Abstract
Long-term data from Port Valdez and short-term data from the northeastern Chukchi Sea were reviewed to gain insights into temporal drivers of macrobenthic biodiversity in Alaskan marine systems. Climate indices associated with oceanographic processes, the Pacific Decadal Oscillation in the North Pacific, and the Arctic Oscillation in the Arctic, were strongly correlated with macrobenthic density and taxon richness, indicating inadequacies of inferences concerning biodiversity from short-term studies as shifts in benthic assemblages are possible when influenced by climate-driven changes in water circulation. Species accumulation curves demonstrate that less than 50 % of biodiversity information may be captured in an area within two years, independent of sampling scale, with 90 % captured in 17 years. Additionally, assumptions of water temperature as a limiting factor for benthic organisms may not hold in Alaska as many organisms are widely dispersed across temperature regimes and, instead, some species may be limited by regional-scale biological interactions. Thus, species-specific investigations of life history and environmental tolerances together with long-term data sets are needed to predict future climate-driven responses to better conserve and manage resources. The two study areas share 33 % of species in common as a result of oceanographic linkages indicating that past geological history is as important as current oceanographic conditions for our understanding of the present. Better availability and retrospective analyses of historic data will contribute to a greater understanding of macroscale ecological patterns. The study also demonstrates the importance of considering broader studies in management decisions, like the long-term Port Valdez study, that can inform decision processes for research in Arctic seas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Understanding temporal elements of biodiversity (species composition, species richness, and distribution patterns) for marine conservation, monitoring, and management of ecosystem functioning is an emerging issue but with poor definition. The increasing magnitude of climate variations in high latitude marine systems are forcing rapid changes resulting in large ecosystem effects (Grebmeier et al. 2010; Doney et al. 2012; Grebmeier 2012). Yet researchers still have, at best, only a basic understanding of how diversity may be affected. Recent sea-ice reduction in US Arctic seas has altered diving bird and marine mammal behavioral patterns and foraging activities (Jay et al. 2012; Gall et al. 2013; Lovvorn et al. 2014; Dunton et al. 2014). Zooplankton communities covary with oceanographic trends shifting to larger copepods and associated increases in pelagic-feeding sea birds with earlier flux of warmer conditions into the northeastern (NE) Chukchi (Day et al. 2013; Gall et al. 2013; Questel et al. 2013; Weingartner et al. 2013). Distributions of some macrobenthic and megabenthic organisms have shifted in the Bering and Chukchi Seas as well, reflecting biological responses to and interactions with varying biological and environmental conditions (Orensanz et al. 2004; Grebmeier 2012). Glacial fjords are also demonstrating ecosystem changes due to increased melting of glaciers (Renaud et al. 2007; Węsławski et al. 2011). Increased glacial melting increases flow of sediment-laden melt water, faster glacier recession, and exposure of new habitat to colonization and erosion. The increased melting of glaciers amplifies the effect of fine sediment on macrofaunal communities, and ultimately, indirect and residual effects of glaciers will become more important over time.
Ecosystem-level management of marine communities requires integrated investigations to understand the factors influencing endpoints and critical ecological interactions. Mechanisms driving marine production affect producers at the functional group level and can drive distributional shifts of species through variations in physical characteristics including temperature, water circulation, and stratification (Drinkwater et al. 2010). Effects of such mechanisms extend to higher trophic levels as well. For example, the range of fishes may directly reflect water temperature variations, and water temperature may also drive distributional changes of some invertebrate megafauna (Mueter and Litzow 2008; Drinkwater et al. 2010). Investigations of the Barents, Bering, and Chukchi Seas have established spatial backgrounds for ecosystem variability (Grebmeier et al. 2006; Day et al. 2013; Hunt et al. 2013), but temporal characteristics of long-term change remain elusive. Ecosystems will respond to variations in global climatic patterns with unpredictable vectors of change as macroscale physical characteristics and oceanographic patterns are perturbed, largely because underlying processes are not fully recognized. Effects from regional-scale variations, however, may be more predictable where the oceanographic drivers and faunal responses are identified (Cloern et al. 2010). In general, however, drivers of temporal variations in observed diversity patterns (project-specific measurements of species assemblages) are not known because, in part, long-term data are lacking, restricting inferences and hindering conservation and management decisions.
Responses of benthic fauna to climatic variations depend on location, oceanographic conditions, and environmental shifts (Węsławski et al. 2011). Distributional shifts in benthic ostracods and bottom fishes reflect physiological limits and water temperature variations in the Bering and Chukchi Seas (Gemery et al. 2013; Kotwicki and Lauth 2013). Compared to the Barents Sea, the low densities of predatory fishes in the Chukchi Sea, a result of low water temperature, probably contributes, to a great extent, to the species composition of the benthos in the latter sea (Hunt et al. 2013; Kotwicki and Lauth 2013). Reduced predation by fishes appears to release the benthos from a top-down control in the Chukchi Sea, as also noted by Stevenson and Lauth (2012) in the Bering Sea. The transport of fauna northward through the Pacific-Arctic Gateway (Woodgate et al. 2012; Budaeva and Rogacheva 2013) results in the dominance of cold-water north Pacific species in muddy sediments of the Chukchi Sea (e.g., Maldane sarsi, Ennucula tenuis, Asterias amurensis, Lethasterias nanimensis, and others; Feder et al. 1994b, 2005; Bluhm et al. 2009; Blanchard et al. 2013a; Budaeva and Rogacheva 2013; Petryashov et al. 2013). Faunal distributions and species composition patterns reflect regional-scale processes driving dispersal but also respond to biological interactions, such as predation, and local variations that influence ecosystem functioning like water circulation and topographic deviations (Blanchard et al. 2013a; Blanchard and Feder 2014).
Regional-scale climate variations can strongly influence oceanographic characteristics and water circulation patterns with significant influences on benthic fauna. The Pacific Decadal Oscillation (PDO) index reflects anomalies in sea surface temperature, and is a measure of climatic variability strongly associated with ecological processes in the North Pacific Ocean (Hare and Mantua 2000; Mundy and Spies 2005; Pinchuk et al. 2008). In warm years (positive PDO values), ecological processes can be altered through varied processes such as increased coastal precipitation that can strengthen stratification in the water column and increase nearshore circulation (Neal et al. 2002; Mundy and Cooney 2005; Weingartner 2005; Blanchard et al. 2010; Cloern et al. 2010). Northward, the Arctic Oscillation (AO) index measures variations in sea level pressure over the Arctic Ocean (Thompson and Wallace 1998). The AO is associated with variations in polar winds over the Arctic, and is strongly coupled to climatic fluctuations over the northern hemisphere as well as variations in water circulation in the Beaufort Gyre (Macdonald et al. 1999; Proshutinsky et al. 2002; Wang et al. 2009; Stroeve et al. 2011). Regional-scale climate processes are now known to indirectly influence biological processes in North Pacific coastal zones (Blanchard et al. 2010; Cloern et al. 2010), and are likely to be drivers of temporal change throughout Alaskan northern seas as well.
There is a paucity of mechanistic information at the species level in Arctic seas explaining how and why macrobenthic communities might vary with climatic patterns (Węsławski et al. 2011). Physiological limits of some benthic organisms are clear and species distributions reflect limiting environmental characteristics (Gemery et al. 2013). It is generally assumed that warming trends will lead to northward invasions (Sirenko and Gagaev 2007; Drinkwater et al. 2010; Węsławski et al. 2011), but as noted by Blanchard et al. (2013a), there is a strong association between species present in southern Alaskan waters and the Chukchi Sea, suggesting that the prior assumption may not always hold. Additionally, latitudinal limits are not always clear as, for example, the blue mussel Mytilus trossulus occurs from California to the Point Barrow, Alaska region of the Chukchi Sea (Blanchard and Feder 1997; Feder et al. 2003). Similarly, the barnacle Semibalanus balanoides and the red King crab Paralithodes camtschaticus can be found from British Columbia to the Chukchi Sea (MacGinitie 1955; Rucker 1983; Feder et al. 1994b; NRC 1996; Blanchard et al. 2013a), and the seastar Pisaster ochraceus extends from Baja California to at least Prince William Sound, Alaska (Feder 1980). Other species with large geographic distributions include, for example, the bivalves Ennucula tenuis and Macoma calcarea, the polychaetes Levinsenia gracilis and Maldane sarsi, and the sea urchin Strongylocentrotus droebachiensis (Hartman 1969; Blake et al. 1996; Coon et al. 2000; Carlton 2007). Thus, the ecological basis for species introductions into the Chukchi Sea lacks clarity as many benthic species throughout Alaska’s coastal waters are widely dispersed latitudinally and have acclimated to high latitude conditions. Consequently, a better understanding is needed for the biological mechanisms by which benthic invertebrate fauna enter the Chukchi Sea and why other species are prevented from colonizing the region. Additionally, the absence of a long-term data record suitable for assessing temporal change in macrobenthic communities precludes inferences about long-term variations in the US Arctic. As noted by Wassmann (2011), the Arctic is not isolated and the bordering sub-arctic environments must also be understood.
