Abstract
The diversity and seasonal variations of two assemblages of marine benthic peracarids were studied between a natural rocky shore and an artificial harbour area over a 12-month period. Samples were obtained monthly in La Estafeta, a rocky intertidal zone with low human impact, and Mar del Plata Harbour, a polluted environment, between March 2011 and March 2012. The two sites differed markedly in the composition and abundance of species across all seasons: the tanaid Tanais dulongii was most abundant in La Estafeta rocky shore, followed by the amphipods Monocorophium acherusicum, Hyale grandicornis, Ampithoe valida, the isopod Idotea balthica, the tanaid Leptochelia sp. and the isopod Sphaeroma serratum. In contrast, M. acherusicum was most abundant in the harbour area, followed by T. dulongii, S. serratum, Ericthonius punctatus, I. balthica, Caprella equilibra and C. dilatata. Total density of peracarids varied between months in La Estafeta rocky shore and Mar del Plata Harbour. In La Estafeta rocky shore mean density increased from March to May 2011 (autumn in the southern hemisphere; ca. 45,000 ind/m2), decreased sharply until August and then increased in January 2012. In Mar del Plata Harbour the mean density was lower from March to October (ca. 500,000 ind/m3), then increased and reached a maximum in January 2012 (more than 1,500,000 ind/m3), and decreased until the following March. This study suggests that the differences in peracarid assemblages, diversity and seasonality could be related to an effect of temperature, but we should not rule out a synergistic effect of other factors, such as pollution, food availability and hydrodynamic factors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The distribution, diversity and density of marine benthic species can vary between habitats due to different environmental conditions, such as temperature, salinity, pH, predation pressure, food or refuge availability and their seasonal changes (Bertness 1999; Wahl 2009; Thiel and Watling 2015). These variations are a consequence of the effects of such factors on the fitness or subsistence of species, which, in turn, alter their reproductive strategies, growth and behaviors (Stearns 1992, 2000).
Harbours are some of the most stressful environments for marine organisms worldwide, characterized by low pH, oxygen concentration and turbulence, and high levels of organic and inorganic pollutants (Darbra et al. 2009). These factors have a strong impact on biodiversity, affecting the life history traits of benthic species and therefore their distribution and abundance (Ward and Hutchings 1996; Wahl 2009). Studies of the associated biota have become very important to monitoring the health status of these environments (e.g. Pearson and Rosenberg 1978; Chintiroglou et al. 2004; Guerra-García and García–Gómez 2004; Martínez–Lladó et al. 2007; Lourido et al. 2008; Sánchez-Moyano and García-Asencio 2010; Esquete et al. 2011; Albano et al. 2013). The studies performed in these areas may be compared with areas with lower anthropogenic impacts that serve as controls in determining the effects of stressful conditions on organisms (Vallarino et al. 2002; Kalkan et al. 2007).
Peracarids are small benthic crustaceans that inhabit marine (e.g. deep sea, tidal flats and estuaries), freshwater and terrestrial environments (Schram 1986; Johnson et al. 2001; Martin and Davis 2006; Thiel and Hinojosa 2009). These organisms are one of the most diverse and dominant groups in marine environments, playing an important role as a food source for many organisms and structuring benthic assemblages (Duffy and Hay 2000; Esquete et al. 2011; Izquierdo and Guerra-García 2011). In addition, peracarids are good candidates for evaluating the effects of environmental conditions on biodiversity and life history of marine benthic macrofauna, due to their reproductive mode associated with life cycles without pelagic larvae, and thus low dispersal rates (Schram 1986; Johnson et al. 2001; Martin and Davis 2006; Thiel and Hinojosa 2009).
There are numerous reports of peracarid assemblages in Southwestern Atlantic harbours and pristine environments (e.g. Brankevich et al. 1988; Scelzo et al. 1996; Excoffon et al. 1999; Adami et al. 2004; López-Gappa et al. 2006; Albano et al. 2006, 2013; López-Gappa and Sueiro 2007; Sueiro et al. 2011; Chiesa and Alonso 2014; Schwindt et al. 2014; Carcedo et al. 2015; among others). However, these studies offer only snapshots of single sites and moments; analyses to compare populations encompassing longer periods of time are necessary in order to assess the effect of contrasting environmental conditions between habitats experiencing low and high human impacts. The main objective of this paper was to compare the assemblage of peracarid species between a natural rocky shore and an artificial harbour area. On the basis of the higher levels of pollutants, lower pH and oxygen concentration, and the less influence of hydrodynamic factors that characterize Mar del Plata harbor, we predict that peracarid assemblages in harbour environments will show lower diversity levels, but higher dominance of certain species less sensitive to environmental stressors compared to La Estafeta, as has been reported for several similar environments subjected to human impact (Pearson and Rosenberg 1978; Chintiroglou et al. 2004; Guerra-García and García–Gómez 2004; Martínez–Lladó et al. 2007; Lourido et al. 2008; Sánchez-Moyano and García-Asencio 2010).
