Abstract
The Chukchi and northern Bering Seas, which serve as the only gateway for Pacific water entering the Arctic Ocean, have experienced profound declines in sea ice cover as well as increases in oceanic temperature. In this region, fish responses to climate change are of widespread concern, but time-series data are limited because of the harsh environment. Four Chinese National Arctic Research Expeditions with stations spanning substantial latitudes in both the Chukchi and northern Bering Seas were conducted in alternating summers (July–September, every 2 years) from 2010 to 2016, indicating that demersal fish had large spatial and interannual fluctuations in species composition and biodiversity. A total of 58 fish species were identified, including several taxa that were rarely sampled previously or repeatedly encountered in the border regions. The significant distributional records of these taxa may correspond to the probabilities that species have extended beyond the documented distribution limits and the interchanges between the Pacific and Atlantic faunas are promoted under the influence of climate change. The fish biodiversity patterns were quantitatively described using seven indices such as species richness and taxonomic diversity indices. The spatial and temporal variations in species composition and biodiversity may be related to differences and interannual oscillations in water mass and tight pelagic-benthic coupling. Additionally, our analyses suggested that fish communities in the Chukchi Sea, where the consequences of climate change seem to be more serious because of the simpler food web and the proximity to the central Arctic Ocean, appeared to be less affected by the currently changing environment than those in the northern Bering Sea. Time-series surveys would establish a foundation for distinguishing between acclimation to climate change and natural fluctuations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Arctic ecosystem is an indicator of global climate change (Carmack and Wassmann 2006; Post et al. 2013). A battery of physical, chemical, and biological variations caused by profound sea-ice retreat and rising ocean temperatures in recent decades known as the Arctic Rapid Change (Post et al. 2013; Smith et al. 2017; Chen et al. 2018) is thought to strongly influence all constituent components of the Arctic marine ecosystem (Pörtner and Farrell 2008; Post et al. 2013; Grebmeier et al. 2015; Mueter et al. 2017). Among these, the impacts on fishes have been widely reported, including changes in species distributions (Perry et al. 2005; Christiansen et al. 2016; Spies et al. 2019), communities (Fossheim et al. 2015; Frainer et al. 2017; Iken et al. 2019), biomass (Stevenson and Lauth 2012, 2019), and biogeography (Mueter and Litzow 2008; Mueter et al. 2009).
The Chukchi (CHS) and northern Bering Seas (NBS) connect the Arctic and Pacific Oceans through the Bering Strait, comprising a biogeographically large marine ecosystem that is defined as the Pacific Arctic Region (PAR) (Mecklenburg et al. 2016; Fish Stock Experts of the Central Arctic Ocean 2018). The PAR would be a preferred area for exploring the ichthyofaunal responses to the Arctic Rapid Change because it has experienced some of the largest increases in surface air and sea surface temperatures (Richter et al. 2019) as well as the declines in the extent and duration of sea ice cover on earth (Post et al. 2013; Stabeno et al. 2019). Evaluating the fish responses depends on how well we understand the current situation and whether we can distinguish acclimation from natural fluctuations that could only be achieved through long-term comprehensive investigations (Iken et al. 2019; Stevenson and Lauth 2019).
Ichthyofaunal knowledge obtained from field surveys in the PAR is limited because of the hostile environment, and the investigations are usually restricted to the summer when seasonal sea ice has melted (Norcross et al. 2013; Mecklenburg and Steinke 2015). After entering the twenty-first century, when the reduced duration and spatial extent of sea ice objectively lowered the logistical requirements, numerous modern and comprehensive investigations were conducted, including the Russian-American Long-Term Census of the Arctic (RUSALCA) in 2004, 2009, and 2012 (Mecklenburg et al. 2011, 2014; Mecklenburg and Steinke 2015, 2016), Studies of Pacific Scientific Research Fisheries Centre (TINRO Centre) in 2008, 2010, and 2012 (Gavrilov and Glebov 2013), the Chukchi Sea Environmental Studies Programme (CSESP) in 2009 and 2010 (Norcross et al. 2013), the Arctic Ecosystem Integrated Surveys (Arctic EIS) in 2007 and 2012 (Logerwell et al. 2015; Mueter et al. 2017), and the Arctic Marine Biodiversity Observing Network (AMBON) in 2015 (Iken et al. 2019). Baselines for species compositions and distributions, biodiversity, and interactions with environmental factors are established but still insufficient, and time-series surveys covering the both seas are scarce. Climate change would bring a series of uncertain consequences to the marine ecosystem; hence, the investigation should be as comprehensive as possible to address this condition.
Four surveys of demersal fish in the Chinese National Arctic Research Expedition (CHINARE) were conducted from 2010 to 2016 in the PAR, and the species compositions, distributions, and biodiversity patterns were analyzed. Species composition is the basis for understanding the characteristics of a fish community. First, a shift in distribution is one of the most intuitive and observable responses, but making this determination requires that we have a good knowledge of species composition across the region. CHINARE was expected to find additional distributions because it was one of the most extensive surveys. Thus, records of species that had seldom been encountered previously or were sampled beyond previously known distribution limits were synthesized. Second, seven of the most commonly used indices were selected to quantify the biodiversity patterns that reflected the productivity, stability, function, and structure of the community (Liu and Ma 2002; Palumbi et al. 2009; Duffy et al. 2013; Wang et al. 2018). Responses of the fish assemblage to changing environment could not always be directly observed. Those empirical indices that are widely used in monitoring and evaluating the impacts of accidents and government policies on marine ecosystems (Warwick and Clarke 1998; Leonard et al. 2006; Wang et al. 2018) could provide specific values, allowing for quantitative description and comparison, and have rarely been applied in the PAR. An opportunity to research the spatial and interannual differences in species composition and biodiversity between the sides of the Bering Strait in the ablation season was provided by the almost simultaneous surveys in both the CHS and NBS.
Materials and methods
Survey area
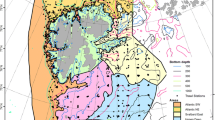
Four surveys were undertaken in the PAR aboard the icebreaker Xue Long (in English, this means “Snow Dragon”) in the summers (July–September) of 2010, 2012, 2014, and 2016 (Fig. 1). A total of 114 stations were successfully monitored (11 stations in the CHS and 12 stations in the NBS with damaged nets were excluded), of which 102 stations were located on the wide and shallow continental shelves (depth < 200 m): 52 stations in the CHS shelf were mainly along the 170°W axis from 65.67°N to 74.61°N; 50 stations in the northern NBS shelf were concentrated around St. Lawrence Island (ranging from 60.65°N and 178.45°W to 64.62°N and 167.12°W). The remaining 12 stations were on the continental slopes (depth ranging from 226 m to 1161 m).