In Alaska, the temporal influence of climatic variations on benthic macrofauna can be evaluated using a long-term database from Port Valdez (Blanchard et al. 2010). Resampling of the same deep benthic sites in the long-term study of the Port Valdez ecosystem (1971–2012) provides a basis for inferences relative to temporal variations of macrofauna and insights into mechanisms for change. Additionally, multidisciplinary investigations in the NE Chukchi Sea from 2008–2012, provide a basis for understanding short-term changes (Blanchard et al. 2013a, b). Thus, temporal trends in faunal characteristics are presented for Port Valdez and the NE Chukchi Sea to better understand how climatic variations might influence benthic fauna in both areas and how future trends might be predicted throughout Alaska’s seas. The emphasis is not on comparing the two systems, but on evaluating each system for trends that may reflect common influences of climatic variations. The environmental background, factors influencing spatial community variability, and major conclusions of each research program are reviewed to provide context and perspective for the presented temporal aspects of biodiversity. This paper contributes to understanding elements of biodiversity (distributional patterns of fauna and richness = number of taxonomic categories) by evaluating historic data for the US portion of the Węsławski et al. (2011) study area in Prince William Sound, Alaska (see also Feder and Blanchard 1998; Hoberg and Feder 2002) and presenting recent data from the NE Chukchi Sea. Drivers of spatial variability for Port Valdez are discussed in detail by Feder and Jewett (1988), Blanchard (2006), and Blanchard et al. (2010). Feder et al. (1994a, b), Grebmeier et al. (2006), and Blanchard et al. (2013a, b) discuss sources for spatial variability in the NE Chukchi Sea.
Climate definitions used here follow Drinkwater et al. (2010) with the phrase climate variations being natural, decadal-scale patterns and climate change referring to long-term changes mainly associated with anthropogenic causes. Climate affects marine systems through multistep processes, but underlying processes are often unclear, particularly when separating climate-driven natural variations from effects of increasing climatic extremes that reflect human contributions.
Study areas and environmental background
Port Valdez, a glacial fjord
Investigations of the marine environment in Port Valdez were initiated in 1968 and multidisciplinary, ecologically oriented studies began in 1971 (Hood et al. 1973; Colonell 1980b; Shaw and Hameedi 1988). Detailed biological studies were performed from 1971 to 2012 encompassing dominant intertidal and subtidal communities (Wiegers et al. 1998; Blanchard et al. 2010). Port Valdez is a glacial outwash fjord in the northeastern corner of Prince William Sound (PWS), Alaska (Fig. 1). The fjord has two sills (at 120 m and 200 m depth) and a relatively flat bottom in the deep basin varying between 230–250 m depth (Hood et al. 1973; Colonell 1980b). Residence time of water in the fjord is estimated as 40 days with slow counterclockwise circulation. Bottom sediments are dominated by silt and clay fractions carried to the fjord in the glacial melt water from several rivers (Sharma and Burbank 1973; Naidu and Klein 1988). Deep basin macrofaunal communities show relatively low density and richness and moderate gradients in community structure associated with higher sediment loads from glacial rivers in the east and greater water depth (Feder and Matheke 1980b; Feder and Jewett 1988; Blanchard et al. 2010). The environmental and biological gradients in Port Valdez are similar to but weaker in strength than those observed in fjords with tidewater glaciers where ice gouging and very high sedimentation rates maintain greater stress (Wlodarska- Kowalczuk et al. 2004, 2005; Wlodarska-Kowalczuk and Pearson 2004; Wlodarska-Kowalczuk et al. 2007; Kedra et al. 2010; Kędra et al. 2013).
The macrobenthos of Port Valdez demonstrates significant spatial and temporal variability in response to natural and anthropogenic perturbations. A magnitude 9.2 earthquake in March, 1964, caused catastrophic disturbance to all marine communities in Prince William Sound (Coulter and Migliaccio 1971; Haven 1971; Hubbard 1971; NRC 1971). Displaced sediments were deposited in the basin of Port Valdez, restructuring sediment habitats and burying benthic organisms. Sills of the fjord were barriers to recruitment of benthic larvae in the deep basin, contributing in part, to the long (26 years) recovery period for macrobenthos of the deep basin from the 1964 earthquake (Blanchard et al. 2010). However, a few species were numerous in Port Valdez after the tsunami resulting from the 1964 earthquake (e.g., Ophiura sarsi) but did not persist in the fjord environment (Blanchard et al. 2010). An additional source of temporal and spatial variability for macrobenthos are releases and returns of hatchery salmon from a shoreside facility that are associated with increased benthic biomass suggesting increased carbon in the fjord. Overlap of stresses from dredging and spoils disposal, discharges from a fish processing plant, and movements of vessels resulted in synergistic effects of these multiple stressors on benthic fauna in Port Valdez (a cumulative effect; Blanchard and Feder 2003). The latter small-scale study demonstrated reduced benthic communities dominated by opportunistic fauna where fish wastes were spread to the disturbed (dredged) sediments by vessel turbulence at a new dock. How overlapping broad-scale environmental changes (the PDO, earthquake, and salmon hatchery releases and returns) influence benthic fauna is unknown. Ultimately, the Port Valdez study was very successful as an early warning system for small-scale anthropogenic effects and as a means to understand and separate natural and anthropogenic effects in the ecosystem.
The northeastern Chukchi Sea, a shallow shelf system
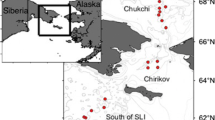
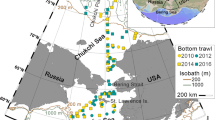
The multidisciplinary Chukchi Sea Environmental Studies Program (CSESP) has investigated the NE Chukchi Sea ecosystem from 2008–2013 (Day et al. 2013) at three focused sites throughout the study period (Klondike, Burger, and Statoil), with additional locations sampled from a larger study area in 2011 and 2012 (Fig. 2; Blanchard et al. 2013a, b). Resampling of the three study areas, Klondike, Burger, and Statoil, from 2008–2012 (2010–2012 for Statoil), provides a short temporal record for the study area.
The NE Chukchi Sea is a shallow sea strongly influenced by water advected north from the Bering Sea. The northward flow of water into the Chukchi Sea is driven by the greater sea level height in the Pacific than in the Arctic Ocean. Water proceeds along three principal pathways associated with bathymetry: the Alaskan Coastal Current flows along the northwest coast of Alaska to Barrow Canyon while Bering Sea Water flows northward through the Central Channel between Herald and Hanna shoals and through Barrow Canyon and Herald Valley in the western Chukchi Sea (Fig. 2; Weingartner et al. 2005; Woodgate et al. 2005). The flow of water through the Pacific-Arctic Gateway from the Bering Sea northward through the Chukchi Sea and into the Arctic Ocean entrains heat, nutrients, and organic carbon contributing to ecological characteristics of the Chukchi Sea including benthic productivity (Carey 1991; Dunton 1992; Feder et al. 1994b; Hunt et al. 2013; Petryashov et al. 2013). Blanchard et al. (2013a) noted strong similarities between the taxonomic composition of sites in the NE Chukchi Sea and those of coastal Alaska and the North Pacific Ocean reflecting the northward transport. General environmental characteristics in the NE Chukchi Sea follow the expected increase in depth and associated increase in the percent of mud of sediments with greater distance offshore (Feder et al. 1994b).
Ecosystem dynamics within the CSESP study area are surprisingly complex over a small spatial extent (Day et al. 2013). As opposed to assumptions that the Chukchi Sea is oceanographically smooth (Weingartner et al. 2005), new findings indicate a more complex system with distributions of benthic fauna reflecting ecologically significant deviations in water circulation (Blanchard et al. 2013a, b; Day et al. 2013; Weingartner et al. 2013). The complex water circulation results in greater macrobenthic biomass and density in and adjacent to Burger underneath a proposed convergence zone. Observed associations between environmental and faunal characteristics covary with sampling scale to the extent that relationships could not be rescaled from mid-scales to large-scales, a direct result of the interactions between water circulation, bottom topography, and ecological processes controlling benthic fauna (Blanchard et al. 2013a, b; Blanchard and Feder 2014). Recent sea ice reductions associated with long-term climate variations is an environmental change potentially with large ecosystem-level effects (Grebmeier et al. 2010; Grebmeier 2012).