Material and methods
Study area
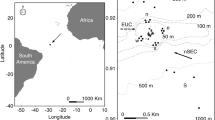
The study was conducted in Mar del Plata Harbour (38° 02′ S, 57° 32′ W) and in the intertidal zone of La Estafeta rocky shore (38°10′ S, 57°38′ W; Fig. 1). Mar del Plata Harbour, built from 1913 to 1924, is a semiclosed area limited by two artificial breakwaters with an opening of approximately 300 m. Mean water depth is around 5 m, ranging between 3 and 10 m, and the bottom is composed of fine and very fine sands near the opening, and silt in the inner parts (Isla and Lasta 2006; Schwindt et al. 2010). The presence of several industries, sewage effluents and intense fishing activity, have favored the formation of a polluted area characterized by high levels of organic matter, hydrocarbons, copper and tributyltin (Penchaszadeh et al. 2001; Goldberg et al. 2004; Rivero et al. 2005; Albano et al. 2013; Laitano et al. 2015). Despite these conditions, artificial structures (e.g. wooden docks and marinas) allowed the development of an extremely diverse biota with ascidians, algae and polychaete tubes that provide refuge for fishes, molluscs, nematodes and crustaceans (Rivero 2005; Albano et al. 2006, 2013; Albano and Obenat 2009). In contrast, La Estafeta rocky shore is an abrasion platform located 15 km southward of Mar del Plata Harbour with a gentle slope (<1%) and numerous tidal pools (depth <0.3 m). This intertidal habitat is 70 m wide when tides recede and the substratum is composed of consolidated sediment (loess) and covered by algae (mainly Ulva rigida and Corallina officinalis), which serve as sites for feeding, breeding and shelter for a variety of benthic organisms, such as echinoderms, annelids, molluscs, nematodes and crustaceans (Baeza et al. 2010; Rumbold et al. 2012). There are no sewage pipes or drains and the site, since it is surrounded by cliffs (height = circa 40 m), is less accessible compared to Mar del Plata Harbour. Consequently, it has a lower degree of human impact (Rumbold et al. 2012, 2015). Both sites are subjected to a microtidal regime with mean amplitude of 0.8 m (Isla 2004).
According to Rumbold et al. (2015), Mar del Plata Harbour showed more anoxic and acidic conditions during the sampling period than La Estafeta rocky shore, indicating clearly that the harbour is a stressful environment. In Mar del Plata Harbour mean values of pH and dissolved oxygen were 8.23 and 8.71 mg/l, respectively, whereas in La Estafeta rocky shore they were 8.55 and 12.69 mg/l. Salinity measurements did not show differences between sites and months sampled (values close to 33 PSU). In the harbour area, though salinity can reach unusual values of 22 PSU, possibly related to heavy rainfall, the presence of a storm water duct and a creek that transports pluvial water from the urban area to the harbour (Schwindt et al. 2010). Moreover, the two environments did not show differences in seawater temperature and presented the same seasonal variation (Rumbold et al. 2015), characterized by lower values in winter and higher values in summer, varying between 9.3 - 23.8 °C. Mean values of the two sites are represented in Fig. 3.
Field sampling and laboratory procedures
Five samples were collected per site per month from March 2011 to March 2012, except in June 2011 in La Estafeta rocky shore and in February 2011 at both sites due to bad weather conditions. The structure of the two habitats is extremely different, so it was not possible to use the same sampling protocol in both sites. In La Estafeta rocky shore samples were taken along a 60 m transect parallel to the coast line (15 m interval) at low intertidal level (ca. 70 m from cliffs). Samples were extracted from the seafloor at very low tide and consisted of algal patches of 0.0225 m2 (using a quadrat of 0.15 × 0.15 m). In Mar del Plata Harbour subtidal samples were taken by hand (depth < 1 m) and consisted of 1000 cm3 of extracted material from the fouling community adhered to the docks (ascidians, algae and polychaetes). Samples were placed in plastic containers and fixed in situ in 98% ethanol. In the laboratory, samples were sieved through a 0.35 mm mesh. All peracarid specimens were sorted, counted and identified to the lowest taxonomic level possible using a stereomicroscope and taxonomic guides (Alonso 1984, 2004; Bastida 2004; Chapman 2007; LeCroy 2007; Perez–Schultheiss 2009). Additionally, organisms were grouped according to functional group: opportunistic, predator/scavenger, suspension-feeder and grazer (Chintiroglou et al. 2004; Valdivia and Thiel 2006; Guerra-García and Tierno de Figueroa 2009; Prato et al. 2012; Rechimont et al. 2013). Density values obtained in La Estafeta rocky shore (individuals/0.0225 m2) and Mar del Plata Harbour (individuals/1000 cm3) were extrapolated as individuals/m2 and individuals/m3, respectively. As densities were expressed in different units, the results obtained were used only to determine seasonal variations and differences in composition of peracarids assemblages between environments.
Data analysis
ANOVAs and post-hoc tests were performed using R statistical software (R Development Core Team 2011), while nMDS, SIMPER analysis and diversity indexes were calculated using the PRIMER 6.1 package (Clarke and Warwick 1994; Clarke and Gorley 2006). Parametric tests were preferably used, but when the assumptions of parametric statistics were seriously violated, an appropriate nonparametric test was applied. Significance was assessed at α = 0.05 (Underwood 1997). To determine if the total density of peracarids varied between months, a one-way ANOVA was used (Underwood 1997). To compare seasonal variations of total peracarids between environments relative abundances (monthly number of individuals of a percentage of total number of individuals) were calculated per month. Nonmetric Multidimensional scaling (nMDS) ordinations were used to show the monthly differences of peracarid assemblages among habitats on square-root transformed total abundance data, to reduce the influence of very abundant species, with a Bray-Curtis similarity matrix (Clarke and Warwick 1994). The similarity percentage analysis (SIMPER) was used to determine the species responsible for the differences in peracarid assemblages between study sites (Clarke and Warwick 1994). Populations that contributed more than 95% of cumulative dissimilarity between habitats were statistically analyzed using one-way ANOVA. To determine differences in diversity indices between study sites: species richness (S), Shannon-Wiener diversity index (H’ log2; Shannon and Wiener 1963) and Pielou’s evenness (J’; Pielou 1966) were calculated monthly from area samples and tested between study sites through a two-way ANOVA (factors: month and study sites; Underwood 1997). Student-Newman-Keuls (SNK) was used for multiple comparisons of means among all months sampled and study sites.