Map of 31 bottom trawls in 2010, 27 trawls in 2012, 28 trawls in 2014, and 28 trawls in 2016 in the Chukchi and northern Bering Seas. Overall, 89.47% of those trawls were located on the continental shelves (depth < 200 m), and nine trawls were located on the Chukchi Plateau in 2010, 2014, and 2016, and three trawls were located on the Bering Sea continental slope in 2012 and 2014
Specimen collection
Fishes were sampled with bottom trawls because of their predominant association with the sea floor in the Arctic (Mecklenburg et al. 2016). Three types of gear were operated in 2010: (1) a 6.5-m triangular net (a triangular structure made of steel with a 2.2-m-wide, 0.65-m-tall opening and a 20-mm-mesh codend liner), (2) a 9.0-m French-type net (with a 2.5-m-wide, 0.5-m-tall opening and a 10-mm-mesh codend liner), and (3) a 3.0-m otter net (with a 1.6-m-wide, 0.5-m-tall opening and a 20-mm-mesh codend liner) (Lin et al. 2012, 2014). In 2012–2016, only a triangular net (the same specifications as above) was used because of its manoeuvrability and durability. Towing speeds were approximately 2.5–3.5 knots, and the distances for each trawl ranged from 0.7 km to 4.4 km. Salinity and temperature were measured by a Seabird CTD Model SBE 911 (Bellevue Sea-Bird Scientific). All specimens were frozen immediately and brought to the laboratory for identification. Species that could only be classified to the genus level were used as the lowest taxon for calculating the biodiversity indices.
Data analysis
The area swept calculated by trawl distance and the net opening width was used to determine catch per unit effort (CPUE) as the number of individuals per square kilometer (ind. km−2). Zero catches were included in the estimation of mean CPUE for all fish taxa.
The following seven biodiversity indices were used to characterize the biodiversity patterns: Margalef species richness index, Shannon species diversity index, Pielou evenness index (Pielou 1975; Ludwig et al. 1988), taxonomic diversity index, taxonomic distinctness index, average taxonomic distinctness index, and variation in taxonomic distinctness index (Warwick and Clarke 1995; Clarke and Warwick 1998, 2001) (Table 1). A species accumulation curve (SAC) was used to assess the properties of diversity for community data and also to illustrate the adequacy of sampling in the survey region. The sampling intensity was deemed sufficient if the SAC smoothed out as the amount of effort increased (Gotelli and Colwell 2001).
Variation in the numbers of species and individuals and biodiversity among years, seas, and gears was examined by statistical tests based on comparisons of means and using the following criteria: whether the data conformed to a normal distribution was checked by Shapiro-Wilk test (accepted if p > 0.05, Royston 1982); homogeneity of variance was checked using Bartlett test (accepted if p > 0.05, Bartlett 1937); comparison between two groups was made using independent two-tailed unpaired t-tests if they conformed to the normal distribution and the Wilcoxon rank sum test (WRS test, David 1972) if they did not; comparison of more than two groups was made by one-way analysis of variance (ANOVA) (Chambers et al. 1992) followed by further pairwise comparisons via Tukey HSD test (Yandell 1997) if they conformed to normal distribution with homogeneous variance and the Kruskal-Wallis test (Hollander and Douglas 1973) followed by further pairwise comparison test via WRS test if they did not (Fig. 2). The four taxonomic indices are less influenced by sampling effort, but the indices of D, H, and J are to some extent sample-dependent (Warwick and Clarke 1995, 1998). Consequently, the analyses for towing distance and numbers of species and individuals between seas and years also used the above processes. Two disclaimers are required. First, the comparison between the CHS and NBS only used stations on the continental shelves. Those stations > 200 m in depth in the CHS Plateau had reached the intersection area of the Pacific, Atlantic, and Arctic water masses (Grebmeier et al. 2006, 2015). Second, the numbers of species and individuals sampled with the three types of gear employed in 2010 were tested to identify the impact of sampling methods, but the number of stations was too small to make separate calculations between the seas. Biodiversity data, difference tests, and figures used Ocean Data View (Schlitzer 2019) and the R project (R Core Team, 2020) with the package “vegan” (Oksanen et al. 2019) and “ggplot2” (Wickham 2016).
Results
Species composition and significant distributional records
A total of 2575 individuals in the PAR were identified to 58 species, and another 22 individuals were identified to genus level. The fish assemblage was mostly composed of the families sculpins (Cottidae), snailfishes (Liparidae), eelpouts (Zoarcidae), pricklebacks (Stichaeidae), and righteye flounders (Pleuronectidae) (Fig. 3) (species list in Online Resource 1). In the CHS, 38 species (65.52% of 58 species) sampled in 61 trawls were affiliated to 26 genera, 11 families, and 6 orders, with the largest number of species being recorded in 2010 (24 species). In the NBS, 48 species (82.76% of 58 species) sampled in 53 trawls were affiliated to 31 genera, 13 families, and 7 orders, with the largest numbers of species being recorded in 2010 (29 species).
Range extension was defined as species extending their distribution beyond formerly known (latitudinal) limits (Mecklenburg and Steinke 2015; Thorsteinson and Love 2016). According to this definition, the significant distributional records were classified into two groups: (1) species that were well known to be widespread in the PAR and/or the North Pacific, and CHINARE had the northernmost records or near to previously announced northernmost limits; (2) species were well known to be widespread in the Atlantic Arctic and/or Circumpolar regions but had rarely been sampled in the PAR (Tables 2 and 3).
Taxa on the continental shelves
Totals of 1136 (37 taxa) and 1239 (51 taxa) individuals were collected at 52 and 50 stations on the CHS and NBS continental shelves, respectively. The analyses of SACs suggested that the trend lines of PAR, CHS, and NBS gradually flattened out (Fig. 4), implying that the sampling intensity was sufficient. In 2010, there were few differences in the species composition of the three gear types: 14 species collected by 5 French nets were also recorded in another 2 nets, only 2 species were unique among the 20 species collected by 11 otter nets, and 10 species were unique among the 29 species collected by 13 triangular nets. The numbers of species (ANOVA, F2, 26 = 0.19, p = 0.8270) and individuals (Kruskal–Wallis test, H2 = 1.71, p = 0.4245) were not significantly different among the three gear types available as well.