Methods
Macrobenthic communities were sampled in Port Valdez and the NE Chukchi Sea using a 0.1 m2 van Veen grab. Data for this study included biomass (g m−2), density (ind. m−2), and taxon richness (richness = the number of taxonomic categories identified). A set of repeatedly sampled stations in the deep basin of Port Valdez (stations 11, 27, 32, 40, 45, and 50; Fig. 1) were selected for detailed analysis from Port Valdez, although fauna from all stations sampled are included in the overall taxon list. Effects from seasonal events are limited at the deep sites (e.g., less exposure to turbulence from winter storms, human activities) allowing a clearer analysis of long-term trends. Limiting the data to 1989–2012 excludes the years when the benthos was recovering from the 1964 earthquake. Nine repeatedly sampled locations from each of the three focused CSESP study areas were selected for detailed analysis in the NE Chukchi from 2008–2012, although all stations sampled from the focused CSESP study areas are included in the calculation of total taxa (Fig. 2).
Linear regression was applied to evaluate the relationship of benthic community parameters with climatic variations. Climatic data were the Pacific Decadal Oscillation Index (Mantua et al. 1997; http://jisao.washington.edu/pdo), the Arctic Oscillation index (Thompson and Wallace 1998; http://jisao.washington.edu/ao/), and average summer precipitation for Port Valdez (www.wrcc.dri.edu). Comparisons between annual average (January to December), summer averages (March through September), or winter averages (November through March) demonstrated that the annual PDO was the strongest predictor of macrobenthic characteristics in Port Valdez whereas the winter AO was the strongest predictor for the Chukchi Sea. Macrobenthic biomass, density, and taxon richness were regressed against the PDO index for Port Valdez where the data record was long enough to draw inferences. Macrobenthic summary measures and biomass and density of selected taxon were also correlated with climate indices. Regression and correlation were performed using Excel, and Primer-e (Clarke and Gorley 2006) was used to estimate species accumulation curves.
Results
Responses of fauna to climate variations
The biological component of the PVESP provides a strong temporal record for evaluating covariances between faunal distributions and climatic variations. Annual variations in climate positively influenced long-term density and richness measures in the deep basin of the fjord, but the PDO was not a significant predictor of overall biomass (Fig. 3; Table 1). Looking closer, however, biomass and densities of five dominant macrofaunal families in Port Valdez demonstrated varying associations with climate indices. Biomass and density of polychaetes in the family Nephtyidae (predominately Nephtys punctata) were strongly and negatively correlated with the PDO index while polychaetes of the family Maldanidae were moderately and positively correlated with the PDO (Table 2). Density and biomass of the polychaete family Lumbrineridae (Scoletoma spp.) and the bivalve families Thyasiridae (largely the small clams Adontorhina cyclia and Axinopsida serricata) and Tellinidae (Macoma spp.) were, at best, moderately correlated with the PDO index. Correlation analysis indicates that biomass and density of Thyasiridae and Tellinidae were highly and negatively correlated with summer precipitation, whereas biomass and density of polychaete families were not (Table 2). This pattern extends to the class level where bivalve biomass and density had a negative correlation, both moderately to strongly, with precipitation while bivalve biomass and polychaete density were moderately correlated with the PDO index.
Macrobenthic fauna in the Chukchi offshore region showed significant differences among years and sites, but changes were poorly reflected in bottom water temperature and salinity (Fig. 4; see also Blanchard et al. 2013a). As in Port Valdez, the significant temporal variability of macrobenthic community density and richness was strongly correlated with climate; the lowest average density and richness values in the CSESP were associated with an all-time low in the Arctic Oscillation (AO) (Fig. 5). Biomass was not strongly correlated with the AO. Although the time series in the Chukchi Sea are too short to draw strong inferences concerning responses of fauna to climatic variability, the similar associations of macrobenthic characteristics with climate indices in two Alaskan study areas suggest common pathways of influence.
Diversity
Similarities of macrofaunal community composition are strong between Port Valdez and the NE Chukchi Sea. Two hundred and seventy-six taxonomic categories were determined from Port Valdez for 1989–2012 over an area of ∼100 km2 (encompassing all stations sampled throughout the fjord for 1989–2012; Fig. 1; Blanchard et al. 2010) comprising 167 distinct species identifications, 109 families, and eight phyla (Fig. 1; Supplementary Material Appendix I). A total of 321 taxonomic categories were determined from the Chukchi Sea CSESP study area in 2008–2012 comprising 189 distinct species identifications (taxonomic verifications in progress to expand the species identifications), 161 families, and 17 phyla over an area of ∼9,000 km2 (Blanchard et al. 2013a; Appendix I).
Species accumulations curves (with removals of higher level categories) provided insights into temporal patterns of observed diversity over multiple years of sampling. For stations in the deep basin of Port Valdez, the species accumulation curve indicated that ∼10 years were needed to adequately document the species composition with an estimated 50 % or less of observed species found in the first 2 years, 77 % found in 10 years, and 90 % observed by year 17 (Fig. 6). The species accumulation curve for the Chukchi Sea (also focusing on the repeatedly sampled stations) has almost twice the number of taxa but demonstrated a similar pattern with the species accumulation curve demonstrating a steep, linear slope after 5 years suggesting that so far, less than 50 % of total species have been documented. Within all years, the species list in Port Valdez was dominated by bivalves and polychaetes and that of the Chukchi Sea from 2008–2012 was dominated by amphipods, bivalves, gastropods, and polychaetes (Figs. 7 and 8). The significant drop in richness for Burger in 2010 (Fig. 4) results largely from a decline in the richness of polychaetes (Fig. 8).
Species accumulation curves of observed species for the Port Valdez Environmental Studies Program for 1989–2012 and Chukchi Sea Environmental Studies Program in 2008–2012. The solid line is the smoothed estimator of observed species. Range = minimum and maximum values of richness estimators (Sobs, Chao1, Chao2, jackknife, and UME)
Discussion
Climate drivers of biodiversity
Biodiversity in the coastal waters and seas of Alaska is strongly influenced by climate variations over short and decadal-scale time periods. The correlations of macrobenthic faunal characteristics with the Pacific Decadal Oscillation (PDO) index in Port Valdez and the Arctic Oscillation (AO) in the Chukchi Sea indicate adjustments of communities to regional climatic variations over short to decadal time scales (Figs. 3 and 5; Blanchard et al. 2010). The PDO index reflects broad spectrum oceanographic influences associated with Alaska’s coastal ecosystem processes such that positive PDO values (warmer sea surface temperatures) covary with increased water circulation and precipitation (the River hypothesis in the PWS ecosystem; Mooers and Wang 1998; Mundy and Cooney 2005). Decreased circulation is associated with negative PDO values (the Lake hypothesis). Blanchard et al. (2010) noted a strong correlation between the PDO and characteristics of macrobenthic communities, as demonstrated in the present study (Fig. 3). A similar relationship between the PDO and benthic community characteristics was observed in San Francisco Bay (Cloern et al. 2010) and is also repeated in the NE Chukchi Sea with the AO. The replicated relationships in multiple environments indicate that regional climate factors can have immediate effects on macrobenthic communities throughout the regions influenced by the North Pacific. It is apparent that a better understanding of species interactions and factors driving potential macrobenthic alternate states is needed to support conclusions of benthic responses to climate variations. Further, short-term studies can mislead investigators concerning faunal distributions and responses to climate variations if water circulation changes underlie shifts between alternate stable states. Total biomass in our study was weakly correlated with climate indices, likely reflecting the persistence of larger individuals, rather than the more immediate responses noted for density and richness reflecting annual recruitment.