Results
Composition of peracarid assemblages
A total of 53,912 peracarid individuals were counted and identified as ten species belonging to three taxa (six Amphipoda, two Isopoda, two Tanaidacea; Table 1). From these species seven were collected in La Estafeta rocky shore and seven in Mar del Plata Harbour, but only four of them were registered at both sites. In La Estafeta rocky shore 27,747 individuals were collected during the study period, with the tanaid Tanais dulongii representing the most abundant species (66.19% of total abundance), followed by the amphipods Monocorophium acherusicum (27.20%), Hyale grandicornis (4.21%), Ampithoe valida (0.98%), the isopod Idotea balthica (0.69%), the tanaid Leptochelia sp. (0.64%), and the isopod Sphaeroma serratum (0.09%). In Mar del Plata Harbour a total of 26,165 individuals were sorted from the samples, and the most abundant species was M. acherusicum (78.74%), followed by T. dulongii (12.59%). The remaining species had lower abundances: S. serratum (5.23%), Ericthonius punctatus (3.22%), I. balthica (0.18%), Caprella equilibra (0.03%) and Caprella dilatata (0.01%). The SIMPER analysis determined that M. acherusicum, T. dulongii, S. serratum, H. grandicornis, E. punctatus, A. valida and Leptochelia sp. were the species contributing most to dissimilarities between the two environments (total cumulative dissimilarity: 95.74%; Tables 2 and 3).
Abundance of peracarid assemblages
Total density of peracarids varied significantly between months in La Estafeta rocky shore and Mar del Plata Harbour (in both cases one-way ANOVA, P < 0.001; Fig. 2; Tables 4 and 5). In La Estafeta rocky shore mean density increased from January to May (mid-autumn), reaching a maximum of ca. 45,000 ind/m2 (SNK-test, P < 0.05), decreased sharply until August and then steadily decreased until January (ca. 1,500-20,000 ind/m2; SNK-test, P > 0.05). In Mar del Plata Harbour the mean density was lower than 500,000 ind/m3 from March to October (SNK-test, P > 0.05), then increased and reached a maximum in January (mid–summer) of more than 1,500,000 ind/m3 and decreased until the following March (SNK-test, P < 0.05).
The comparison of relative abundances of total peracarids between environments showed a marked difference in the seasonal variation, characterized by highest percentages in autumn and early winter in La Estafeta rocky shore and only in summer in Mar del Plata Harbour (Fig. 3). In addition, nMDS ordination plot of total abundances of peracarid assemblages established that samples of La Estafeta rocky shore and Mar del Plata Harbour showed a clear difference between sites (Fig. 4) and between months in each site. In La Estafeta rocky shore, Ampithoe valida was absent in November, Sphaeroma serratum from April to December and Idotea balthica during August and November. In Mar del Plata Harbour, Caprella equilibra and C. dilatata were absent from March to January, I. balthica from March to May and from September to October, and E. punctatus from August to November.
The analysis of functional groups showed that suspension-feeders were dominant in both environments, with a relative abundance of ca. 95% (Fig. 5), followed by grazers in La Estafeta rocky shore and opportunistic species in Mar del Plata Harbour (5-6%). The other functional groups showed a lower percentage (<1%) (Fig. 6).
Variation in densities of most abundant species
The annual variation of densities of the seven species that contributed to 95.74% of total cumulative dissimilarity in peracarid assemblages is shown in Fig. 7. Except for Ampithoe valida in La Estafeta rocky shore, all the species showed significant differences between months sampled (one-way ANOVA, P < 0.05; Tables 4 and 5). Overall individual numbers of Tanais dulongii remained below 15,000 ind/m2 (SNK-test, P > 0.05; Fig. 7a), but from May to July 2011 this population reached its maximum density in La Estafeta rocky shore (ca. 20,000-40,000 ind/m2; SNK-test, P < 0.05). In contrast, the population of Mar del Plata Harbour remained homogeneous during the study period (ca. 1,000-100,000 ind/m3; SNK-test, P > 0.05), except for March 2012 where it exceeded 400,000 ind/m3.
Monthly variation of total mean density (mean ± standard error) of peracarid species that most contributed to dissimilarities (>95% of cumulative dissimilarity) between La Estafeta rocky shore (LE) and Mar del Plata Harbour (MdP): Tanais dulongii (a), Monocorophium acherusicum (b), Hyale grandicornis (c), Sphaeroma serratum (d), Amphitoe valida (e), Ericthonius punctatus (f) and Leptochelia sp. (g)
Monocorophium acherusicum showed their highest densities only in March 2012 in La Estafeta rocky shore (ca. 20,000 ind/m2; SNK-test, P < 0.05; Fig. 7b) and remained below 12,000 ind/m2 during the rest of the months sampled (SNK-test, P > 0.05). In Mar del Plata Harbour the mean density was lower than 500,000 ind/m3 (SNK-test, P > 0.05), but in December 2011 and January 2012 this population reached its maximum values (ca. 1,600,000 ind/m3; SNK-test, P < 0.05).
In La Estafeta rocky shore, Sphaeroma serratum varied between 100-150 ind/m2 during March 2011 and January 2012 (SNK-test, P < 0.05; Fig. 7d), and was absent the rest of study period. In Mar del Plata Harbour, this species reached its maximum values in March and April 2011 (ca. 120,000 ind/m3; SNK-test, P < 0.05), while during the remaining months values were between 400-30,000 ind/m3 (SNK-test, P > 0.05).