Species accumulation curves (SACs) with 95% confidence intervals for continental shelves of the Pacific Arctic Region (PAR, gray shade, a total of 59 taxa in 102 trawls), Chukchi Sea (CHS, green shade, a total of 37 taxa in 52 trawls), and northern Bering Sea (NBS, red shade, a total of 51 taxa in 50 trawls)
The similarities of the same taxa among surveys and seas were relatively low. The percentages of shared taxa in CHS and NBS to the total of each year were 39.39% (13 of 33 taxa), 33.33% (9 of 27 taxa), 29.73% (11 of 37 taxa), and 34.48% (10 of 29 taxa) in 2010, 2012, 2014, and 2016, respectively. The percentages of shared taxa in adjacent surveys (2010 and 2012, 2012 and 2014, 2014 and 2016) to the total were 30.43%, 33.33%, and 34.48% in the CHS and 30.00%, 33.33%, and 26.32% in the NBS, respectively. There were five species, Arctic alligatorfish (Aspidophoroides olrikii), polar cod (Boreogadus saida), Arctic staghorn sculpin (Gymnocanthus tricuspis), Bering flounder (Hippoglossoides robustus), and gelatinous seasnail (Liparis fabricii), that were encountered in all cruises of the CHS and seven species, Pacific sand lance (Ammodytes hexapterus), Arctic alligatorfish, Arctic staghorn sculpin, Bering flounder, spatulate sculpin (Icelus spatula), gelatinous seasnail, and Alaska plaice (Pleuronectes quadrituberculatus), that were collected every year in the NBS.
The most abundant taxa varied among surveys (Fig. 5). In the CHS, these were shorthorn sculpin (Myoxocephalus scorpius) in 2010 (3589.09 ± 10,166.35 ind. km−2 [SD, n = 11]), Arctic staghorn sculpin in 2012 (1803.95 ± 4080.47 ind. km−2 [SD, n = 13]) and 2016 (1454.67 ± 2091.85 ind. km−2 [SD, n = 14]), and Bering flounder in 2014 (2161.77 ± 3011.11 ind. km−2 [SD, n = 14]). In the NBS, these were Bering flounder in 2010 (2298.21 ± 3724.60 ind. km−2 [SD, n = 18]), Arctic staghorn sculpin in 2012 (366.25 ± 1198.11 ind. km−2 [SD, n = 12]) and 2016 (1541.24 ± 3045.96 ind. km−2 [SD, n = 9]), and gelatinous seasnail in 2014 (2003.80 ± 5191.46 ind. km−2 [SD, n = 11]). Summarily, the Bering flounder, Arctic staghorn sculpin, and gelatinous seasnail were the most abundant species in the NBS, and the Arctic staghorn sculpin, polar cod, and Bering flounder are the ones in the CHS during the investigations.
Interannual characteristics of biodiversity on the continental shelves
In the NBS, the numbers of species (ANOVA, F3, 46 = 4.65, p = 0.0064) and individuals (Kruskal-Wallis test, H3 = 12.09, p = 0.0071) and H (Kruskal-Wallis test, H3 = 9.03, p = 0.0289) had significant differences among years. In contrast, there was no significant difference among surveys in the CHS (p > 0.05, H by ANOVA; the remaining indices by Kruskal-Wallis tests). The towing distances were largely dependent on sea ice conditions, with significant differences among surveys in both the CHS (Kruskal-Wallis test, H3 = 13.67, p = 0.0034) and the NBS (Kruskal-Wallis test, H3 = 9.31, p = 0.0255), and according to further pairwise comparisons they were 2010 and 2014 (WRS test, W = 121.0, p = 0.0168), 2012 and 2014 (WRS test, W = 146.0, p = 0.0080) in the CHS, and 2010 and 2016 (WRS test, W = 136.0, p = 0.0050), 2012 and 2016 (WRS test, W = 100.0, p = 0.0012) in the NBS. In summary, the indices between years with significantly different towing distance had no significant difference, implying that the impact of towing distance on biodiversity indices may be negligible because of the randomness in our surveys.
The biodiversity indices fluctuated interannually on both CHS and NBS shelves, and their mean values had a consistent trend of “high-low-high-low.” That is, they were higher in 2010 and 2014 than in 2012 and 2016 (Tables 4 and 5). It appears that 2010 and 2014 were instances of a high biodiversity pattern and 2012 and 2016 were instances of a low biodiversity pattern; thus, we compared the fish assemblages between the two biodiversity patterns. The towing distances between the patterns no longer had a significant difference in either the CHS (WRS test, W = 254.0, p = 0.1274) or the NBS (WRS test, W = 330.5, p = 0.6161). However, indices with significant difference clearly increased, including numbers of species (WRS test, W = 464.5, p = 0.0192) and individuals (WRS test, W = 459.0, p = 0.0264), D (WRS test, W = 456.0, p = 0.0302), ∆* (WRS test, W = 481.5, p = 0.0077), and ∆+ (WRS test, W = 474.5, p = 0.0112) in the CHS and the numbers of species (t-test, t48 = 3.50, p = 0.0010) and individuals (WRS test, W = 479.0, p = 0.0006), D (WRS test, W = 420.5, p = 0.0229), H (WRS test, W = 440.0, p = 0.0078), and ∆* (WRS test, W = 411.0, p = 0.0368) in the NBS (Fig. 6) (additional results of difference tests are given in Online Resource 2).
Spatial characteristics of biodiversity on the continental shelf
Several indices reflected the dissimilarities in biodiversity between the CHS and NBS shelves, especially in 2010 and 2014. D, H, ∆*, and ∆+ (p < 0.05, WRS test) in 2010 and ∆* and ∆+ (p < 0.05, WRS test) in 2014 were significantly different, while towing distance (p > 0.05; 2010, 2012, and 2014 by WRS test, 2016 by t-test), numbers of species (p > 0.05; 2010, 2012, and 2016 by t-test, 2014 by WRS test) and individuals (p > 0.05, WRS test) were not among all cruises between the CHS and NBS. In other words, only in years of high biodiversity pattern could significant differences be detected in the biodiversity indices. Considering all trawls sampled from 2010 to 2016, the number of species and six of seven biodiversity indices (J was the only exception) were significantly different between the CHS and NBS shelves (p < 0.05, WRS test; Fig. 7).