Physical mechanisms by which the AO may influence Arctic physical oceanography and ecosystem characteristics are not understood. The positive phase of the AO results in stormy weather in the north because air pressure is weaker while the negative AO phase is associated with higher air pressure in the Arctic and warmer weather in high latitudes (Thompson and Wallace 1998). The negative phase is also associated with strengthened anticyclonic circulation of the Beaufort Gyre in the Beaufort Sea, but effects on circulation in the Chukchi Sea are not yet known (Proshutinsky and Johnson 1997; Proshutinsky et al. 2002; Wang et al. 2009; Stroeve et al. 2011). Nevertheless, variations in water flow associated with pressure changes may be one pathway for the AO (or the related dipole anomaly; Wang et al. 2014) to influence Arctic ecosystems, as with the PDO. Variability in water circulation over the Chukchi shelf and exchange between the Chukchi and Beaufort Seas may have Arctic-wide influences (Kawaguchi et al. 2012; Williams et al. 2014). Conversely, Williams et al. (2014) suggest that circulation in the Beaufort Gyre may influence shelf-break exchange and circulation dynamics as well. Water circulation may link the AO and macrobenthic fauna variations in the NE Chukchi by removing larvae (increased circulation removing waters into the Arctic Ocean), preventing larvae from entering the region (increased strength of the Beaufort Gyre blocking circulation of water north), or perturbing food delivery patterns, although numerous other pathways are possible.
On very long time scales, sub-Arctic and Arctic shallow shelf benthic habitats are recent marine habitats (via deglaciation) and all marine biota in these environments are considered geologically-recent invaders (Nelson et al. 2014). Based on the geological history of the present study areas, the colonization of geologically recent marine habitats should have resulted in a high similarity of species as the sediment habitats and benthic communities adjusted over time. The faunal similarities between Port Valdez and the Chukchi Sea (>30 % overlap) suggest a common population source, likely due in part, to the movement of some species into cold-water refugia (Orensanz et al. 2004; Norton and Feder 2006). The climatic and geological history that shaped the linkages observed in the present is, thus, as relevant to current biodiversity patterns as present oceanographic conditions (Ogasawara 2002; Hardy et al. 2011a). It is critical, therefore, to understand how linkages may drive biological change in open marine systems like the NE Chukchi Sea to better predict effects from climatic variations. Currently, the broader historical data necessary to fully support large-scale inferences about changes in Arctic macrobenthos are unavailable.
Physiological limits, biotic interactions, and biodiversity in Alaskan seas
Temperature effects on benthic fauna are not always clear, and many species are able to adapt to large water temperature changes over the ranges of their distribution (Węsławski et al. 2011). For example, the mussel Mytilus trossulus acclimates to large shifts in environmental and climatic conditions along the North Pacific coast from California to Point Barrow, Alaska by adjusting the timing of reproductive events and growth (Blanchard and Feder 1997; Feder et al. 2003). Similar acclimations to large-scale oceanographic variations are noted for other species along the Pacific coast to Alaska as well (MacGinitie 1955, 1959; Feder 1980; Rucker 1983; Feder et al. 1994a, b; Blanchard and Feder 2000a; Blanchard et al. 2013b). Overall, the overlap of benthic fauna from arctic waters with communities of the North Pacific Coast is broad (see Feder et al. 1980; Feder and Matheke 1980a; Feder and Jewett 1986, 1988; Hoberg and Feder 2002). Thus, water temperature may not be as strong a limiting factor as assumed for many species in the Chukchi Sea because of plasticity in physiology, ecology, and life histories, as noted for Arctic species more generally by Węsławski et al. (2011). In particular, the biological bases for range expansions in association with climatic variations and environmental variables are not known, except for a few species where information is available (e.g., Mytilus trossulus, Chionoecetes opilio, Elipidia, and Paradiopatra; Blanchard and Feder 1997, 2000b; Orensanz et al. 2004; Hardy et al. 2011b; Budaeva and Rogacheva 2013; Hollowed et al. 2013; see also MacGinitie 1955). Cold water does, however, limit the distribution of benthic-feeding fishes in the northeastern Bering and Chukchi Seas, releasing benthic communities from some measure of top-down control (Stevenson and Lauth 2012; Day et al. 2013; Kotwicki and Lauth 2013; Norcross et al. 2013), and cold water (∼2–6 ° C) is also characteristic of Port Valdez (Colonell 1980a). There is a general decline in the presence of boreal species from mid-latitudes to the Arctic (Węsławski et al. 2011) and certainly, some invertebrate species will migrate north with climate warming, but the biological basis for understanding distributional patterns is lacking.
It is possible that some benthic fauna restricted to the Chukchi are poor competitors and may represent residual organisms from the original source population at the time of deglaciation, rather than water temperature effects alone. A species distribution may contract northward due to predation or other factors, but the northward flowing water could prevent their movement southward after relaxation of predation pressures (Orensanz et al. 2004). Such an altered distribution pattern would be confounded by a covariance with water temperature as warmer water allows a northward movement of predators, limiting prey distributions to colder waters. Predation on invertebrates at all life stages can be a significant source of variability and a structuring factor in macrobenthic communities at various scales (Olafsson et al. 1994; Feder et al. 2005, 2007). Modification of faunal distributions due to biological/environmental interactions following disturbance is common at small scales (Carroll and Highsmith 1996; Blanchard 2006), so interactions at larger scales should be expected (Orensanz et al. 2004). These unmeasured effects of biological interactions on macrobenthic communities may play large roles in the geographic distribution of animals, and evaluation of species interactions over regional scales may be an enlightening direction for research.
Given the overlap in macrobenthic species composition between the two study areas, inferences about invasions of benthic invertebrates into the US Arctic must be viewed with caution. The northward flow of water into the NE Chukchi leads to the prediction that species introductions would occur as waters warm following global climate trends. Three epifaunal species are suggested as recent invaders (Sirenko and Gagaev 2007; Węsławski et al. 2011), although two of the species were identified in primary sources for the region dating to 1948 (MacGinitie 1955; Sparks and Pereyra 1966; Feder et al. 1994a, b, 2005, 2007). Norton and Feder (2006) demonstrated that the lack of observation of an animal (e.g., not presented in taxon lists) is not proof of its absence; a species may be rare, living in refugia, sampling may be inadequate relative to the spatial scale of the study area, not all habitats may be sampled, or the species may be listed in sources difficult to access. Poor availability of earlier work contributes directly to confusion concerning recent invaders into the Chukchi, and further biodiversity studies are suggested (Sirenko and Gagaev 2007; Blanchard et al. 2013b). The present study demonstrates that new species will continue to be observed for the next decade and beyond, simply as a result of the accumulation of species over time (Fig. 6), as well as greater sampling effort across varying habitats. Statistically rigorous, long-term sampling of representative habitats in the Chukchi Sea, rather than of unique habitats alone, will be necessary to understand long-term trends and interrelationships with environmental and oceanographic features (Grebmeier et al. 2010; Blanchard et al. 2013a). Further, all spatial scales should be considered (Blanchard and Feder 2014). Since macrofaunal community characteristics are directly linked to short-term and long-term variability of large-scale physical processes, oceanographic variations will confound biodiversity investigations relying on a few spatially and temporally dispersed sampling events.
Data gaps and future needs in Arctic seas
Data gaps confound attempts to draw inferences of long-term trends, and are concerns for researchers in the Arctic. Currently the understanding of Pan-Arctic ecosystems is progressing due to integrated studies evaluating macroecological processes. Studies encompass macroecological predictors of benthic community structure to factors driving ecosystem variability (Cusson and Bourget 2005; Cusson et al. 2007; Drinkwater et al. 2010; Blanchard et al. 2013a, b; Dunton et al. 2005, 2014). Investigations of Arctic benthic systems have evaluated linkages at macroscales to Pan-Arctic scales (Grebmeier et al. 2006; Piepenburg et al. 2011; Węsławski et al. 2011; Grebmeier 2012). Yet, numerous data gaps remain, having arisen from sources as varied as spatial and temporal sampling gaps, no information on spatial and temporal interactions, mismatches in sampling locations, and inadequate information on the distributions and life histories of invertebrates in Alaska. Adding to the growing body of knowledge, the information from Port Valdez and the NE Chukchi Sea leads to a series of propositions that may provide direction for future investigations to fill data gaps for Arctic seas. The propositions are: (1) effects from climatic variations need to be understood in terms of directed studies of macrofaunal life history stages; (2) faunal composition and richness will demonstrate ecologically significant temporal variations due to normally varying oceanographic variations influencing biological communities, potentially shifting between alternate stable states; and (3) biotic/environmental interactions may limit ranges of macrofaunal communities at regional scales through environmental tolerances of predators.
