongoing debate. Initially, Antholoba was assigned to the family Paractidae (Hertwig 1882; McMurrich 1893; Carlgren 1893, 1899; Stephenson 1921). In his comprehensive treatment of all Actiniaria, Carlgren (1949) reclassified the genus into the family Actinostolidae Carlgren 1893, justifying it by shared morphological characteristics, such as the presence of a mesogloeal sphincter, the absence of acontia, the arrangement of tentacles in cycles, and mesenteries that are not divisible into well-developed and poorly-developed mesenteries. The last attribute was mis-interpreted: members of Antholoba could have additional poorly-developed mesenteries distally (see above). Further morphological studies affirmed the placement of Antholoba within Actinostolidae (Rodríguez et al. 2008). However, Rodríguez et al. (2008) noted that Antholoba did not associate with the taxa aligned with Carlgren’s (1949) two-group scheme within Actinostolidae. In contrast, molecular studies consistently place sequences of Antholoba achates within superfamily Metridioidea, along with other species previously classified in Actinostoloidea Carlgren 1893, such as Paranthus niveus (Lesson, 1830) (see Rodríguez and Daly 2010; Lauretta et al. 2014; Rodríguez et al. 2014; Grajales and Rodríguez 2016; Gusmão et al. 2018, 2019, 2020; Gusmão and Rodríguez 2021a; Yoshikawa et al. 2022). This phylogenetic assignment implies a loss of acontia. Milne-Edwards (1857) foreshadowed this classification when he allied Antholoba and Metridium based on similarities in their column and disc shape.
Based on highly supported molecular phylogenetic trees, we propose the repositioning of genus Antholoba within the superfamily Metridioidea. Recognizing that there is no support for an association between Antholoba and Actinostola Verrill, 1883, the type genus of Actinostolidae, we propose the establishment of the new family, Antholobidae fam. nov., and classify it as a member of Metridiodea. Concerning its relationships within Metridiodea, Antholobidae fam. nov. is part of the Acuticulate clade, sister to all the other acuticulate taxa we studied (Fig. 7, Supplementary material 7).
Our trees provide insight into a few other open questions in actiniarian systematics. Gusmão and Rodríguez (2021a) placed family Halcampoididae Appellöf, 1896 in superfamily Actinostoloidea. In contrast, both our phylogenetic analysis and that of Sanamyan et al. (2021) suggest that Halcampoididae is a sister group to Metridioidea. Furthermore, when comparing our results with those of Sanamyan et al. (2021), we note that the representative of family Sicyonidae Hertwig 1882 does not cluster within Actinostoloidea; instead, it forms a sister group to the superfamily Actinioidea Rafinesque, 1815. The trees of Sanamyan et al. (2021) did not sample broadly enough to determine the affinity of this group, although they recognized it as distinct from other Actinostoloidea.
References
Acuña FH, Excoffon AC, Scelso MA (2003) Mutualism between the sea anemone Antholoba achates (Drayton, 1846) (Cnidaria: Actiniaria: Actinostolidae) and the spider crab Libinia spinosa Milne-Edwards, 1834 (Crustacea: Decapoda: Majidae). Belg J Zool 133:85–87
Andres A (1883) Le Attinie. Salviucci, Rome, 460 pp
Anisimova M, Gascuel O (2006) Approximate likelihood-ratio test for branches: a fast, accurate, and powerful alternative. Syst Biol 55:539–552. https://doi.org/10.1080/10635150600755453