The difference between the neighboring seas was also reflected by ∆+ that represented an average taxonomic path length. For 37 taxa in the CHS, the value was 86.87, and for 51 taxa in the NBS, the value was 73.37. The value of ∆+ at each station in each year tended to be lower than expected in the years after 2010 in the NBS (Fig. 8). The biodiversity index J showed no significant differences in all years examined and was the most insensitive index. In addition, species composition was also different between the seas. Similarity of same species (ratio of the shared species to the total) under the same biodiversity pattern (2010 and 2014: 45.00%, 2012 and 2016: 56.00%) was larger than under different biodiversity patterns (27.03%–34.29%) in the NBS, but it was not in the CHS (2010 and 2014: 31.25%, 2012 and 2016: 33.33%, others: 23.33–46.88%).
Plots of ∆+ against the number of species (stations of “∆+ = 0” are not shown) on the Chukchi Sea continental shelf (CHS, blue) and northern Bering Sea continental shelf (NBS, red). Straight lines are the expected values in the CHS (86.87) and NBS (73.37), and the curves are the 95% confidence funnels based on 51 taxa recorded in the NBS and 37 taxa in the CHS
Characteristics of bottom temperature and salinity
Bottom temperature and salinity were collected from a total of 210 stations during our investigations (60.70°N–75.43°N; 159.71°W–178.49°W), including 71 stations in 2010, 51 in 2012, 47 in 2014, and 41 in 2016. There are four major currents in the survey region: the warm (2–13 °C) and fresh (salinity < 31.8) Alaskan Coastal Current following the Alaskan coast northward to the Barrow Canyon; the cold (< 0 °C) and marine saline (32.8–33.2) Anadyr Water and warm (> 0 °C) and moderately saline (~ 32.5) Bering Water, emanating into two branches in the CHS from the central and western Bering Sea with higher levels of nutrients, and the warm (> 0 °C), highly saline (> 34), and deep (> 200 m) Atlantic Water (Grebmeier et al. 2006, 2015; Smith et al. 2017; Thorsteinson and Love 2016). Our data suggested that bottom temperature and salinity typically varied between seas and years, and water flowing through the Bering Strait had a higher bottom temperature in 2010, 2014, and 2016 and lower salinity in 2014 and 2016 (Fig. 9).
Discussion
Our results are consistent with previous studies confirming that fishes sampled in the PAR are dominated by sculpins, snailfishes, eelpouts, pricklebacks, and righteye flounders (Gavrilov and Glebov 2013; Mecklenburg and Steinke 2015). Most notably, there are major similarities in the composition of the fish community across studies despite using different type of sampling gear. The shared species in the CHINARE (58 species) and surveys of RUSALCA (54 species) mainly in Russian waters in 2004, 2009, and 2012 (Mecklenburg and Steinke 2015), Shelf Habitat and Ecology of Fish and Zooplankton project (30 species) in the northeastern Chukchi Sea in 2013 (Logerwell et al. 2018), CSESP (29 species) in three closely spaced areas in the northeastern Chukchi Sea during 2009–2010 (Norcross et al. 2013), and the VNIRO Transarctic expedition (42 species) in western Chukchi Sea in 2019 (Orlov et al. 2020) reached 75.93%, 83.33%, 86.21%, and 71.43% of their total species, respectively. Both these high similarities and the results of SAC implied the effectiveness of the sampling and that the absence of a species in a year may be due to its low abundance in the summer.
Significant distributional records in the PAR
Establishing baselines for species composition and distribution is especially important for the PAR, as it is currently experiencing the most dramatic climate change. The PAR had attracted scientific interest in syntheses of fish inventories (Chernova 2011; Maslenikov et al. 2013; Christiansen and Reist 2013; Datsky 2015a, b; Thorsteinson and Love 2016; Mecklenburg et al. 2016, 2018; Orlov et al. 2020), and the CHINARE had the advantage of latitudinal range.
We found some species with the northernmost records or near the northern distribution limits. As the single gateway for Pacific boreal marine fauna entering the Arctic, the Bering Strait is dominated by northward flowing waters with essential sources of heat, freshwater, and nutrients due to a sea level gradient (Grebmeier et al. 2006, 2015; Mueter et al. 2017). This provides naturally favorable conditions for the northward movement of fish, because ocean warming and sea ice retreat could create living spaces for organisms in the temperate and sub-Arctic ecosystems to enter the sub-Arctic and Arctic regions (Mueter and Litzow 2008; Cheung et al. 2009; Hollowed et al. 2013). Moreover, it appears that the populations in marginal habitats must be maintained by migration from the core, because they are expected to be less abundant and to have lower survival and reproductive rates than those in core habitats (Kawecki 2008; Spies et al. 2019). Hence, the multiple discovery of a species at known boundary areas may represent its stable colonization in the new habitats, indicating the potential for northward shift.
We found some Atlantic species having few records in the PAR. This may be a sign of increasing interchanges between fishes in the Atlantic and Pacific sectors. The Chukchi Plateau is the intersection area of Atlantic and Pacific waters, and thus it could be expected that fish encountered in the region are also distributed in the Atlantic Arctic waters (Mecklenburg et al. 2014). Now, however, fish interchanges between Atlantic and Pacific sectors are being promoted by climate change (Wisz et al. 2015). A dramatic environmental change, causing the Arctic fish communities to be rapidly replaced by boreal species, was observed in the Atlantic Arctic from 2004 to 2012 (Wiedmann et al. 2014; Wisz et al. 2015; Frainer et al. 2017). The timing was coincidental with fish surveys of CHINARE, for it took the warming Atlantic waters approximately 6–8 years to enter the Chukchi borderland from the Barents Sea and Fram Strait (Pickart 2010; Mecklenburg et al. 2014). This may help surveys in the PAR to find more Atlantic species. However, in 2012 when the Bering Strait was dominated by cold and moderately saline water that was most likely from the Anadyr Water, no such species were recorded. Therefore, the distribution of Atlantic species in the PAR might also be related to the intensity of northward Pacific waters.
Distribution shifts could be regarded as a part of constant movement, as it is widely thought that globally distributed fishes sharing common centers of origin and dispersal is a dynamic process (Briggs 2003). Old groups of Arctic fish were eliminated during the rapid cooling of the Middle Miocene, and new groups of sculpins, snailfishes, eelpouts, pricklebacks, and righteye flounders that represent a large fraction of fish in our study, together with the poachers (Agonidae) and Salmonidae, originated from the Northern Pacific (Briggs 2003; Mecklenburg et al. 2011; Christiansen and Reist 2013). Accommodation is essential for speciation and changes in biogeography (Briggs 2010). Fish distributions are influenced by many factors, and different fish species as well as different sex and age groups often have different environmental preferences (Mueter et al. 2017; Stevenson and Lauth 2019). Nowadays, northward Pacific waters and sea-ice melting are strongly influenced by climate change (Logerwell et al. 2018). Consequently, these significant distributional records may represent shifts in the direction and rate of the constant movement of fish assemblages affected by climate change. This may have multitude impacts on global fish distribution patterns because of the more rapid speciation rate for marine fish in polar regions than expected (Rabosky et al., 2018).