Quantitative approaches to evaluating marine communities are a strong component of environmental studies like the PVESP and CSESP. In contrast, statistical power, ranges of inference, and sampling designs have sometimes been inadequately considered for benthic research in Arctic Seas. Because it is difficult and expensive to sample macrobenthos, particularly in high latitudes, there is a tendency to utilize any data available, including opportunistic sampling of single data points and pilot studies. Inferences based on trends of single or a few data points where standard errors cannot be calculated should be considered at best, as preliminary, and not accepted as proof of trends. Simply put, low power studies without adequate variance estimates do not provide a basis for inferences concerning climate change or any environmental/biological relationship. Additionally, failure to adapt sampling to increased understanding of ecological processes can result in a mismatch with too few samples in areas with important environmental gradients, preventing determination of patterns at multiple scales (Blanchard and Feder 2014). In concert with funding gaps, sampling issues have contributed to large data gaps in Arctic seas through inadequate spatial and temporal sampling. Recent efforts are contributing to a stronger, long-term database for understanding spatial and temporal interactions including the Distributed Biological Observatory and the CSESP (Grebmeier et al. 2010; Blanchard et al. 2013a).
The lack of access to historical work is an outstanding issue for research and management of Alaska and other marine habitats. While it is not clear what sampling effort will be required to adequately document spatial and temporal biodiversity patterns, it can be expected that new species will be recorded in the regions for decades to come, but historical work can shed light on species previously known. At present, too little is known about the composition, life histories, and ecology of benthic fauna in the Chukchi Sea to infer change, and direct or indirect climate-related distributional shifts cannot be separated from other sources of variation. Given the status of all fauna in Port Valdez and the NE Chukchi as invaders (relative to geological history and repeated glaciation events), it is imperative to understand true drivers of spatial and temporal variability in the distribution of individual organisms, as opposed to the proxies for drivers such as sediment grain size and water depth, a question to which retrospective analysis of historical data may directly contribute.
Considerations for management and conservation in Arctic benthic systems
Managing Arctic communities in light of unpredictable changes and large data gaps is a great challenge. Recent projections of benthic community adjustments to climatic variations suggest variable responses as communities are reshaped under complex environmental changes (Węsławski et al. 2011; Doney et al. 2012; Lurgi et al. 2012). While the covariance of biodiversity with habitat heterogeneity and environmental disturbance is well known (Magurran 2004), the wide environmental tolerances of many Alaskan and worldwide Arctic benthic fauna make projecting future changes even more difficult. Additionally, temporal aspects of biodiversity are poorly known but are a key to understanding benthic community variability. As shown in the present study, short-term studies from which biodiversity inferences are made, are likely capturing less than 50 % of the faunal information needed within 2 years and at best, ∼75 % within 7 years (Fig. 6). Additionally, the observed correlations of taxon richness with climate indices in multiple environments suggest high potential for confounding of conclusions from short-term studies with background variability. The poor availability of historical resources and low power studies also contribute to reduced expectations for inferences concerning Arctic seas, highlighting the need for consistent, long-term studies to adequately document species assemblages with common sampling frames. Predictability of future benthic responses to environmental variations will remain low until confounding issues (such as the biological bases for change and sampling designs) are clarified. Given the importance of benthic fauna to higher trophic level predators in the Arctic (extending to coastal communities depending on subsistence harvests of marine mammals), understanding drivers of spatial and temporal variations in prey communities will be one key for managing resources.
Monitoring biodiversity and ecosystem functioning is an increasingly useful tool, but requires knowledge of past and present conditions to predict future changes. The present study demonstrates the importance of climatic variations as a temporal predictor of benthic community characteristics, a role usually attributed to primary production variations, suggesting new directions of research may be required. In the long-term, introductions of new benthic predators with increased water warming will be a natural experiment for understanding large-scale changes, but changes will only be detectable with adequate sampling that is designed with forethought related to the eventual long-term data sets and analyses (e.g., Blanchard 2006; Blanchard et al. 2010). It is suggested; therefore, that the current emphasis on research at the oceanographic scales in the NE Chukchi and other Arctic seas be adjusted to include long-term monitoring of representative habitats, as well as species-specific studies to determine distribution-limiting factors, as performed in Port Valdez (Blanchard and Feder 1997, 2000a, b, 2003; Blanchard et al. 2003, 2010).
As equally important, the definition of a basis for management of benthic resources and biodiversity is needed in many Alaskan and other Arctic coastal communities, particularly as anthropogenic stressors increase. A basic framework can be described for the Chukchi Sea, but significant elements are still lacking. Environmental managers of the Port Valdez environment faced a similar need to make significant decisions in the absence of adequate data to predict anthropogenic effects. The multidisciplinary PVESP provided that information by first understanding the ecosystem and important physical drivers, identifying key biological resources for further study and then focusing on those endpoints relevant to local stressors under consideration with diversity as one endpoint. In the Chukchi Sea, the northward flow of water will maintain biodiversity but ecosystem functioning may be altered by changes in water circulation, even if the species composition remains the same. The potential for large, indirect effects from changes in water circulation to unduly influence benthic-feeding predators remains undefined (Blanchard and Feder 2014). Nevertheless, declines in species density and richness with strong negative PDO and AO values indicate some potential exists in the Chukchi Sea. The underlying similarities of benthic fauna and key habitat characteristics suggest that the decision processes, evolution, and projection of the PVESP into the present may be of value for current management decisions at larger scales where data are still accumulating and much is unknown (Shaw and Hameedi 1988; Blanchard et al. 2010).
References
Blake JA, Hilbig B, Scott PH (eds) (1996) Taxonomic atlas of the Santa Barbara channel, volume 6, Annelida - part 3 Polychaeta: Orbiniidae to Cossuridae. Santa Barbara Museum of Natural History, Santa Barbara
Blanchard AL (2006) Retrospective analysis of marine biological data from Port Valdez, Alaska: a case study in long-term monitoring. PhD dissertation
Blanchard A, Feder HM (1997) Reproductive timing and nutritional storage cycles of Mytilus trossulus Gould, 1850, in Port Valdez, Alaska, site of a marine oil terminal. Veliger 40(2):120–130