Anisimova M, Gil M, Dufayard JF, Dessimoz C, Gascuel O (2011) Survey of branch support methods demonstrates accuracy, power, and robustness of fast likelihood-based approximation schemes. Syst Biol 60:685–699. https://doi.org/10.1093/sysbio/syr041
Averbuj A, Palomo G, Brogger MI, Penchaszadeh PE (2012) Diet and feeding of the nassariid Buccinanops cochlidium from northern Patagonia, Argentina. Aquat Biol 17:261–268. https://doi.org/10.3354/ab00480
Beneti JS, Stampar SN, Maronna MM, Morandini AC, Silveira FLD (2015) A new species of Diadumene (Actiniaria: Diadumenidae) from the subtropical coast of Brazil. Zootaxa 4021:156–168. https://doi.org/10.11646/zootaxa.4021.1.6
Bigatti G, Sanchez Antelo C, Miloslavich P, Penchaszadeh PE (2009) Feeding behavior of Adelomelon ancilla (Ligfoot, 1786): A predatory neogastropod (Gastropoda: Volutidae) in Patagonian benthic communities. The Nautilus 123:159–165
Brugler MR, González-Muñoz R, Tessler M, Rodríguez E (2018) An EPIC journey to locate single-copy nuclear markers in sea anemones. Zool Scr 47:756–776. https://doi.org/10.1111/zsc.12309
Carlgren O (1934) Zur revision der Actiniarien. Arkiv För Zoologi 26:1–36
Carlgren O (1959) Corallimorpharia and Actiniaria with description of a new genus and species from Peru. Reports of the Lund University Chile Expedition 1948–49, Nr. 38. Acta Univ Lund 56:1–39
Carlgren O (1893) Studien über Nordische Actinien. K Svenska Vetenskapsakad Handl 25:1–148
Carlgren O (1899) Zoantharien. Ergebnisse der Hamburger Magalhaensische Sammelreise 4:1–48
Carlgren O (1949) A survey of Ptychodactiria, Corallimorpharia and Actiniaria. K Svenska Vetenskapsakad Handl 1:1–121
Carter D (1965) Actinias de Montemar, Valparaíso. Rev Biol Mar 12:129–157
Castresana J (2000) Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol Biol Evol 17:540–552. https://doi.org/10.1093/oxfordjournals.molbev.a026334
Daly M, Gusmão LC, Reft AJ, Rodríguez E (2010) Phylogenetic signal in mitochondrial and nuclear markers in sea anemones (Cnidaria, Actiniaria). Integr Comp Biol 50:371–388. https://doi.org/10.1093/icb/icq081
Daly M, Crowley LM, Larson P, Rodríguez E, Heestand Saucier E, Fautin DG (2017) Anthopleura and the phylogeny of Actinioidea (Cnidaria: Anthozoa: Actiniaria). Org Divers Evol 17:545–564. https://doi.org/10.1007/s13127-017-0326-6
Dana JD (1846) Zoophytes: 740 in: United States Exploring Expedition. During the years 1838, 1839, 1840, 1841, 1842. Under the command of Charles Wilkes, U.S.N.— Lea and Blanchard, Philadelphia
Dereeper A, Guignon V, Blanc G, Audic S, Buffet S, Chevenet F, Gascuel O (2008) Phylogeny. fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res 36(suppl_2):W465–W469
Deserti MI, Zamponi MO, Riestra G (2012) Las anémonas de mar (Cnidaria; Anthozoa; Actiniaria) de la plataforma continental uruguaya. Revista Real Academia Galega de Ciencias 31:115–136
Estrada-Flores E, Peralta L, Rivas P (1982) Manual de técnicas histológicas. AGT Press
Fautin DG (1984) More Antarctic and Subantarctic Sea Anemones (Coelenterata: Cörallimorpharia and Actiniaria). Antarct Res Ser 41:1–42
Fautin DG (2016) Catalog to families, genera, and species of orders Actiniaria and Corallimorpharia (Cnidaria: Anthozoa). Zootaxa 4145:1–449. https://doi.org/10.11646/zootaxa.4145.1.1
Fautin DG, Hickman CP, Daly M, Molodtsova T (2007) Shallow-water sea anemones (Cnidaria: Anthozoa: Actiniaria) and tube anemones (Cnidaria: Anthozoa: Ceriantharia) of the Galápagos Islands. Pac Sci 61:549–573. https://doi.org/10.2984/1534-6188(2007)61[549:SSACAA]2.0.CO;2
GBIF (2023) Global Biodiversity Information Facility. Antholoba Hertwig, 1882. https://www.gbif.org/species/2256332. Accessed 21 Sept 2023