Spatiotemporal variations in the fish assemblage on both continental shelves
The species composition and biodiversity pattern varied between the seas and years. The spatial difference was reflected by several lines of evidence: first, the same species in both seas had low proportions (around 30%) of the total of each year; second, in the NBS there were more species recorded, longer step length between hierarchical levels on average with higher volatility, and a concentrating trend of ∆+ being less than the expected value; third, in the CHS, unlike in the NBS, no index had a significant difference among years, possibly implying a more stable community structure and fewer disturbed factors at present (Warwick and Clarke 1995; Leonard et al. 2006). In addition to the explanation that the differences between the CHS and NBS are affected by differently hydrographic and biological characteristics of water masses (Grebmeier et al. 2006; Woodgate et al. 2015), the narrow (~ 85 km) and shallow (~ 50 m) Bering Strait is also a key factor for substantial numbers of fishes being unable to cross this geographical barrier (Mecklenburg et al. 2018). However, the top abundant species in both seas included Arctic staghorn sculpin, polar cod, shorthorn sculpin, Bering flounder, gelatinous seasnail, and slender eelblenny (Lumpenus fabricii), but not the most abundant walleye pollock (Theragra chalcogrammus) in Russian water (Orlov et al. 2020), showing a major similarity, and this situation had been maintained for many years (Cui et al. 2009; Norcross et al. 2013; Mecklenburg and Steinke 2015). Thus, it was more likely that the taxa with lesser abundance had greater spatial and interannual variation.
Geographically, the CHS is closer to the central Arctic Ocean, where the ecosystem is more vulnerable because of the simpler trophic interaction structure (Mueter et al. 2009); fortunately, it was less affected than NBS according to our analyses.
The consistent trend in the mean values of all indices across years as well as the increase in the number of indices with statistically significant difference between high and low biodiversity patterns in both the CHS and NBS indicated obvious interannual variation. Nevertheless, we were not sure that this variation was part of a long-term trend or rather was a short-term phenomenon caused by interannual fluctuations in the climate regime and water properties, which were well known for the glacial oscillations, and the extent and prominence of Pacific waters advected through the Bering Strait (Mueter and Litzow 2008; Stabeno et al. 2012; Stevenson and Lauth 2012, 2019; Pisareva et al. 2015). According to our results, there was no obvious correspondence between biodiversity patterns and bottom temperature or salinity as we expected; for example, the biodiversity was higher at higher temperatures. Alternatively, there may be indirect relationships: under the tight pelagic-benthic coupled ecosystem in the PAR, any changes in the components of the ecosystem that are susceptible and vulnerable to environmental change, such as the size class of phytoplankton (Danielson et al. 2016), the biomass and abundance of zooplankton (Ershova et al. 2015; Sigler et al. 2017), and the recruitment of fish population (Mueter et al. 2009; Hollowed et al 2012), could be quickly transmitted to the benthic food web (Mueter et al. 2017). It has been hypothesized that ecosystems in the PAR are shifting from tight pelagic-benthic coupling to systems with more pelagic fishes (Grebmeier et al. 2006; Post et al. 2013; Mueter et al. 2017), which may disrupt the tight pelagic-benthic coupling and then buffer the direct impacts on demersal fish. If so, the biodiversity would experience a decrease in interannual fluctuations.
The fish biodiversity patterns in the PAR during the survey period are described through seven indices, suggesting a low level of biodiversity in the PAR. This has been explained by several hypotheses, such as the decreasing number of species with increasing latitude (Mittelbach et al. 2007), the incident solar energy and temperature, and the overall instability of the climate (Currie et al. 2004; Stevenson and Lauth 2012). Therefore, the ecosystem of the PAR may be vulnerable to external influences. Understanding biodiversity patterns is essential to developing conservation strategies and monitoring conservation goals (Gaston 2000), and a proper level of biodiversity can promote productivity and secure multiple functions of the ecosystem (Palumbi et al. 2009; Duffy et al. 2013; Iken et al. 2019). However, species composition and distribution are the basis of diversity, and if the several significant distributional records we have observed do represent shifts in species distribution under the influence of climate change, then future changes in diversity are not yet known. Unfortunately, our study was limited by an inadequate number of stations, a factor that did not allow us to make parametric tests or to distinguish between seas when assessing the different nets. Fish surveys by CHINARE are continuing, and we should have the chance to increase our research effort in the PAR.
Data availability
Electronic supplementary materials include species lists and results of analysis.
Code availability
Not applicable.
References
GBIF (2021) Global biodiversity information facility. https://www.gbif.org/. Accessed 10 April 2021.