Hyale grandicornis, Leptochelia sp. and A. valida were collected from La Estafeta rocky shore. H. grandicornis reached its maximum value in March 2011 (ca. 4,000 ind/m2; SNK-test, P < 0.05; Fig. 7c) and during the remaining months numbers were below 1,500 ind/m2 (SNK-test, P > 0.05), while Leptochelia sp. showed its highest values in May 2011 (ca. 15 ind/m2; SNK-test, P < 0.05; Fig. 7g) and remained below 5 ind/m2 the rest of the study period (SNK-test, P > 0.05). In contrast, the mean density of A. valida was less variable (ca. 500 ind/m2; one-way ANOVA, P = 0.514; Fig. 7e), reaching its maximum in May 2011 (ca. 1000 ind/m2).
Ericthonius puncatus showed a mean density lower than 3,500 ind/m3 in Mar del Plata Harbour (SNK-test, P > 0.05; Fig. 7f), reaching its highest values in April 2011 (ca. 220,000 ind/m3; SNK-test, P < 0.05).
Diversity
S, H′ and J′ indices showed significant differences between study sites (two-way ANOVA, P < 0.001; Table 3). On one hand, the pattern of annual variation of S was always higher in La Estafeta rocky shore than in Mar del Plata Harbour. It reached its lowest values in spring (except for November in La Estafeta rocky shore) and the highest in summer (SNK-test, P < 0.05; Fig. 6a). On the other hand, the pattern of annual variation of H′ and J′ differed markedly between sites. It was higher in La Estafeta rocky shore than in Mar del Plata Harbour in autumn and the beginning of winter, while the trend was reversed in the rest of sampling months, with a marked decrease of both indices during spring (SNK-test, P < 0.05; Fig. 6b and c).
Discussion
In our study, the superorder Peracarida was represented by the orders Amphipoda, Isopoda and Tanaidacea in both environments, as reported from other intertidal and subtidal hard-bottom communities studied in Argentina (Scelzo et al. 1996; Adami et al. 2004; Cuevas et al. 2006; Sueiro et al. 2011; Genzano et al. 2011; Albano 2012; Mendez et al. 2015). However, we found differences in numerical dominance between the two sites studied: amphipods were most abundant in the harbour, followed by tanaidaceans, while it was the opposite in La Estafeta rocky shore, suggesting a differential response of these orders to environmental conditions, which is concordant with patterns observed in other harbour areas around the world (Chintiroglou et al. 2004; Guerra-García and García–Gómez 2004; Martínez–Lladó et al. 2007; Lourido et al. 2008; Sánchez-Moyano et al. 2010).
Suspension-feeding was the dominant trophic habit in both environments. Several authors proposed that hydrodynamic factors are most relevant to the structure of intertidal and subtidal marine communities and the establishment of species with a particular trophic habit (Sepúlveda et al. 2003; Guerra-García et al. 2009; Izquierdo and Guerra-García 2011; Bueno et al. 2016). For example, in environments exposed to strong wave action (e.g. intertidal zones) organic matter is continuously suspended, favoring the presence of suspension-feeders, while in habitats subjected to weaker currents (e.g. harbours), more organic matter is deposited and the presence of grazers and opportunistic species increases (McQuaid and Branch 1984; Sepúlveda et al. 2003; Izquierdo and Guerra-García 2011; Bueno et al. 2016). However, in the present study this pattern was not observed and so the presence of suspension-feeders in Mar del Plata Harbour is likely related to the continuous supply of organic material from industrial and sewage effluents (Bastida et al. 1971; Rivero et al. 2005; Albano et al. 2013).
Species richness was lower in Mar del Plata Harbour than in La Estafeta rocky shore. According to Bueno et al. (2016) environments exposed to hydrodynamic factors are subjected to water renewal, which would improve the oxygen levels of the environment, favoring species settlement and increasing richness values. Environmental conditions of Mar del Plata Harbour would favor a decrease in species richness related to the presence of toxic chemicals, higher eutrophication levels, and the low salinity and oxygen values registered in this habitat (Penchaszadeh et al. 2001; Goldberg et al. 2004; Rivero et al. 2005; Albano et al. 2013; Laitano et al. 2015), as has been suggested by reports from several harbours around the world (Chintiroglou et al. 2004; Martínez–Lladó et al. 2007; Darbra et al. 2009; Chen et al. 2010).
Diversity and evenness indices showed a seasonal variation that differed slightly between sites: maximal values during winter and minimal ones in summer in Mar del Plata Harbour, while in La Estafeta rocky shore both indices decreased during autumn and winter. Higher temperatures play an important role in the annual pattern of peracarid species, increasing their reproductive activity (i.e. promoting juvenile growth and sexual maturity) and consequently increasing their density (Pöckl 1992; McKenney and Celestial 1995; Maranhão and Marques 2003; Fockedey et al. 2005; Tsoi et al. 2005; Henninger et al. 2010; Hosono 2011). In the current study, the high densities of Monocorophium acherusicum in Mar del Plata Harbour registered during spring and summer, coinciding with their highest reproductive and recruitment period, as has been previously reported by Rumbold et al. (2016), could explain the dominance and a marked decrease of diversity and evenness. By way of contrast, in La Estafeta rocky shore, lower values of diversity and evenness indices during autumn-winter could be explained by the fact that in intertidal environments some species reach their highest densities during the colder seasons (e.g. M. acherusicum, Tanais dulongii and Ampithoe valida) which could be related to more favorable conditions for reproduction and recruitment. In other species, though, lower temperatures increase mortality rates, resulting in a reduction of density (Kneib 1984; Bertness 1999; Rumbold et al. 2012). Thus, temperature could affect peracarid species in different ways, producing changes in their reproductive traits and densities, which would explain the seasonal differences between density peaks, diversity and evenness indices between environments. On the other hand, several studies have shown that stressful conditions of environments subjected to high human impact can affect the development, reproduction, lifespan and mortality rates of peracarid species, altering their seasonal variation (Sánchez-Moyano et al. 2000; Chintiroglou et al. 2004; Guerra-García and García–Gómez 2004; Martínez–Lladó et al. 2007). The effects of specific pollutants and ecological factors, such as predation pressure, shelter sites or food availability would require more field and laboratory surveys (Stearns 2000).