Blanchard A, Feder HM (2000a) Distribution, reproduction, and shell growth of limpets in Port Valdez, Alaska. Veliger 43(4):289–301
Blanchard A, Feder HM (2000b) Shell growth of Mytilus trossulus Gould, 1850, in Port Valdez, Alaska. Veliger 43(1):34–42
Blanchard AL, Feder HM (2003) Adjustment of benthic fauna following sediment disposal at a site with multiple stressors in Port Valdez, Alaska. Mar Pollut Bull 46(12):1590–1599. doi:10.1016/S0025-326X(03)00325-4
Blanchard AL, Feder HM (2014) Interactions of habitat complexity and environmental characteristics with macrobenthic community structure at multiple spatial scales in the northeastern Chukchi Sea. Deep-Sea Res II Top Stud Oceanogr 102:132–143. doi:10.1016/j.dsr2.2013.09.022
Blanchard AL, Feder HM, Shaw DG (2003) Variations in benthic fauna underneath an effluent mixing zone at a marine oil terminal in Port Valdez, Alaska. Mar Pollut Bull 46(12):1583–1589. doi:10.1016/S0025-326X(03)00324-2
Blanchard AL, Feder HM, Hoberg MK (2010) Temporal variability of benthic communities in an Alaskan glacial fjord, 1971–2007. Mar Environ Res 69(2):95–107
Blanchard AL, Parris CL, Knowlton AL, Wade NR (2013a) Benthic ecology of the northeastern Chukchi Sea. Part I. Environmental characteristics and macrofaunal community structure, 2008–2010. Cont Shelf Res 67:52–66. doi:10.1016/j.csr.2013.04.021
Blanchard AL, Parris CL, Knowlton AL, Wade NR (2013b) Benthic ecology of the northeastern Chukchi Sea. Part II. Spatial variation of megafaunal community structure, 2009–2010. Cont Shelf Res 67:67–76. doi:10.1016/j.csr.2013.04.031
Bluhm BA, Iken K, Hardy SM, Sirenko BI, Holladay BA (2009) Community structure of epibenthic megafauna in the Chukchi Sea. Aquat Biol 7(3):269–293. doi:10.3354/ab00198
Budaeva NE, Rogacheva AV (2013) Colonization of the Arctic Ocean by two cosmopolitan genera of marine invertebrates. Invertebr Zool 10(1):127–142
Carey AG Jr (1991) Ecology of North American Arctic continental shelf benthos: a review. Cont Shelf Res 11(8–10):865–883. doi:10.1016/0278-4343(91)90083-i
Carlton JT (ed) (2007) The light and Smith manual: intertidal invertebrates from Central California to Oregon, 4th edn. University of California Press, Berkeley
Carroll ML, Highsmith RC (1996) Role of catastrophic disturbance in mediating Nucella-Mytilus interactions in the Alaskan rocky intertidal. Mar Ecol Prog Ser 138:125–133. doi:10.3354/meps138125
Clarke KR, Gorley RN (2006) PRIMER v6: user manual/tutorial. Primer-E, Plymouth
Cloern JE, Hieb KA, Jacobson T, Sansó B, Di Lorenzo E, Stacey MT, Largier JL, Meiring W, Peterson WT, Powell TM, Winder M, Jassby AD (2010) Biological communities in San Francisco Bay track large-scale climate forcing over the North Pacific. Geophys Res Lett 37(21), L21602. doi:10.1029/2010gl044774
Colonell JM (1980a) Physical oceanography. In: Colonell JM (ed) Port Valdez, Alaska environmental studies, 1976–1979. Institute of Marine Science, University of Alaska, Fairbanks, pp 9–36
Colonell JM (1980b) Port Valdez, Alaska. Environmental studies, 1976–1979. Institute of Marine Science, University of Alaska, Fairbanks
Coon EV, Scott PV, Bernard FR (2000) Bivalve seashells of Western North America. Santa Barbara Museum of Natural History, Santa Barbara
Coulter HW, Migliaccio RR (1971) Effects at Valdez. In: National Research Council, The Great Alaska Earthquake of 1964 Geology, Part A. National Academy of Science, Washington, pp 8–34
Cusson M, Bourget E (2005) Global patterns of macroinvertebrate production in marine benthic habitats. Mar Ecol Prog Ser 297:1–14
Cusson M, Archambault P, Aitken A (2007) Biodiversity of benthic assemblages on the Arctic continental shelf: historical data from Canada. Mar Ecol Prog Ser 331:291–304. doi:10.3354/meps331291
Day RH, Weingartner TJ, Hopcroft RR, Aerts LAM, Blanchard AL, Gall AE, Gallaway BJ, Hannay DE, Holladay BA, Mathis JT, Norcross BL, Questel JM, Wisdom SS (2013) The offshore northeastern Chukchi Sea, Alaska: a complex high-latitude ecosystem. Cont Shelf Res 67:147–165. doi:10.1016/j.csr.2013.02.002
Doney SC, Ruckelshaus M, Duffy JE, Barry JP, Chan F, English CA, Galindo HM, Grebmeier JM, Hollowed AB, Knowlton N, Polovina J, Rabalais NN, Sydeman WJ, Talley LD (2012) Climate change impacts on marine ecosystems. Annu Rev Mar Sci 4(1):11–37. doi:10.1146/annurev-marine-041911-111611
Drinkwater KF, Beaugrand G, Kaeriyama M, Kim S, Ottersen G, Perry RI, Pörtner H-O, Polovina JJ, Takasuka A (2010) On the processes linking climate to ecosystem changes. J Mar Syst 79(3–4):374–388. doi:10.1016/j.jmarsys.2008.12.014
Dunton KH (1992) Arctic biogeography: the paradox of the marine benthic fauna and flora. Tree 7:183–189
Dunton KH, Goodall JL, Schonberg SV, Grebmeier JM, Maidment DR (2005) Multi-decadal synthesis of benthic-pelagic coupling in the western arctic: role of cross-shelf advective processes. Deep-Sea Res II Top Stud Oceanogr 52(24–26):3462–3477. doi:10.1016/j.dsr2.2005.09.007
Dunton KH, Grebmeier JM, Trefry JH (2014) The benthic ecosystem of the northeastern Chukchi Sea: an overview of its unique biogeochemical and biological characteristics. Deep-Sea Res II Top Stud Oceanogr 102:1–8. doi:10.1016/j.dsr2.2014.01.001
Feder HM (ed) (1980) Asteroidea: the Sea Stars. Intertidal invertebrates of California. Stanford University Press, Stanford
Feder HM, Blanchard A (1998) The deep benthos of Prince William Sound, Alaska, 16 months after the Exxon Valdez oil spill. Mar Pollut Bull 36(2):118–130. doi:10.1016/s0025-326x(97)87420-6
Feder HM, Jewett SC (1986) The subtidal benthos. In: Hood DW, Zimmerman ST (eds) The Gulf of Alaska. Physical Environment and Biological Resources. U. S. Dept. of Commerce. U. S. Government Printing Office, Washington, D. C., pp 347–396
Feder HM, Jewett SC (1988) The subtidal benthos. In: Shaw DG, Hameedi MJ (eds) Environmental studies in Port Valdez, Alaska. A basis for management. Springer-Verlag, New York, pp 165–202
Feder HM, Matheke GEM (1980a) Distribution, abundance, community structure, and trophic relationships of the benthic infauna of the northeast Gulf of Alaska. Biological Studies. Institute of Marine Science, Fairbanks
Feder HM, Matheke GEM (1980b) Subtidal benthos. In: Colonell JM (ed) Port Valdez, Alaska: environmental studies 1976–1979. Institute of Marine Science, University of Alaska, Fairbanks, pp 235–318
Feder HM, Haflinger K, Hoberg M, McDonald J (1980) The infaunal invertebrates of the southeastern Bering Sea. Institute of Marine Science, Fairbanks
Feder HM, Foster NR, Jewett SC, Weingartner TJ, Baxter R (1994a) Mollusks in the northeastern Chukchi Sea. Arctic 47(2):145–163
Feder HM, Naidu AS, Jewett SC, Hameedi JM, Johnson WR, Whitledge TE (1994b) The northeastern Chukchi Sea: benthos-environmental interactions. Mar Ecol Prog Ser 111:171–190
Feder HM, Norton DW, Geller JB (2003) A review of apparent 20th century changes in the presence of mussels (Mytilus trossulus) and macroalgae in arctic Alaska, and of historical and paleontological evidence used to relate mollusc distributions to climate change. Arctic 56(4):391–407
Feder HM, Jewett SC, Blanchard A (2005) Southeastern Chukchi Sea (Alaska) epibenthos. Polar Biol 28(5):402–421. doi:10.1007/s00300-004-0683-4
Feder HM, Jewett SC, Blanchard AL (2007) Southeastern Chukchi Sea (Alaska) macrobenthos. Polar Biol 30(3):261–275. doi:10.1007/s00300-006-0180-z
Gall AE, Day RH, Weingartner TJ (2013) Structure and variability of the marine-bird community in the northeastern Chukchi Sea. Cont Shelf Res 67:96–115. doi:10.1016/j.csr.2012.11.004
Gemery L, Cronin TM, Cooper LW, Grebmeier JM (2013) Temporal changes in benthic ostracode assemblages in the Northern Bering and Chukchi Seas from 1976 to 2010. Deep-Sea Res II Top Stud Oceanogr 94:68–79. doi:10.1016/j.dsr2.2013.03.012