Bartlett MS (1937) Properties of sufficiency and statistical tests. P Roy Soc Lond A Mat 160:268–282. https://doi.org/10.1098/rspa.1937.0109
Briggs JC (2003) Marine centres of origin as evolutionary engines. J Biogeogr 30:1–18. https://doi.org/10.1046/j.1365-2699.2003.00810.x
Briggs JC (2010) Marine biology: the role of accommodation in shaping marine biodiversity. Mar Biol 157:2117–2126. https://doi.org/10.1007/s00227-010-1490-9
Carmack AE, Wassmann BP (2006) Food webs and physical-biological coupling on pan-arctic shelves: unifying concepts and comprehensive perspectives. Prog Oceanogr 71:446–477. https://doi.org/10.1016/j.pocean.2006.10.004
Chambers JM, Freeny A, Heiberger RM (1992) Statistical models in S, chapter 5: analysis of variance; designed experiments. Wadsworth & Brooks/Cole, Boca Raton
Yandell BS (1997) Practical data analysis for designed experiments. Chapman & Hall, Atlanta
Chen JF, Jin HY, Bai YC, Zhuang YP, Li HL, Li YJ, Ren J (2018) Marine ecological and environmental responses to the Arctic rapid change. Hai Yang Xue Bao 40:22–31. https://doi.org/10.3969/j.issn.0253-4193.2018.10.003
Cheung WW, Lam VW, Sarmiento JL, Kearney K, Waston R, Pauly D (2009) Projecting global marine biodiversity impacts under climate change scenarios. Fish Fish 10:235–251. https://doi.org/10.1111/j.1467-2979.2008.00315.x
Christiansen JS, Reist JD (2013) Arctic biodiversity assessment chapter 6: fishes. Conservation of Arctic Flora and Fauna, Akureyri
Christiansen JS, Bonsdorff E, Byrkjedal I, Fevolden SE, Karamushko OV, Lynghammar A, Mecklenburg CW, Møller PDR, Nielsen J, Nordström MC, Præbel K, Wienerroither RM (2016) Novel biodiversity baselines outpace models of fish distribution in Arctic waters. Sci Nat 103:8. https://doi.org/10.1007/s00114-016-1332-9
Clarke KR, Warwick RM (1998) A taxonomic distinctness index and its statistical properties. J Appl Ecol 35:523–531. https://doi.org/10.1046/j.1365-2664.1998.3540523.x
Clarke KR, Warwick RM (1999) The taxonomic distinctness measure of biodiversity: weighting of step lengths between hierarchical levels. Mar Ecol Prog Ser 184:21–29
Clarke KR, Warwick RM (2001) A further biodiversity index applicable to species list: variation in taxonomic distinctness. Mar Ecol Prog Ser 216:265–278. https://doi.org/10.3354/meps216265
Cui XH, Grebmeier JM, Cooper LW, Lovvorn JR, North CA, Seaver WL, Kolts JM (2009) Spatial distributions of groundfish in the northern Bering Sea in relation to environmental variation. Mar Ecol Prog Ser 393:147–160. https://doi.org/10.3354/meps08275
Currie DJ, Mittelbach GG, Cornell HV, Field R, Guégan JF, Hawkins BA, Kaufman DM, Kerr JT, Oberdorff T, O’Brien TE, Turner JRG (2004) Predictions and tests of climate-based hypotheses of broad–scale variation in taxonomic richness. Ecol Lett 7:1121–1134. https://doi.org/10.1111/j.1461-0248.2004.00671.x
Danielson SL, Eisner L, Ladd C, Mordy C, Sousa L, Weingartner TJ (2016) A comparison between late summer 2012 and 2013 water masses, macronutrients, and phytoplankton standing crops in the northern Bering and Chukchi Seas. Deep-Sea Res Pt II 135:7–26. https://doi.org/10.1016/j.dsr2.2016.05.024
Datsky AV (2015a) Fish fauna of the Chukchi Sea and perspectives of its commercial use. J Ichthyol+ 55:185–209
Datsky AV (2015b) Ichthyofauna of the Russian exclusive economic zone of the Bering Sea: 1 Taxonomic diversity. J Ichthyol+ 55:792–826
David FB (1972) Constructing confidence sets using rank statistics. J Am Stat Assoc 67:687–690. https://doi.org/10.2307/2284469
Duffy JE, Amaral-Zettler LA, Fautin DG, Paulay G, Rynearson TA, Sosik HM, Stachowicz JJ (2013) Envisioning a marine biodiversity observation network. Bioscience 63:350–361. https://doi.org/10.1525/bio.2013.63.5.8
Ershova EA, Hopcroft RR, Kosobokova KN (2015) Inter-annual variability of summer mesozooplankton communities of the western Chukchi Sea: 2004–2012. Polar Biol 38:1461–1481. https://doi.org/10.1007/s00300-015-1709-9
Fish Stock Experts of the Central Arctic Ocean (FiSCAO) (2018) Final report of the fifth meeting of Scientific Experts on Fish Stocks in the Central Arctic Ocean. Ottawa
Fossheim M, Primicerio R, Johannesen E, Ingvaldsen RB, Aschan MM, Dolgov AV (2015) Recent warming leads to a rapid borealization of fish communities in the Arctic. Nat Clim Change 5:673
Frainer A, Primicerio R, Kortsch S, Auneb M, Dolgovc AV, Fossheimd M, Aschana MM (2017) Climate-driven changes in functional biogeography of Arctic marine fish communities. P Natl Acad Sci USA 114:12202–12207. https://doi.org/10.1073/pnas.1706080114
Gaston KJ (2000) Global patterns in biodiversity. Nature 405:220–227. https://doi.org/10.1038/35012228
Gavrilov GM, Glebov II (2013) Bottom ichthyocene of the Chukchi shelf in the Russian economic zone of the Bering Sea on the results of studies of «TINRO Centre» in 2008, 2010 and 2012. Advances in Current Natural Sciences 7:13–17 (In Russian)
Gotelli NJ, Colwell RK (2001) Quantifying biodiversity: procedures and pitfalls in measurement and comparison of species richness. Ecol Lett 4:379–391. https://doi.org/10.1046/j.1461-0248.2001.00230.x
Grebmeier JM, Cooper LW, Feder HM, Sirenko BI (2006) Ecosystem dynamics of the Pacific–influenced northern Bering and Chukchi Seas in the Amerasian Arctic. Prog Oceanogr 71:331–361. https://doi.org/10.1016/j.pocean.2006.10.001
Grebmeier JM, Bluhm BA, Cooper LW et al (2015) Ecosystem characteristics and processes facilitating persistent macrobenthic biomass hotspots and associated benthivory in the Pacific Arctic. Prog Oceanogr 136:92–114. https://doi.org/10.1016/j.pocean.2015.05.006
Hollander M, Douglas AW (1973) Nonparametric statistical methods. Wiley, New York
Hollowed AB, Barbeaux SJ, Cokelet ED, Cokelet ED, Farley E, Kotwicki S, Ressler PH, Spital C, Wilson CD (2012) Effects of climate variations on pelagic ocean habitats and their role in structuring forage fish distributions in the Bering Sea. Deep-Sea Res Pt II 65:230–250. https://doi.org/10.1016/j.dsr2.2012.02.008