Multivariate analysis of peracarid species revealed that species composition differed markedly between the two environments as well as between months (Fig. 4). The amphipods Hyale grandicornis, A. valida and the tanaid Leptochelia sp. were absent in Mar del Plata Harbour, while the amphipods Caprella dilatata, Caprella equilibra and Ericthonius punctatus were absent in La Estafeta rocky shore. All the species recorded in Mar del Plata Harbour had previously been reported from environments contaminated with organic or/and inorganic matter, and some of them are considered as bioindicators of contamination (Reizopouloua and Nicolaidou 2004; Lee and Lee 2005; Kalkan et al. 2007; Guerra-García et al. 2010; Sánchez-Moyano and García-Asencio 2010; El-Din et al. 2014). The lack of Leptochelia sp. (reported as Leptognathia sp. by Albano and Obenat 2009) and H. grandicornis in the harbour samples is not indicative that both species are sensitive to contaminants, because they had been detected in other areas of Mar del Plata Harbour before (Alonso 2004; Albano and Obenat 2009). On the contrary, laboratory bioassays with A. valida have determined that this species is sensitive to hydrocarbons, which could explain the absence of specimens in the harbour (Lee et al. 1981). The absence of caprellids in La Estafeta rocky shore should be viewed with care and may represent a particular spatial or temporal circumstance, since Albano (2012) have reported the presence of C. equilibra at this site and C. dilatata was detected at other marine intertidal sites located to the North and South of La Estafeta (López-Gappa et al. 2006). On the other hand, the lack of E. punctatus in La Estafeta rocky shore may be related to suboptimal conditions for its settlement, such as the presence of stronger competitors, greater numbers of predators, inadequate shelter and high exposure to wave action, among others (Galil et al. 2011). Although its population dynamics have already been studied by Rumbold et al. (2016) in Mar del Plata Harbour, more monitoring studies are needed in the coming years to determine its invasive potential. Finally, some species showed a discontinuity in their monthly density in La Estafeta rocky shore (A. valida, S. serratum and I. balthica) and Mar del Plata Harbour (C. dilatata, C. equilibra, I balthica and E. punctatus). This variation could possibly be related to higher mortality rates (e.g. lower temperatures, predation pressure and pollutant concentration) or migration to other areas (Rumbold et al. 2016), but the explanation of the proximal causes of the observed differences would require more studies and detailed laboratory and field experiments.
The present study suggests that the differences in peracarid assemblages and the seasonal variations between environments, characterized by highest densities in autumn and early winter in La Estafeta rocky shore and only in summer in Mar del Plata Harbour, could be related to a differential effect of temperature on reproductive traits of these organisms. However, we should not rule out a synergistic effect of pollutants, food availability and hydrodynamic factors. Further studies are necessary to identify the factors in detail, and to demonstrate experimentally their effect and their impact on the life history traits of these species.
References
Adami ML, Tablado A, López-Gappa JL (2004) Spatial and temporal variability in intertidal assemblages dominated by the mussel Brachidontes rodriguezii (d’Orbigny, 1846). Hydrobiologia 520:49–59
Albano MJ (2012) Patrones de distribución y abundancia de invertebrados bentónicos exóticos en áreas naturales y portuarias de la provincia de Buenos Aires. Dissertation, Universidad Nacional de Mar del Plata
Albano MJ, Obenat SM (2009) Assemblage of benthic macrofauna in the aggregates of the tubiculous worm Phyllochaetopterus socialis in the Mar del Plata harbour, Argentina. J Mar Biol Assoc UK 89:1099–1108
Albano MJ, Seco Pon J, Obenat S (2006) Macrozoobentos asociado a los agregados de Phyllochaetopterus socialis Claparède, 1870 en el puerto de Mar del Plata, Argentina. Rev Invest Mar 34:197–203
Albano MJ, Lana P, Bremec C, Elías R, Martins C, Venturini N, Muniz P, Rivero S, Vallarino EA, Obenat S (2013) Macrobenthos and multi–molecular markers as indicators of environmental contamination in a South American port (Mar del Plata, Southwest Atlantic). Mar Pollut Bull 73:102–114
Alonso GM (1984) Anfipodos Gammarideos litorales del Mar Austral Argentino (Crustacea Amphipoda Gammaridea). Dissertation, Universidad de Buenos Aires
Alonso GM (2004) Crustáceos anfípodos. In: Boschi EE, Cousseau MB (eds) La vida entre mareas: vegetales y animales de las costas de Mar del Plata, Argentina. Publicaciones Especiales INIDEP, Mar del Plata, pp 169–178
Baeza JA, Farías NE, Luppi TA, Spivak ED (2010) Refuge size, group living and symbiosis: testing the “resource economic monopolization” hypothesis with the shrimp Betaeus lilianae and description of its partnership with the crab Platyxanthus crenulatus. J Exp Mar Biol Ecol 389:85–92