Glon H, Quattrini A, Rodríguez E, Titus BM, Daly M (2021) Comparison of sequence-capture and ddRAD approaches in resolving species and populations in hexacorallian anthozoans. Mol Phylogenet Evol 163:107233. https://doi.org/10.1016/j.ympev.2021.107233
González-Muñoz R, Simões N, Mascaro M, Tello-Musi JL, Brugler MR, Rodríguez E (2015) Morphological and molecular variability of the sea anemone Phymanthus crucifer (Cnidaria, Anthozoa, Actiniaria, Actinioidea). J Mar Biol Assoc 95:69–79. https://doi.org/10.1017/s0025315414000988
Gosse PH (1855) Description of Peachia hastata, a new genus and species of the Class Zoophyta; with observations on the Family Actiniadae. Trans Linn Soc 21:267–276
Gosse PH (1860) Actinologia Britannica: A History of the British Sea Anemones and Corals. Van Voorst, London
Grajales A, Rodríguez E (2016) Elucidating the evolutionary relationships of the Aiptasiidae, a widespread cnidarian-dinoflagellate model system (Cnidaria: Anthozoa: Actiniaria: Metridioidea). Mol Phylogenet Evol 94:252–263. https://doi.org/10.1016/j.ympev.2015.09.004
Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O (2010) New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol 59:307–321. https://doi.org/10.1093/sysbio/syq010
Gusmão LC, Rodríguez E (2021a) Two sea anemones (Cnidaria: Anthozoa: Actiniaria) from the Southern Ocean with evidence of a deep-sea, polar lineage of burrowing sea anemones. Zool J Linn 193:1392–1415. https://doi.org/10.1093/zoolinnean/zlaa176
Gusmão LC, Rodríguez E (2021b) Deep-sea Anemones (Cnidaria: Anthozoa: Actiniaria) from the South Atlantic. Bull Am Mus Nat Hist 444:1–69. https://doi.org/10.1206/0003-0090.444.1.1
Gusmão LC, Grajales A, Rodríguez E (2018) Sea anemones through X-rays: visualization of two species of Diadumene (Cnidaria, Actiniaria) using micro-CT. Am Mus Novit 2018:1–47. https://doi.org/10.1206/3907.1
Gusmão LC, Berniker L, Van Deusen V, Harris O, Rodríguez E (2019) Halcampulactidae (Actiniaria, Actinostoloidea), a new family of burrowing sea anemones with external brooding from Antarctica. Polar Biol 42:1271–1286. https://doi.org/10.1007/s00300-019-02516-1
Gusmão LC, Van Deusen V, Daly M, Rodríguez E (2020) Origin and evolution of the symbiosis between sea anemones (Cnidaria, Anthozoa, Actiniaria) and hermit crabs, with additional notes on anemone-gastropod associations. Mol Phylogenet Evol 148:106805. https://doi.org/10.1016/j.ympev.2020.106805
Häussermann V, Försterra G (2009) Actiniaria-Sea Anemones. Marine Benthic Fauna of Chilean Patagonia. Nature in Focus, Puerto Montt
Hertwig R (1882) Die Actinien der Challenger expedition. Gustav Fischer, Jena
Hoang DT, Chernomor O, Von Haeseler A, Minh BQ, Vinh LS (2018) UFBoot2: improving the ultrafast bootstrap approximation. Mol Biol Evol 35:518–522. https://doi.org/10.1093/molbev/msx281
Kalyaanamoorthy S, Minh BQ, Wong TK, Von Haeseler A, Jermiin LS (2017) ModelFinder: fast model selection for accurate phylogenetic estimates. Nat Methods 14:587–589. https://doi.org/10.1038/nmeth.4285
Katoh K, Asimenos G, Toh H (2009) Multiple alignment of DNA sequences with MAFFT. Methods Mol Biol 537:39–64. https://doi.org/10.1007/978-1-59745-251-9_3
Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, Thierer T, Ashton B, Meintjes P, Drummond A (2012) Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28:1647–1649. https://doi.org/10.1093/bioinformatics/bts199
Kimura M (1980) A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120