Grebmeier JM (2012) Shifting patterns of life in the Pacific Arctic and sub-Arctic Seas. Annu Rev Mar Sci 4(1):63–78. doi:10.1146/annurev-marine-120710-100926
Grebmeier JM, Cooper LW, Feder HM, Sirenko BI (2006) Ecosystem dynamics of the Pacific-influenced Northern Bering and Chukchi seas in the Amerasian Arctic. Prog Oceanogr 71(2–4):331–361. doi:10.1016/j.pocean.2006.10.001
Grebmeier JM, Moore SE, Overland JE, Frey KE, Gradinger R (2010) Biological response to recent Pacific Arctic Sea ice retreats. Eos Trans AGU 91(18). doi:10.1029/2010eo180001
Hardy SM, Carr CM, Hardman M, Steinke D, Corstorphine E, Mah C (2011a) Biodiversity and phylogeography of Arctic marine fauna: insights from molecular tools. Mar Biodivers 41(1):195–210. doi:10.1007/s12526-010-0056-x
Hardy SM, Lindgren M, Konakanchi H, Huettmann F (2011b) Predicting the distribution and ecological niche of unexploited snow crab (Chionoecetes opilio) populations in Alaskan waters: a first open-access ensemble model. Integr Comp Biol 51(4):608–622. doi:10.1093/icb/icr102
Hare SR, Mantua NJ (2000) Empirical evidence for North Pacific regime shifts in 1977 and 1989. Prog Oceanogr 47(2–4):103–145. doi:10.1016/s0079-6611(00)00033-1
Hartman O (1969) Atlas of the Sedentariate Polychaetous Annelids from California. Allan Hancock Foundation, University of Southern California, Los Angeles
Haven SB (1971) Effects of land-level changes on intertidal invertebrates, with discussion of post earthquake ecological succession. In: National Research Council, The Great Alaska Earthquake of 1964, Biology. National Academy of Science, Washington, pp 82–126
Hoberg M, Feder H (2002) The macrobenthos of sites within Prince William Sound, Alaska, prior to the Exxon Valdez oil spill. Int Rev Hydrobiol 87(1):25–45
Hollowed AB, Planque B, Loeng H (2013) Potential movement of fish and shellfish stocks from the sub-Arctic to the Arctic Ocean. Fish Oceanogr 22(5):355–370. doi:10.1111/fog.12027
Hood DW, Shiels WE, Kelley EJ (eds) (1973) Environmental studies of Port Valdez. Institute of Marine Science, University of Alaska, Fairbanks
Hubbard JD (1971) Distribution and Abundance of intertidal invertebrates at Olsen Bay in Prince William Sound, Alaska, one year after the 1964 earthquake. In: National Research Council, The Great Alaska Earthquake of 1964, Biology. National Academy of Science, Washington, pp 137–157
Hunt GL, Blanchard AL, Boveng P, Dalpadado P, Drinkwater KF, Eisner L, Hopcroft RR, Kovacs KM, Norcross BL, Renaud P, Reigstad M, Renner M, Skjoldal HR, Whitehouse A, Woodgate RA (2013) The Barents and Chukchi Seas: comparison of two Arctic shelf ecosystems. J Mar Syst 109–110:43–68. doi:10.1016/j.jmarsys.2012.08.003
Jay CV, Fischbach AS, Kochnev AA (2012) Walrus areas of use in the Chukchi Sea during sparse sea ice cover. Mar Ecol Prog Ser 468:1–13. doi:10.3354/meps10057
Kawaguchi Y, Itoh M, Nishino S (2012) Detailed survey of a large baroclinic eddy with extremely high temperatures in the Western Canada Basin. Deep-Sea Res I Oceanogr Res Pap 66:90–102
Kedra M, Gromisz S, Jaskula R, Legezynska J, Maciejewska B, Malec E, Opanowski A, Ostrowska K, Wlodarska- Kowalczuk M, Weslawski JM (2010) Soft bottom macrofauna of an all taxa biodiversity site: Hornsund (77N, Svalbard). Pol Polar Res 31(4):18
Kędra M, Pabis K, Gromisz S, Węsławski J (2013) Distribution patterns of polychaete fauna in an Arctic fjord (Hornsund, Spitsbergen). Polar Biol 36(10):1463–1472. doi:10.1007/s00300-013-1366-9
Kotwicki S, Lauth RR (2013) Detecting temporal trends and environmentally-driven changes in the spatial distribution of bottom fishes and crabs on the eastern Bering Sea shelf. Deep-Sea Res II Top Stud Oceanogr 94:231–243. doi:10.1016/j.dsr2.2013.03.017
Lovvorn JR, Anderson EM, Rocha AR, Larned WW, Grebmeier JM, Cooper LW, Kotts JM, North CA (2014) Variable wind, pack ice, and prey dispersion affect the long-term adequacy of protected areas for an Arctic sea duck. Ecol Appl 24:396–412
Lurgi M, López BC, Montoya JM (2012) Novel communities from climate change. Phil Trans R Soc B Biol Sci 367(1605):2913–2922. doi:10.1098/rstb.2012.0238
Macdonald RW, Carmack EC, McLaughlin FA, Falkner KK, Swift JH (1999) Connections among ice, runoff and atmospheric forcing in the Beaufort Gyre. Geophys Res Lett 26(15):2223–2226. doi:10.1029/1999GL900508
MacGinitie GE (1955) Distribution and ecology of the marine invertebrates of Point Barrow, Alaska. Smithson Misc Collect 128(9):1–201
MacGinitie N (1959) Marine molluscs of point barrow, Alaska. Proceedings of the United States National Museum 109, pp 59–208
Magurran AE (2004) Measuring biological diversity. Blackwell Publishing, Malden
Mantua NJ, Hare SR, Zhang Y, Wallace JM, Francis RC (1997) A Pacific interdecadal climate oscillation with impacts on salmon production. Bull Am Meteorol Soc 78(6):1069–1079
Mooers CNK, Wang J (1998) On the implementation of a three-dimensional circulation model for Prince William Sound, Alaska. Cont Shelf Res 18(2–4):253–277. doi:10.1016/S0278-4343(97)00058-7
Mueter FJ, Litzow MA (2008) Sea ice retreat alters the biogeography of the Bering Sea continental shelf. Ecol Appl 18(2):309–320. doi:10.1890/07-0564.1
Mundy PR, Cooney RT (2005) Physical and biological background. In: Mundy PR (ed) The gulf of Alaska biology and oceanography. Exxon Valdez Oil Spill Trustee Council, Alaska Sea Grant College Program, University of Alaska Fairbanks, pp 15–24
Mundy PR, Spies R (2005) Introduction. In: Mundy PR (ed) The Gulf of Alaska Biology and Oceanography. Exxon Valdez Oil Spill Trustee Council, Alaska Sea Grant College Program, University of Alaska Fairbanks, pp 1–14
Naidu AS, Klein LH (1988) Sedimentation processes. In: Shaw DG, Hameedi MJ (eds) Environmental studies in Port Valdez, Alaska. A basis for management. Springer-Verlag, New York, pp 69–91
National Research Council (1971) The great Alaska earthquake of 1964, biology. National Academy of Science, Washington
National Research Council (1996) The Bering Sea ecosystem. The National Academies Press, Washington, DC
Neal EG, Todd Walter M, Coffeen C (2002) Linking the pacific decadal oscillation to seasonal stream discharge patterns in Southeast Alaska. J Hydrol 263(1–4):188–197. doi:10.1016/s0022-1694(02)00058-6
Nelson RJ, Ashjian C, Bluhm B, Conlan K, Gradinger R, Grebmeier J, Hill V, Hopcroft R, Hunt B, Joo H, Kirchman D, Kosobokova K, Lee S, Li W, Lovejoy C, Poulin M, Sherr E, Young K (2014) Biodiversity and biogeography of the lower trophic fauna of the pacific arctic region – sensitivities to climate change. In: Grebmeier J, Maslawski W (eds) The Pacific Arctic Region ecosystem status and trends in a rapidly changing environment. Springer, Dordrecht, pp 269–336
Norcross BL, Raborn SW, Holladay BA, Gallaway BJ, Crawford ST, Priest JT, Edenfield LE, Meyer R (2013) Northeastern Chukchi Sea demersal fishes and associated environmental characteristics, 2009–2010. Cont Shelf Res 67:77–95. doi:10.1016/j.csr.2013.05.010
Norton D, Feder HM (2006) Mytilus thermophily? Mar Ecol Prog Ser 309:301–303
Ogasawara K (2002) Responses of Japanese Cenozoic molluscs to Pacific gateway events. Rev Mex Cienc Geológicas 19(3):206–214
Olafsson E, Peterson CH, Ambrose W (1994) Does recruitment limitation structure populations and communities of macro-invertebrates in marin soft sediments: the relative significance of pre-and post settlement processes. Oceanogr Mar Biol Annu Rev 32:65–109
Orensanz J, Ernst B, Armstrong DA, Stabeno P, Livingston P (2004) Contraction of the geographic range of distribution of snow crab (Chionoecetes opilio) in the eastern Bering Sea: an environmental ratchet? CalCOFI
Petryashov VV, Vassilenko SV, Voronkov AY, Sirenko BI, Smirnov AV, Smirnov IS (2013) Biogeographical analysis of the Chukchi Sea and adjacent waters based on fauna of some macrobenthos taxa. Invertebr Zool 10(1):49–68