Bastida RO (2004) Crustáceos isópodos. In: Boschi EE, Cousseau MB (eds) La vida entre mareas: vegetales y animales de las costas de Mar del Plata, Argentina. Publicaciones Especiales INIDEP, Mar del Plata, pp 187–204
Bastida RO, Capezzani DAA, Torti MR (1971) Los organismos incrustantes del Puerto de Mar del Plata. I. Siphonaria lessoni (Blainville, 1824). Aspectos ecológicos y biométricos. LEMIT Serie II 149:200–233
Bertness MD (1999) The ecology of Atlantic shorelines. Sinauer Associates, Sunderland
Brankevich G, Bastida R, Lemmi C (1988) A comparative study of biofouling settlements in different sections of Necochea power plant (Quequén Port, Argentina). Biofouling 1:113–135
Bueno M, Dena-Silva SA, Flores AAV, Leite FPP (2016) Effects of wave exposure on the abundance and composition of amphipod and tanaidacean assemblages inhabiting intertidal coralline algae. Mar Biol Assoc UK 96:761–767
Carcedo C, Fiori S, Bremec C (2015) Macrobenthic surf zone communities of temperate sandy beaches: Spatial and temporal patterns. Mar Ecol 36:326–336
Chapman JW (2007) Amphipoda. In: Carlton JT (ed) The Light and Smith Manual: Intertidal Invertebrates from Central California to Oregon. The University of California Press, Richmond, pp 545–630
Chen K, Tian S, Jiao JJ (2010) Macrobenthic community in Tolo Harbour, Hong Kong and its relations with heavy metals. Estuar Coast 33:600–608
Chiesa IL, Alonso GM (2014) Amphipoda. In: Calcagno JA (ed) Los Invertebrados Marinos. Fundación de Historia Natural Félix de Azara. Editorial Vazquez Mazzini, Buenos Aires, pp 265–276
Chintiroglou CC, Antoniadou C, Baxevanis A, Damianidis P, Karalis P, Vafidis D (2004) Peracarida populations of hard substrate assemblages in ports of the NW Aegean Sea (eastern Mediterranean). Helgoland Mar Res 58:54–61
Clarke K, Gorley R (2006) PRIMER v6. User Manual/Tutorial. PRIMER-E, Plymouth
Clarke K, Warwick R (1994) Change in Marine Communities: An Approach to Statistical Analysis and Interpretation. PRIMER–E, Plymouth
Cuevas JM, Martin JP, Bastida R (2006) Benthic community changes in a patagonian intertidal: a forty years later comparison. Thalassas 22:29–37
Darbra RM, Pittam N, Royston KA, Darbra JP, Journee H (2009) Survey on environmental monitoring requirements of European ports. J Environ Manage 90:1396–1403
R Development Core Team (2011) R: A Language and environment for statistical computing. R foundation for statistical computing. Version R 2.13.0. Vienna: R Development Core Team. Computer program
Duffy JE, Hay ME (2000) Strong impacts of grazing amphipods on the organization of a benthic community. Ecol Monogr 70:237–263
El-Din MIS, Sakiko Y, Mohamed SZ, Bedir MA, Bahgat IM, Nishimura O (2014) Investigating the use of Sphaeroma serratum (Crustacea, Isopoda) as bio–indicator for heavy metals pollution in Lake Timsah, Suez Canal using alkaline comet assay technique. Egypt Acad J Biol Sci 6:7–26
Esquete P, Moreira J, Troncoso JS (2011) Peracarid assemblages of Zostera meadows in an estuarine ecosystem (O Grove inlet, NW Iberian Peninsula): spatial distribution and seasonal variation. Helgoland Mar Res 65:445–455
Excoffon AC, Genzano GN, Zamponi MO (1999) Macrobentos asociado con una población de Antothoe chilensis (Lesson. 1830) (Cnidaria, Actiniaria) en el puerto de Mar del Plata, Argentina. Cienc Mar 25:177–191
Fockedey N, Meesa J, Vangheluwe M, Verslycked T, Janssen CR, Vincxa M (2005) Temperature and salinity effects on postmarsupial growth of Neomysis integer (Crustacea: Mysidacea). J Exp Mar Biol Ecol 326:27–47
Galil BS, Clark PF, Carlton JT (2011) In the wrong place - alien marine crustaceans: distribution, biology and impacts. Invading Nature-Springer Series in Invasion Ecology 6. Springer, Dordrecht
Genzano G, Giberto D, Bremec C (2011) Benthic survey of natural and artificial reefs off Mar del Plata, Argentina. Lat Am J Aquat Res 39:553–566
Goldberg RN, Averbuj A, Cledón M, Luzzatto D, Sbarbati–Nudelman N (2004) Search for triorganotins along the Mar del Plata (Argentina) marine coast: finding of tributyltin in egg capsules of a snail Adelomelon brasiliana (Lamarck, 1822) population showing imposex effects. Appl Organomet Chem 18:117–123
Guerra-García JM, García–Gómez JC (2004) Crustacean assemblages and sediment pollution in an exceptional case study: a harbour with two opposing entrances. Crustaceana 77:353–370
Guerra-García JM, Tierno de Figueroa JM (2009) What do caprellids (Crustacea: Amphipoda) feed on? Mar Biol 156:1881–1890
Guerra-García JM, Ros M, Sánchez JA (2009) Isopods, tanaids and cumaceans (Crustacea, Peracarida) associated to the seaweed Stypocaulon scoparium in the Iberian Peninsula. Zool Baetica 20:35–48
Guerra-García JM, Cabezas MP, Baeza–Rojano E, García–Gómez JC (2010) Na, K, Ca and Mg of intertidal caprellids (Crustacea: Amphipoda). Mar Biol Res 6:321–326
Henninger TO, Froneman PW, Booth AJ, Hodgson AN (2010) Growth and longevity of Exosphaeroma hylocoetes (Isopoda) under varying conditions of salinity and temperature. J Afr Zool 45:41–51
Hosono T (2011) Effect of temperature on growth and maturation pattern of Caprella mutica (Crustacea, Amphipoda): does the temperatura size rule function in caprellids? Mar Biol 158:363–370