Larson P (2017) Brooding sea anemones (Cnidaria: Anthozoa: Actiniaria): paragons of diversity in mode, morphology, and maternity. Invertebr Biol 136:92–112. https://doi.org/10.1111/ivb.12159
Larson PG, Daly M (2016) Phylogenetic analysis reveals an evolutionary transition from internal to external brooding in Epiactis Verrill (Cnidaria: Anthozoa: Actiniaria) and rejects the validity of the genus Cnidopus Carlgren. Mol Phylogenet Evol 94:548–558. https://doi.org/10.1016/j.ympev.2015.10.008
Lauretta D, Häussermann V, Brugler MR, Rodríguez E (2014) Isoparactis fionae sp. nov. (Cnidaria: Anthozoa: Actiniaria) from Southern Patagonia with a discussion of the family Isanthidae. Org Divers Evol 14:31–42. https://doi.org/10.1007/s13127-013-0149-z
Luzzatto D, Pastorino G (2006) Adelomelon brasiliana and Antholoba achates: a phoretic association between a volutid gastropod and a sea anemone in Argentine waters. Bull Mar Sci 78:281–286
McMurrich JP (1893) Report on the Actiniæ collected by the United States Fish Commission Steamer Albatross during the winter of 1887–1888. Proc US Natl Mus 16:119–216
McMurrich JP (1904) The Actiniae of the Plate Collection (in Plate Fauna Chilensis 3. 2). Zool Jahrb Jena Suppl 6(2): 215–306
Milne-Edwards H (1857) Zoanthaires. In Histoire Naturelle des Coralliaires ou Polypes Proprement Dits, I. 295–310
Minh BQ, Schmidt HA, Chernomor O, Schrempf D, Woodhams MD, Von Haeseler A, Lanfear R (2020) IQ-TREE 2: new models and efficient methods for phylogenetic inference in the genomic era. Mol Biol Evol 37:1530–1534. https://doi.org/10.1093/molbev/msaa015
Pereira AM, Brito C, Sanches J, Sousa-Santos C, Robalo JI (2014) Absence of consistent genetic differentiation among several morphs of Actinia (Actiniaria: Actiniidae) occurring in the Portuguese coast. Zootaxa 3893:595–600. https://doi.org/10.11646/zootaxa.3893.4.9
Perroca JF, Rodrigues Filho JL, Fransozo A, Costa RC (2022) Variations in pink-shrimps Farfantepenaeus brasiliensis and F. paulensis juvenile abundance: clarifying ecological patterns and providing subsidies to management in shallow marine ecosystems. Fish Res 256:106482. https://doi.org/10.1016/j.fishres.2022.106482
Rees OM (1913) Notes on Actinostola callosa (Verrill) = Dysactis crassicornis (Hertwig). Ann Mag Nat Hist 12:382–387. https://doi.org/10.1080/00222931308693413
Reimer JD, Ono S, Takishita K, Tsukahara J, Maruyama T (2006) Molecular evidence suggesting species in the zoanthid genera Palythoa and Protopalythoa (Anthozoa: Hexacorallia) are congeneric. Zool Sci 23:87–94. https://doi.org/10.2108/zsj.23.87
Rheindt FE, Bouchard P, Pyle RL, Welter-Schultes F, Aescht E, Ahyong ST, Ballerio A, Bourgoin T, Ceríaco LMP, Dmitriev D, Evenhuis N, Grygier MJ, Harvey MS, Kottelat M, Kluge N, Krell FT, Kojima JI, Kullander SO, Lucinda P, Lyal CHC, Scioscia CL, Whitmore D, Yanega D, Zhang ZQ, Zhou HZ, Pape T (2023) Tightening the requirements for species diagnoses would help integrate DNA-based descriptions in taxonomic practice. PLoS Biol 21(8):e3002251. https://doi.org/10.1371/journal.pbio.3002251
Rodríguez E, Daly M (2010) Phylogenetic relationships among deep-sea and chemosynthetic sea anemones: Actinoscyphiidae and Actinostolidae (Actiniaria: Mesomyaria). PLoS ONE 5:e10958. https://doi.org/10.1371/journal.pone.0010958
Rodríguez E, Castorani CN, Daly M (2008) Morphological phylogeny of the family Actinostolidae (Anthozoa: Actiniaria) with description of a new genus and species of hydrothermal vent sea anemone redefining the family Actinoscyphiidae. Invertebr Syst 22:439–452. https://doi.org/10.1071/is07053