Piepenburg D, Archambault P, Ambrose W, Blanchard A, Bluhm B, Carroll M, Conlan K, Cusson M, Feder H, Grebmeier J, Jewett S, Lévesque M, Petryashev V, Sejr M, Sirenko B, Włodarska-Kowalczuk M (2011) Towards a pan-Arctic inventory of the species diversity of the macro- and megabenthic fauna of the Arctic shelf seas. Mar Biodivers 41(1):51–70. doi:10.1007/s12526-010-0059-7
Pinchuk AI, Coyle KO, Hopcroft RR (2008) Climate-related variability in abundance and reproduction of euphausiids in the northern Gulf of Alaska in 1998–2003. Prog Oceanogr 77(2–3):203–216. doi:10.1016/j.pocean.2008.03.012
Proshutinsky AY, Johnson MA (1997) Two circulation regimes of the wind-driven Arctic Ocean. J Geophys Res Oceans 102(C6):12493–12514. doi:10.1029/97JC00738
Proshutinsky A, Bourke RH, McLaughlin FA (2002) The role of the Beaufort Gyre in Arctic climate variability: seasonal to decadal climate scales. Geophys Res Lett 29(23):2100. doi:10.1029/2002GL015847
Questel JM, Clarke C, Hopcroft RR (2013) Seasonal and interannual variation in the planktonic communities of the northeastern Chukchi Sea during the summer and early fall. Cont Shelf Res 67:23–41. doi:10.1016/j.csr.2012.11.003
Renaud P, Włodarska-Kowalczuk M, Trannum H, Holte B, Węsławski J, Cochrane S, Dahle S, Gulliksen B (2007) Multidecadal stability of benthic community structure in a high-Arctic glacial fjord (van Mijenfjord, Spitsbergen). Polar Biol 30(3):295–305. doi:10.1007/s00300-006-0183-9
Rucker TL (1983) The life history of the intertidal barnacle Balanus balanoides (L.) in Port Valdez, Alaska
Sharma GD, Burbank DC (1973) Geological oceanography. In: Hood DW, Shiels WE, Kelley EJ (eds) Environmental studies of Port Valdez. Institute of Marine Science, University of Alaska, Fairbanks, pp 15–100
Shaw DG, Hameedi MJ (1988) Environmental studies in Port Valdez, Alaska. A basis for management. Springer-Verlag, New York
Sirenko BI, Gagaev SY (2007) Unusual abundance of macrobenthos and biological invasions in the Chukchi Sea. Russ J Mar Biol 33(6):355–364. doi:10.1134/s1063074007060016
Sparks AK, Pereyra WT (1966) Benthic invertebrates of the southeastern Chukchi Sea. In: Wilimovsky NJ, Wolfe JN (eds) Environment of the Cape Thompson Region, Alaska. US Atomic Energy Commission, Oak Ridge, pp 817–838
Stevenson DE, Lauth RR (2012) Latitudinal trends and temporal shifts in the catch composition of bottom trawls conducted on the eastern Bering Sea shelf. Deep-Sea Res II Top Stud Oceanogr 65–70:251–259. doi:10.1016/j.dsr2.2012.02.021
Stroeve JC, Maslanik J, Serreze MC, Rigor I, Meier W, Fowler C (2011) Sea ice response to an extreme negative phase of the Arctic Oscillation during winter 2009/2010. Geophys Res Lett 38(2), L02502. doi:10.1029/2010GL045662
Thompson DWJ, Wallace JM (1998) The Arctic oscillation signature in the wintertime geopotential height and temperature fields. Geophys Res Lett 25(9):1297–1300. doi:10.1029/98gl00950
Wang J, Zhang J, Watanabe E, Ikeda M, Mizobata K, Walsh JE, Bai X, Wu B (2009) Is the dipole anomaly a major driver to record lows in Arctic summer sea ice extent? Geophys Res Lett 36(5), L05706. doi:10.1029/2008GL036706
Wang J, Eicken H, Yu Y, Bai X, Zhang J, Hu H, Wang D-R, Ikeda M, Mizobata K, Overland JE (2014) Abrupt climate changes and emerging ice-ocean processes in the Pacific Arctic Region and the Bering Sea. In: Grebmeier J, Maslawski W (eds) The Pacific Arctic Region ecosystem status and trends in a rapidly changing environment. Springer, Dordrecht, pp 65–99
Wassmann P (2011) Arctic marine ecosystems in an era of rapid climate change. Prog Oceanogr 90:1–17. doi:10.1016/j.pocean.2011.02.002
Weingartner T (2005) Physical and geological oceanography: coastal boundaries and coastal and ocean circulation. In: Mundy PR (ed) The Gulf of Alaska Biology and Oceanography. Exxon Valdez Oil Spill Trustee Council, Alaska Sea Grant College Program, University of Alaska Fairbanks, pp 35–48
Weingartner T, Aagaard K, Woodgate R, Danielson S, Sasaki Y, Cavalieri D (2005) Circulation on the north central Chukchi Sea shelf. Deep-Sea Res II Top Stud Oceanogr 52(24–26):3150–3174. doi:10.1016/j.dsr2.2005.10.015
Weingartner T, Dobbins E, Danielson S, Winsor P, Potter R, Statscewich H (2013) Hydrographic variability over the northeastern Chukchi Sea shelf in summer-fall 2008–2010. Cont Shelf Res 67:5–22. doi:10.1016/j.csr.2013.03.012
Węsławski JM, Kendall MA, Włodarska-Kowalczuk M, Iken K, Kędra M, Legezynska J, Sejr MK (2011) Climate change effects on Arctic fjord and coastal macrobenthic diversity—observations and predictions. Mar Biodivers 41(1):71–85. doi:10.1007/s12526-010-0073-9
Wiegers JK, Feder HM, Mortensen LS, Shaw DW, Wilson VJ, Landis WG (1998) A regional multi-stressor rank-based ecological risk assessment for the fjord of Port Valdez, Alaska. Hum Ecol Risk Assess 4(5):1125–1173
Williams WJ, Shroyer E, Kinney JC, Itoh M, Maslowski W (2014) Shelf-break exchange in the Bering, Chukchi and Beaufort Seas. In: Grebmeier J, Maslawski W (eds) The Pacific Arctic Region ecosystem status and trends in a rapidly changing environment. Springer, Dordrecht, pp 133–165
Wlodarska-Kowalczuk M, Pearson T (2004) Soft-bottom macrobenthic faunal associations and factors affecting species distributions in an Arctic glacial fjord (Kongsfjord, Spitsbergen). Polar Biol 27(3):155–167. doi:10.1007/s00300-003-0568-y
Wlodarska-Kowalczuk M, Kendall MA, Weslawski JM, Klages M, Soltwedel T (2004) Depth gradients of benthic standing stock and diversity on the continental margin at a high-latitude ice-free site (off Spitsbergen, 791N). Deep sea Research Part 1 51:12
Wlodarska-Kowalczuk M, Pearson TH, Kendall MA (2005) Benthic response to chronic natural physical disturbance by glacial sedimentation in an Arctic fjord. Mar Ecol Prog Ser 303:11
Wlodarska-Kowalczuk M, Szymelfenig M, Zajiczkowski M (2007) Dynamic sedimentary environments of an Arctic glacier-fed river estuary (Adventfjorden, Svalbard). II: Meio- and macrobenthic fauna. Estuar Coast Shelf Sci 74(1–2):274–284. doi:10.1016/j.ecss.2007.04.017
Woodgate RA, Aagaard K, Weingartner TJ (2005) A year in the physical oceanography of the Chukchi Sea: moored measurements from autumn 1990–1991. Deep-Sea Res II Top Stud Oceanogr 52(24–26):3116–3149. doi:10.1016/j.dsr2.2005.10.016
Woodgate RA, Weingartner TJ, Lindsay R (2012) Observed increases in Bering Strait oceanic fluxes from the Pacific to the Arctic from 2001 to 2011 and their impacts on the Arctic Ocean water column. Geophys Res Lett 39(24), L24603. doi:10.1029/2012GL054092
Acknowledgments
The Port Valdez Environmental Studies Program was funded by Alyeska Pipeline Service Co. The Chukchi Sea Environmental Studies Program was funded by ConocoPhillips Company, Shell Exploration & Production Co., and Statoil USA E & P, Inc. I thank Dr. Ann Knowlton and two anonymous reviewers for reviewing the manuscript. Taxonomic consultants included Ken Coyle (University of Alaska Fairbanks; amphipods and crustaceans), Sarah Gerken (University of Alaska Anchorage; cumaceans), Steven Hulsman (SGH Group; other taxa), Nora Foster (NRF Taxonomic Services; mollusks), R. Eugene Ruff (Ruff Systematics; polychaetes), and Chip Barrett (polychaetes), Matt Hill (other taxa), and Angela Eagleston (molluscs) of EcoAnalysts, Inc. I thank the many technicians who assisted with these projects. The viewpoints expressed in this manuscript are those of the author alone, and do not necessarily reflect the views of the research sponsors.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 280 kb)
Rights and permissions
About this article
Cite this article
Blanchard, A.L. Variability of macrobenthic diversity and distributions in Alaskan sub-Arctic and Arctic marine systems with application to worldwide Arctic Systems. Mar Biodiv 45, 781–795 (2015). https://doi.org/10.1007/s12526-014-0292-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12526-014-0292-6