Isla FI (2004) Geología del sudeste de Buenos Aires. In: Boschi EE, Cousseau MB (eds) La vida entre mareas: vegetales y animales de las costas de Mar del Plata, Argentina. Publicaciones Especiales INIDEP, Mar del Plata, pp 19–28
Isla IF, Lasta CA (2006) Manual de manejo costero para la Provincia de Buenos Aires. Eudem, Mar del Plata
Izquierdo D, Guerra-García JM (2011) Distribution patterns of the peracarid crustaceans associated with the alga Corallina elongata along the intertidal rocky shores of the Iberian Peninsula. Helgoland Mar Res 65:233–243
Johnson WS, Stevens M, Watling L (2001) Reproduction and development of Marine Peracaridans. Adv Mar Biol 39:105–260
Kalkan E, Karhan SÜ, Mutlu E, Simboura N, Bekbölet M (2007) Application of the bentix index in assessing ecological quality of hard substrata: a case study from the Bosphorus Strait, Turkey. Mediterr Mar Sci 8–1:15–29
Kneib RT (1984) Patterns of invertebrate distribution and abundance in the intertidal salt marsh: causes and questions. Estuaries 7:392–412
Laitano MV, Castro IB, Costa PG, Fillmann G, Cledón M (2015) Butyltin and PAH contamination of Mar del Plata port (Argentina) sediments and their influence on adjacent coastal regions. Bull Environ Contam Toxicol 95:513–520
LeCroy S (2007) An illustrated identification guide to the nearshore marine and estuarine gammaridean Amphipoda of Florida. Families Anamixidae, Eusiridae, Hyalellidae, Hyalidae, Iphimedidae, Ischyroceridae, Lysianassidae, Megaluropidae and Melphidippidae. United States Environmental Protection Agency. http://publicfiles.dep.state.fl.us/dear/labs/biology/biokeys/amphipoda_fl_v4.pdf. Accessed 29 November 2015
Lee JS, Lee KT (2005) Delayed mortality of benthic amphipods Monocorophium acherusicum exposed to various pollutants in seawater (Cd, Cu, Hg, TBT, ammonia and phenanthrene). J Environ Toxicol 20:133–141
Lee WY, Macko SA, Nicol JAC (1981) Changes in nesting behavior and lipid content of a marine amphipod (Amphithoe valida) to the toxicity of no. 2 fuel oil. Water Air Soil Poll 15:185–195
López-Gappa J, Sueiro MC (2007) The subtidal macrobenthic assemblages of Bahía San Sebastián (Tierra Del Fuego, Argentina). Polar Biol 30:679–687
López-Gappa J, Alonso GM, Landoni NA (2006) Biodiversity of benthic Amphipoda (Crustacea: Peracarida) in the Southwest Atlantic between 35°S and 56°S. Zootaxa 1342:1–66
Lourido A, Moreira J, Troncoso JS (2008) Assemblages of peracarid crustaceans in subtidal sediments from the Ría de Aldán (Galicia, NW Spain). Helgoland Mar Res 62:289–301
Maranhão P, Marques JC (2003) The influence of temperature and salinity on the duration of embryonic development, fecundity and growth of the amphipod Echinogammarus marinus Leach (Gammaridae). Acta Oecol 24:5–13
Martin JW, Davis GE (2006) Historical trends in crustacean systematics. Crustaceana 79:1347–1368
Martínez–Lladó X, Gibert O, Martí V, Díez S, Romo J, Bayona JM, De Pablo J (2007) Distribution of polycyclic aromatic hydrocarbons (PAHs) and tributyltin (TBT) in Barcelona harbour sediments and their impact on benthic communities. Environ Pollut 149:104–113
McKenney CL, Celestial DM (1995) Interactions among salinity, temperature, and growth of the estuarine mysid Mysidopsis bahia reared in the laboratory in the complete life cycle. 1. Body mass and age specific growth rate. J Crustacean Biol 15:169–178
McQuaid CD, Branch GM (1984) Influence of sea temperature, substratum and wave exposure on rocky intertidal communities – an analysis of faunal and floral biomass. Mar Ecol Prog Ser 19:145–151
Mendez MM, Schwindt E, Bortolus A, Roche A, Maggioni M, Narvarte M (2015) Ecological impacts of the austral–most population of Crassostrea gigas in South America: a matter of time? Ecol Res 30:979–987
Pearson TH, Rosenberg R (1978) Macrobenthic succession in relation to organic enrichment and pollution of the marine environment. Oceanogr Mar Biol 16:229–311
Penchaszadeh PE, Averbuj A, Cledón M (2001) Imposex in Gastropods from Argentina (south–western Atlantic). Mar Pollut Bull 42:790–791
Perez–Schultheiss J (2009) Composición y diversidad de la fauna de Amphipoda (Crustacea, Peracarida) asociada a instalaciones acuícolas de Bahía Metri, Región de Los Lagos, Chile. Informe final. Centro de Estudios en Biodiversidad, Chile (CEBCh). https://centroestudiosbiodiversidad.files.wordpress.com/2009/08/informe_amphipoda_metri1.pdf. Accessed 27 November 2015
Pielou EC (1966) The measurement of diversity in different types of biological collections. J Theor Biol 13:131–144
Pöckl M (1992) Effects of temperature, age and body size on moulting and growth in the freshwater amphipods Gammarus fossarum and G. roeseli. Freshwater Biol 27:211–225
Prato E, Danieli A, Maffia M, Biandolino F (2012) Lipid Contents and Fatty Acid Compositions of Idotea baltica and Sphaeroma serratum (Crustacea: Isopoda) as Indicators of Food Sources. Zool Stud 51:38–50
Rechimont ME, Galván DE, Sueiro MC, Casas G, Piriz ML, Diez ME, Primost M, Zabala MS, Márquez F, Brogger M, Alfaya JEF, Bigatti G (2013) Benthic diversity and assemblage structure of a north Patagonian rocky shore: a monitoring legacy of the NaGISA project. J Mar Biol Assoc UK 93:2049–2058