Hollowed AB, Planque B, Loeng H (2013) Potential movement of fish and shellfish stocks from the sub–arctic to the Arctic ocean. Fish Oceanogr 22:355–370. https://doi.org/10.1111/fog.12027
Iken K, Mueter F, Grebmeier JM, Cooper LW, Danielson S, Bluhm B (2019) Developing an observational design for epibenthos and fish assemblages in the Chukchi Sea. Deep-Sea Res Pt II 162:180–190. https://doi.org/10.1016/j.dsr2.2018.11.005
Kawecki TJ (2008) Adaptation to marginal habitats. Annu Rev Ecol Evol S 39:321–342. https://doi.org/10.1146/annurev.ecolsys.38.091206.095622
Leonard DRP, Clarke KR, Somerfield PJ, Warwick RM (2006) The application of an indicator based on taxonomic distinctness for UK marine biodiversity assessments. J Environ Manage 78:52–62. https://doi.org/10.1016/j.jenvman.2005.04.008
Lin LS, Liao YC, Zhang J, Zheng SL, Xiang P, Yu XG, Wu RS, Shao KT (2012) Composition and distribution of fish species collected during the fourth Chinese National Arctic Research Expedition in 2010. Advances in Polar Science 23:116–127
Lin LS, Chen YJ, Liao YC, Zhang J, Song PQ, Yu XG, Wu RS, Shao KT (2014) Composition of fish species in the Bering and Chukchi Seas and their responses to changes in the ecological environment. Acta Oceanol Sin 33:63–73. https://doi.org/10.1007/s13131-014-0490-x
Liu CR, Ma KP (2002) Diversity ordering: methods and an example. Chin J Plan Ecolo 26:63–67
Logerwell E, Busby M, Carothers C et al (2015) Fish communities across a spectrum of habitats in the western Beaufort Sea and Chukchi Sea. Prog Oceanogr 136:115–132. https://doi.org/10.1016/j.pocean.2015.05.013
Logerwell E, Rand K, Danielson S, Sousa L (2018) Environmental drivers of benthic fish distribution in and around Barrow Canyon in the northeastern Chukchi Sea and western Beaufort Sea. Deep-Sea Res Pt II 152:170–181. https://doi.org/10.1016/j.dsr2.2017.04.012
Ludwig JA, Quartet L, Reynolds JF, Reynolds JF (1988) Statistical ecology: a primer in methods and computing, vol 1. Wiley, New York
Maslenikov KP, Orr JW, Stevenson DE (2013) Range extensions and significant distributional records for eighty–two species of fishes in Alaskan marine waters. Northwest Nat 4:1–21. https://doi.org/10.1898/12-23.1
Mecklenburg CW, Steinke D (2015) Ichthyofaunal baselines in the Pacific Arctic region and RUSALCA study area. Oceanography 28:158–189. https://doi.org/10.5670/oceanog.2015.64
Mecklenburg CW, Møller PR, Steinke D (2011) Biodiversity of arctic marine fishes: taxonomy and zoogeography. Mar Biodivers 41:109–140. https://doi.org/10.1007/s12526-010-0070-z
Mecklenburg CW, Byrkjedal I, Karamushko OV, Møller PR (2014) Atlantic fishes in the Chukchi borderland. Mar Biodivers 44:127–150. https://doi.org/10.1007/s12526-013-0192-1
Mecklenburg CW, Mecklenburg TA, Sheiko BA, Steinke D (2016) Pacific Arctic marine fishes. Conservation of Arctic Flora and Fauna, Akureyri
Mecklenburg CW, Lynghammar A, Johannesen E, Byrkjedal I, Christiansen JS, Dolgov AV, Karamushko OV, Mecklenburg TA, Møller PR, Steinke D, Wienerroither RM (2018) Marine fishes of the arctic region. Conservation of Arctic Flora and Fauna, Akureyri
Mittelbach GG, Schemske DW, Cornell HV et al (2007) Evolution and the latitudinal diversity gradient: speciation, extinction and biogeography. Ecol Lett 10:315–331. https://doi.org/10.1111/j.1461-0248.2007.01020.x
Møller PR (2001) Redescription of the Lycodes pallidus species complex (Pisces, Zoarcidae), with a new species from the Arctic/North Atlantic Ocean. Copeia 4:972–996. https://doi.org/10.1643/0045-8511(2001)001[0972:ROTLPS]2.0.CO;2
Mueter FJ, Litzow MA (2008) Sea ice retreat alters the biogeography of the Bering Sea continental shelf. Ecol Appl 18:309–320. https://doi.org/10.1890/07-0564.1
Mueter FJ, Broms C, Drinkwater KF, Friedland K, Hare J, Hunt G, Melle W, Taylor M (2009) Ecosystem responses to recent oceanographic variability in high–latitude Northern Hemisphere ecosystems. Prog Oceanogr 81:93–110. https://doi.org/10.1016/j.pocean.2009.04.018
Mueter FJ, Weems J, Farley EV, Sigler MF (2017) Arctic ecosystem integrated survey (Arctic Eis): marine ecosystem dynamics in the rapidly changing Pacific Arctic Gateway. Deep-Sea Res Pt II 135:1–6. https://doi.org/10.1016/j.dsr2.2016.11.005
Norcross BL, Raborn SW, Holladay BA, Gallaway BJ, Crawford ST, Priest JT, Edenfield LE, Meyer R (2013) Northeastern Chukchi Sea demersal fishes and associated environmental characteristics, 2009–2010. Cont Shelf Res 67:77–95. https://doi.org/10.1016/j.csr.2013.05.010
Oksanen J, Blanchet FG, Friendly Michael, et al (2019) vegan: Community Ecology Package. R package version 2.5–6. https://CRAN.R-project.org/package=vegan
Orlov AM, Savin AB, Gorbatenko КM, Benzik AN, Morozov TB, Rybakov MO, Terentiev DA, Vedishcheva EV, Kurbanov YK, Nosov MA, Orlova SY (2020) Biological studies in the Russian Far Eastern and Arctic seas in the VNIRO Transarctic expedition. Trudy VNIRO 181:102–143. https://doi.org/10.36038/2307-3497-2020-181-102-143 (In Russian)
Orr JW, Drumm DT, Hoff GR, Stevenson D E (2014) Species identification confidence in the Bering Sea Slope surveys (1976–2010). AFSC Processed Report, Alaska Fisheries Science Center, NOAA, Seattle
Palumbi SR, Sandifer PA, Allan JD, Beck MW, Fautin DG, Fogarty MJ, Halpern BS, Incze LS, Leong JA, Norse E, Stachowicz JJ, Wall DH (2009) Managing for ocean biodiversity to sustain marine ecosystem services. Front Ecol Environ 7:204–211. https://doi.org/10.1890/070135
Perry AL, Low PJ, Ellis JR, Reynolds JD (2005) Climate change and distribution shifts in marine fishes. Science 308:1912–1915. https://doi.org/10.1126/science.1111322
Pickart RS (2010) Hydrographic context for Chukchi slope fish trawls during RUSALCA 2009. Presentations from RUSALCA Workshop, Woods Hole Oceanographic Institution, Montenegro
Pielou EC (1975) Ecological diversity. Wiley, New York
Pisareva MN, Pickart RS, Iken K et al (2015) The relationship between patterns of benthic fauna and zooplankton in the Chukchi Sea and physical forcing. Oceanography 28:68–83. https://doi.org/10.5670/oceanog.2015.58