Rodríguez E, Barbeitos M, Daly M, Gusmao LC, Häussermann V (2012) Toward a natural classification: phylogeny of acontiate sea anemones (Cnidaria, Anthozoa, Actiniaria). Cladistics 28:375–392. https://doi.org/10.1111/j.1096-0031.2012.00391.x
Rodríguez E, Barbeitos MS, Brugler MR, Crowley LM, Grajales A, Gusmão L, Häussermann V, Reft A, Daly M (2014) Hidden among sea anemones: the first comprehensive phylogenetic reconstruction of the order Actiniaria (Cnidaria, Anthozoa, Hexacorallia) reveals a novel group of Hexacorals. PLoS ONE 9:e96998. https://doi.org/10.1371/journal.pone.0096998
Rodríguez E, Fautin D, Daly M (2023) World List of Actiniaria. https://www.marinespecies.org/actiniaria. Accessed 11 July 2023. https://doi.org/10.14284/568
Sanamyan NP, Sanamyan KE, Galkin SV, Ivin VV, Bocharova ES (2021) Deep water Actiniaria (Cnidaria: Anthozoa) Sicyonis, Ophiodiscus and Tealidium: re-evaluation of Actinostolidae and related families. Invertebr Zool 18:385–449. https://doi.org/10.15298/invertzool.18.4.01
Shearer TL, Van Oppen MJH, Romano SL, Wörheide G (2002) Slow mitochondrial DNA sequence evolution in the Anthozoa (Cnidaria). Mol Ecol 11:2475–2487. https://doi.org/10.1046/j.1365-294X.2002.01652.x
Spano C, Flores V (2013) Staining protocol for the histological study of sea anemones (Anthozoa: Actiniaria) with recommendations for anesthesia and fixation of specimens. Lat Am J Aquat Res 41:1019–1024. https://doi.org/10.3856/vol41-issue5-fulltext-23
Stampar SN, Maronna MM, Vermeij MJA, Silveira FLD, Morandini AC (2012) Evolutionary diversification of banded tube-dwelling anemones (Cnidaria; Ceriantharia; Isarachnanthus) in the Atlantic Ocean. PLoS ONE 7:e41091. https://doi.org/10.1371/journal.pone.0109481
Stephenson TA (1921) On the classification of Actiniaria. Part II. Consideration of the whole group and its relationships, with special reference to forms not treated in Part I. J Cell Sci 65:493–576. https://doi.org/10.1242/jcs.s2-65.260.493
Stotz WB (1979) Functional morphology and zonation of three species of sea anemones from rocky shores in southern Chile. Mar Biol 50:181–188. https://doi.org/10.1007/BF00397825
Stöver BC, Müller KF (2010) TreeGraph 2: combining and visualizing evidence from different phylogenetic analyses. BMC Bioinform 11:7. https://doi.org/10.1186/1471-2105-11-7
Tamura K, Stecher G, Kumar S (2021) MEGA 11: Molecular evolutionary genetics analysis version 11. Mol Biol Evol 38:3022–3027. https://doi.org/10.1093/molbev/msab120
Vaidya G, Lohman DJ, Meier R (2011) SequenceMatrix: concatenation software for the fast assembly of multi-gene datasets with character set and codon information. Cladistics 27:17–180. https://doi.org/10.1111/j.1096-0031.2010.00329.x
Vences M, Miralles A, Brouillet S, Ducasse J, Fedosov A, Kharchev V, Kostadinov I, Kumari S, Patmanidis S, Scherz, MD, Puillandre N, Renner SS (2021) iTaxoTools 0.1: Kickstarting a specimen-based software toolkit for taxonomists. Megataxa 6:77–92. https://doi.org/10.11646/megataxa.6.2.1
Verrill AE (1869) Review of the corals and polyps of the west coast of America. Trans Conn Acad Arts Sci 1:377–596
Verrill AE (1882) Notice of the remarkable marine fauna occupying the outer banks off the southern coast of New England, No 4. Am J Sci 23:216–225
Verrill AE (1899) Descriptions of imperfectly known and new Actinians, with critical notes on other species, III. Am J Sci 7:143–146
Widersten B (1976) Ceriantharia, Zoanthidea, Corallimorpharia, and Actiniaria from the continental shelf and slope off the eastern coast of the United States. Fish Bull 74:857–878