Reizopouloua S, Nicolaidou A (2004) Benthic diversity of coastal brackish–water lagoons in western Greece. Aquat Conserv 14:S93–S102
Rivero MS, Elías R, Vallarino EA (2005) First survey of macroinfauna in the Mar del Plata Harbour (Argentina), and the use of polychaetes as pollution indicators. Rev Biol Mar Oceanogr 40:101–108
Rumbold CE, Obenat SM, Spivak ED (2012) Life history of Tanais dulongii (Tanaidacea: Tanaidae) in an intertidal flat in the Southwestern Atlantic. J Crustacean Biol 32:891–898
Rumbold CE, Obenat SM, Spivak ED (2015) Comparison of life history traits of Tanais dulongii (Tanaidacea: Tanaididae) in natural and artificial marine environments of the south-western Atlantic. Helgoland Mar Res 69:231–242
Rumbold CE, Ruíz-Barlett T, Gavio MA, Obenat SM (2016) Population dynamics of two invasive amphipods in the Southwestern Atlantic: Monocorophium acherusicum and Ericthonius punctatus (Crustacea). Mar Biol Res 12:268–277
Sánchez-Moyano JE, García-Asencio I (2010) Crustacean assemblages in a polluted estuary from South–Western Spain. Mar Pollut Bull 60:1890–1897
Sánchez-Moyano JE, García-Adiego EM, Estacio FJ, García–Gómez JC (2000) Effect of environmental factors on the spatial distribution of the epifauna of the alga Halopteris scoparia in Algeciras Bay, Southern Spain. Aquatic Ecol 34:355–367
Scelzo MA, Elias R, Vallarino EA, Lucero N (1996) Variación estacional de la fauna acompañante del mejillin (Brachydontes rodriguezi) en Mar del Plata, provincia de Buenos Aires, Argentina. Frente Marítimo 16:149–156
Schram FR (1986) Crustacea. Oxford University Press, New York
Schwindt E, Darrigan G, Repizo H (2010) Evaluación nacional de situación en materia del agua de lastre en el litoral marino y fluvial, Argentina - Informe Final. Proyecto Globallast. http://www.ambiente.gov.ar/archivos/web/GTRA/file/Aves%20marinas/Informe%20Final%20ENSMAL%20marino%20y%20fluvial.pdf. Accessed 27 November 2015
Schwindt E, López-Gappa J, Raffo MP, Tatián M, Bortolus A, Orensanz JM, Alonso G, Diez ME, Doti B, Genzano G, Lagger C, Lovrich G, Piriz ML, Mendez MM, Savoya V, Sueiro MC (2014) Marine fouling invasions in ports of Patagonia (Argentina) with implications for legislation and monitoring programs. Mar Environ Res 99:60–68
Sepúlveda R, Cancino JM, Thiel M (2003) The peracarid epifauna associated with the ascidian Pyura chilensis (Molina, 1782) (Ascidiacea: Pyuridae). J Nat Hist 37:1555–1569
Shannon CE, Wiener W (1963) The mathematical theory of communications. University of Illinois, Urbana
Stearns SC (1992) The evolution of life histories. Oxford University Press, New York
Stearns SC (2000) Life history evolution: successes, limitations, and prospects. Naturwissenschaften 87:476–486
Sueiro MC, Bortolus A, Schwindt E (2011) Habitat complexity and community composition: relationships between different ecosystem engineers and the associated macroinvertebrate assemblages. Helgoland Mar Res 65:467–477
Thiel M, Hinojosa I (2009) Peracarida - anfípodos, isópodos, tanaidáceos y cumáceos. In: Försterra G (ed) Häussermann V. Fauna marina bentónica de la patagonia chilena, Santiago de Chile, pp 671–738
Thiel M, Watling L (2015) Lifestyles and Feeding Biology. Oxford University Press, New York
Tsoi KH, Chiu KM, Chu KH (2005) Effects of temperature and salinity on survival and growth of the amphipod Hyale crassicornis (Gammaridea, Hyalidae). J Nat Hist 39:325–336
Underwood AJ (1997) Experiments in ecology: their logical design and interpretation using analysis of variance. Cambridge University Press, Cambridge
Valdivia N, Thiel M (2006) Effects of point-source nutrient addition and mussel removal on epibiotic assemblages in Perumytilus purpuratus beds. J Sea Res 56:271–283
Vallarino EA, Rivero MS, Gravina MC, Elías R (2002) The community–level response to sewage impact in intertidal mytilid beds of the Southwestern Atlantic and the use of the Shannon index to assess pollution. Rev Biol Mar Oceanogr 37:25–33
Wahl M (2009) Marine hard bottom communities, ecological studies Vol 206. Springer–Verlag, Berlin–Heidelberg
Ward TJ, Hutchings PA (1996) Effects of trace metals on infaunal species composition in polluted intertidal and subtidal marine sediments near a lead smelter, Spencer Gulf, South Australia. Mar Ecol Prog Ser 135:123–135
Acknowledgements
This paper was funded by Grants from the Universidad Nacional de Mar del Plata (EXA 610/12, EXA705/14) and from the Consejo Nacional de Investigaciones Científicas y Técnicas de Argentina (CONICET) (PIP 112-201101-00830). Finally, we appreciate the important comments and suggestions made by Dr. Stefanie Kaiser and three anonymous referees that largely improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. S. M. Kaiser
Rights and permissions
About this article
Cite this article
Rumbold, C., Obenat, S., Velazquez, S.N. et al. Seasonal variation of peracarid assemblages in natural and artificial marine environments of the Southwestern Atlantic Ocean. Mar Biodiv 48, 1743–1754 (2018). https://doi.org/10.1007/s12526-017-0663-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12526-017-0663-x