Pörtner HO, Farrell AP (2008) Physiology and climate change. Science 322:690–692. https://doi.org/10.1126/science.1163156
Post E, Bhatt US, Bitz CM, Brodie JF, Fulton TL, Hebblewhite M, Kerby J, Kutz SJ, Stirling I, Walker DA (2013) Ecological consequences of sea–ice decline. Science 341:519–524. https://doi.org/10.1126/science.1235225
R Core Team (2020) R: A language and environment for statistical computing. R Foundation for R Foundation for Statistical Computing, Vienna. https://www.R-project.org/. Accessed 25 Feb 2020
Rabosky DL, Chang J, Title PO, Cowman PF, Sallan L, Friedman M, Kaschner K, Garilao C, Near TJ, Coll M, Alfaro ME (2018) An inverse latitudinal gradient in speciation rate for marine fishes. Nature 559:392–395. https://doi.org/10.1038/s41586-018-0273-1
Richter MJ, Druckenmiller ML, Jeffries M (2019) Arctic Report Card, https://www.arctic.noaa.gov/Report–Card/. Accessed 20 Feb 2020
Royston P (1982) An extension of Shapiro and Wilk’s W test for normality to large samples. Appl Stat–J roy St C 31:115–124
Schlitzer R (2019) Ocean Data View. https://odv.awi.de
Sigler MF, Mueter FJ, Bluhm BA, Busby MS, Cokelet ED (2017) Late summer zoogeography of the northern Bering and Chukchi seas. Deep-Sea Res Pt II 135:168–189. https://doi.org/10.1016/j.dsr2.2016.03.005
Smith MA, Goldman MS, Knight EJ, Warrenchuk JJ (2017) Ecological Atlas of the Bering, Chukchi, and Beaufort Seas, 2nd edn. Audubon Alaska, Anchorage
Spies I, Gruenthal KM, Drinan DP, Hollowed AB, Stevenson DE, Tarpey CM, Hauser L (2019) Genetic evidence of a northward range expansion in the eastern Bering Sea stock of Pacific cod. Evol Appl 13:1–14. https://doi.org/10.1111/eva.12874
Stabeno PJ, Kachel NB, Moore SE, Nappd JM, Siglere M, Yamaguchif A, Zerbini AN (2012) Comparison of warm and cold years on the southeastern Bering Sea shelf and some implications for the ecosystem. Deep-Sea Res Pt II 65:31–45. https://doi.org/10.1016/j.dsr2.2012.02.020
Stabeno PJ, Bell SW, Bond NA, Kimmel DG, Mordy CW, Sullivan ME (2019) Distributed biological observatory region 1: physics, chemistry and plankton in the northern Bering Sea. Deep-Sea Res Pt II 162:8–21. https://doi.org/10.1016/j.dsr2.2018.11.006
Stevenson DE, Hoff GR (2009) Species identification confidence in the eastern Bering Sea shelf survey (1982–2008). Alaska Fisheries Science Center Processed Report, Seattle
Stevenson DE, Lauth RR (2012) Latitudinal trends and temporal shifts in the catch composition of bottom trawls conducted on the eastern Bering Sea shelf. Deep-Sea Res Pt II 65:251–259
Stevenson DE, Lauth RR (2019) Bottom trawl surveys in the northern Bering Sea indicate recent shifts in the distribution of marine species. Polar Biol 42:407–421. https://doi.org/10.1007/s00300-018-2431-1
Thorsteinson LK, Love MS (2016) Alaska Arctic marine fish ecology catalog: U.S. Geological Survey Scientific Investigations Report 2016–5038 (OCS Study, BOEM 2016–048). https://doi.org/10.3133/sir20165038.
Wang ZY, Tang LN, Qiu QY, Chen HX, Wu T, Shao GF (2018) Assessment of regional ecosystem health—a case study of the golden triangle of southern Fujian province. China Int J Environ Res Public Health 15:802. https://doi.org/10.3390/ijerph15040802
Warwick RM, Clarke KR (1995) New ‘biodiversity’ measures reveal a decrease in taxonomic distinctness with increasing stress. Mar Ecol Prog Ser 129:301–305
Warwick RM, Clarke KR (1998) Taxonomic distinctness and environmental assessment. J Appl Ecol 35:532–543
Wickham H (2016) ggplot2: elegant graphics for data analysis. Springer, New York
Wiedmann MA, Primicerio R, Dolgov A, Ottesen CA, Aschan M (2014) Life history variation in Barents Sea fish: implications for sensitivity to fishing in a changing environment. Ecol Evol 4:3596–3611
Wisz MS, Broennimann O, Grønkjær P, Møller PR, Pellissier L (2015) Arctic warming will promote Atlantic Pacific fish interchange. Nat Clim Change 5:261–265. https://doi.org/10.1038/nclimate2500
Woodgate RA, Stafford KM, Prahl FG (2015) A synthesis of year–round interdisciplinary mooring measurements in the Bering Strait (1990–2014) and the RUSALCA years (2004–2011). Oceanography 28:46–67. https://doi.org/10.5670/oceanog.2015.57
Acknowledgements
We are grateful to the Chinese Arctic and Antarctic Administration of the State Oceanic Administration (CAA) and the Polar Research Institute of China (PRIC) for their organization and logistical support during the expeditions. We also appreciate the scientists and crew of R/V Xuelong for their generous help and assistance in the fish sampling. We thank Yunchih Liao and Kwangtsao Shao for their analyses of species in 2010 and the Chinese National Arctic and Antarctic Data Center (NADC) for providing the environmental data. We acknowledge the comprehensive and constructive comments of Heino Fock, Dr. Andrey V. Dolgov (Polar branch of VNIRO, Murmansk, Russia), and an anonymous reviewer that greatly improved this manuscript. We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (grant number 41876176), the Scientific Research Foundation of Third Institute of Oceanography, MNR (grant number 2016011), the Chinese Arctic and Antarctic Administration, and the Chinese Polar Environment Comprehensive Investigation & Assessment Program (grant numbers CHINARE 2012-2016-03-05 and CHINARE 2012-2016-04-03).
Author information
Authors and Affiliations
Contributions
LSL and PQS conceived and designed the research. RZ, PQS, HL, and LL conducted the surveys. RZ, PQS, YL, RW, LSL, and XM contributed species identification. SXD and LSL provided ideas and guidance. RZ analyzed fish data and wrote and edited the manuscript. All authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, R., Song, P., Li, H. et al. Spatio-temporal characteristics of demersal fish community in the Chukchi and northern Bering Seas: significant distributional records and interannual variations in species composition and biodiversity. Polar Biol 45, 259–273 (2022). https://doi.org/10.1007/s00300-021-02980-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-021-02980-8