Yoshikawa A, Yasuda A, Izumi T, Yanagi K (2022) A novel epibiotic association in the benthic community: The sea anemone Verrillactis sp. (Actiniaria: Sagartiidae) on the necto-benthic fish, Inimicus japonicus. Plankton Benthos Res 17:208–213. https://doi.org/10.3800/pbr.17.208
Zabala S, Bigatti G, Botto F, Iribarne OO, Galván DE (2013) Trophic relationships between a Patagonian gastropod and its epibiotic anemone revealed by using stable isotopes and direct observations. Mar Biol 160:909–919. https://doi.org/10.1007/s00227-012-2143-y
Zabala MS (2012) Ecología trófica, crecimiento y reproducción en el gasterópodo Adelomelon ancilla en el Golfo Nuevo. PhD Dissertation, Universidad de Buenos Aires
Zamponi MO, Excoffon AC (1995) La anemonofauna de Bahía Concepción (Chile) I. Algunos aportes a la distribución y biología de los géneros Phlyctenactis Stuckey, 1909 (Actiniaria: Actiniidae) y Antholoba Hertwig, 1882 (Actiniaria: Actinostolidae). Physis 50A:1–6
Acknowledgements
We extend our gratitude to Ana Clara Santana and Dr. Sergio Pereira for their assistance in the histological protocol for the study samples. We also appreciate the valuable contributions of Dr. André Morandini for donating the Antholoba achates collections and Dr. Maximiliano M. Maronna for his enriching contributions to genetic analysis. Our thanks extend to Professor Dr. Rogério Caetano da Costa and his groups of students at LABCAM (Laboratory of Biology of Marine and Freshwater Shrimps) at the São Paulo State University, for their involvement in the sampling conducted in Ubatuba Bay. Funding support for this research was provided through resources from the Center for Scientific Computing (NCC/GridUNESP) at the Universidade Estadual Paulista (UNESP). Comments from Karen Sanamyan, Estefanía Rodríguez, and an anonymous reviewer improved this manuscript.
Funding
This study was supported by São Paulo Research Foundation (FAPESP), grant numbers 2020/16589–7 and 2022/09430-7 to JDF. SNS was supported by São Paulo Research Foundation (FAPESP), grant numbers 2019/03552-0 and 2022/16193-1, and CNPq (Research Productivity Scholarship) grant number 307340/2019-8 and 304267/2022-8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed by the authors.
Sampling and field studies
All necessary permits for sampling and observational field studies have been obtained by the authors from the competent authorities and are mentioned in the acknowledgements, if applicable.
Data availability
The data generated and analyzed during this study are included in the Electronic Supplementary Material files.
Author contribution
JDF, RGM, and MD conceptualized, analyzed data. JDF wrote the manuscript. JDF and SNS conceived and designed research. RGM, MD, and SNS edited and reviewed the manuscript. SNS procured funds and managed the project. All authors read and approved the manuscript.
Additional information
Communicated by B. W. Hoeksema
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is registered in Zoobank under https://zoobank.org/References/82f829b3-698f-4855-bfbc-db38e7eb9722.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Durán-Fuentes, J.A., González-Muñoz, R., Daly, M. et al. Antholoba fabiani sp. nov. (Actiniaria, Metridioidea, Antholobidae fam. nov.), a new species and family of sea anemone of the southwestern Atlantic, Brazil. Mar. Biodivers. 54, 40 (2024). https://doi.org/10.1007/s12526-024-01433-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12526-024-01433-9