Abstract
Radon (222Rn) is radium’s immediate radioactive decay product (226Ra) in the uranium decay series. Radon is classified as a group 1 carcinogen due to its hazard to human health, and the evaluation of radon concentrations has become essential. Therefore, an advanced method was applied to a liquid scintillation counter (Hidex 300SL) to measure the radon concentration in groundwater. The estimated radon concentration was between 0.1 and 3.20 Bq/L, with an average of 0.96 Bq/L and with a standard deviation of 0.82 Bq/L. The estimated radon concentrations are in the safe range recommended by the United States Environmental Protection Agency (USEPA) and European Atomic Energy Community (EAEC). However, the values are far below the safe range set by the United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCARE), European Commission (EC), and World Health Organization (WHO) standards. Nevertheless, the estimated radon concentration by this advanced application ensured no risk of radon exposure from the groundwater of the tested area.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Radon gas and its progeny are the most significant natural radionuclides present in the air, water, ground, underground, groundwater, and surface water (Aleissa et al. 2012; Aleissa et al. 2013; Gebresilasie et al. 2021; Küçükönder and Gümbür 2022). The human body can be exposed to the radon gas directly by inhalation and by ingestion of water (Aleissa et al. 2013; Althoyaib and El-Taher 2015). Radon can expose most of the natural radiation to humans and poses the most significant risk for humans by causing several types of cancer, such as liver, colon, and lung cancer (Harrison 2021; Kendall and Smith 2002; UNSCEAR 1988; USEPA 1991). The average annual radon dose is approximately half of the average natural dose and twice that of all other artificial radiation sources (Mehra and Bala 2014; USEPA 1991). Hence, the measurement of radon activity has become a major essential issue for researchers worldwide (El-Araby 2018; Kuo 2023; Maringer et al. 2013; Massoud et al. 2020). International organizations set different radon concentration levels as safety standards to be aware of radon exposure. For example, the United States Environmental Protection Agency (USEPA) and European Atomic Energy Community (EAEC) have set a value of 11.1 Bq/L as a safe level for drinking water (EC 2019). The World Health Organization (WHO) recommends 100 Bq/L (WHO 2007, 2011) as a safe level of radon in water. For any water source, if the radon concentration exceeds the safety level, the WHO recommends treating the water until the concentration is reduced to below the safety level.
Water is essential for living beings, so the measurement of radon concentration, its annual consumption, and the corresponding health risk assessment are crucial. Many countries worldwide have aimed to estimate the radon concentration in water using various techniques (Hopke et al. 2000; Malik et al. 2015), such as ionization chambers, liquid scintillation counting, Lucas cells, gamma spectroscopy, solid-state alpha detection, and aeration from water (De Simone et al. 2015). The liquid scintillation counter is a modern and convenient device for measuring radon concentrations. A Hidex 300 SL scintillation counter is suitable for its high efficiency in measuring radon concentrations. The liquid scintillation counter has a low background count rate and minimum detectable activity. The device is equipped with an autosampler to handle many samples simultaneously. The analytical conditions, background count rate, and counting efficiency are controllable for the liquid scintillation counter. The advantage of a liquid scintillation counter is that it can count even low radon activity. Various types of samples, for example, solids, liquids, suspensions, and gels, can be accommodated with an LSC counter. Moreover, different isotopes can be counted simultaneously. The data analysis, efficiency correction, graph plotting, various calculations, and radioimmunoassay are programmable and can be controlled using a built-in computer.
For the importance of the above, the present work represents the estimation of the radon concentration of groundwater located in the eastern area of Saudi Arabia, and those wells are being used for drinking and domestic purposes. The estimated radon concentrations are compared with the international safety standards set by various international organizations. The results estimated using a liquid scintillation counter are verified with other standard techniques. To the best of our knowledge, this report is the first attempt to estimate the radon concentration in groundwater using a liquid scintillation counter in this area.
Materials and method
Study areas
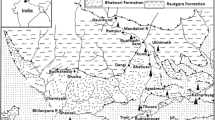
The present area of investigation is located in the northeastern part of Saudi Arabia. Three cities are covered in this study: Hafr Al Batin, Thybiyah, and Qaisumah, as shown in Fig. 1. Hafr Al Batin (Al Batin valley) is named for its location in the dry valley of Wadi Al Batin. Wadi Al Batin is a part of Wadi al Rummah, the largest and longest dry river in the Arabian Peninsula, approximately 2000 km long (Aly et al. 2015; El-Taher and Al-Turki 2016; El-Taher et al. 2020). The central part of Wadi Al Rummah comes through the Al Qassim oasis, one of the leading agricultural regions (Aly et al. 2015). During summer days, the climate is arid and hot (40–50 °C), and it falls during winter nights to (−2–8 °C). It rarely rains during winter, and no rainfall is recorded during summer.
Geology of the study areas
Hafr Al Batin, located in northeastern Saudi Arabia, exhibits a geological landscape dominated by sedimentary rock formations. These sedimentary rocks, including sandstone, limestone, shale, and siltstone, bear testament to the area’s ancient geological history, with the potential for valuable insights through fossil discoveries. The region is renowned for the Saq Aquifer, an essential groundwater source stored within porous sedimentary layers. Geological structures, such as fault lines and fractures, can influence groundwater dynamics and the distribution of potential mineral resources such as limestone. While situated within the generally stable Arabian Plate, occasional seismic activity remains possible due to its tectonic setting. In light of its geological properties, Hafr Al Batin presents opportunities for further exploration, resource assessment, and geological hazard evaluation, ensuring responsible land use and environmental preservation in this unique geological landscape. The areas have good water quality due to their aquifers and geological structure (Vincent 2008). Based on the report of Vincent and Alsharhan et al. the main aquifers are based on primary and secondary origins. The primary origins are Quaternary sands, including quartz sandstones and conglomerates with primary porosity. The secondary porosity with oolitic limestone (Alsharhan et al. 2001; Vincent 2008). Hafr Al Batin is located on a Quaternary sand aquifer, where deep wells supply relatively good water compared to shallow wells. Radon can be generated between the rocks as an intermediate radioactive decay of uranium/thorium radioactive substances. Therefore, radon can be present in aquifer rocks dissolved in groundwater. It was found that groundwater associated with granite and metamorphic rocks has relatively higher radon concentrations (Durrani 1999). Since radon gas is dissolved in groundwater, it might escape from water during contact with the atmosphere. Serious attention was taken during the sampling process to obtain the actual radon concentration. Groundwater samples from 18 source wells were collected into plastic bottles and capped immediately. For better performance, in most cases, the measurements were carried out within 4 h of sample collection.
Radon measurement by LSC
The Hidex 300SL is one of the most useful liquid scintillation devices based on the triple-to-double coincidence ratio (TDCR). A photograph of such a measurement device in our laboratory is shown in Fig. 2a. The device has three photomultiplier tubes on the same plane at 120 degrees (Fig. 2b, c). The device is based on the triple-to-double coincidence ratio (TDCR) (Arun et al. 2021; Broda 2003). A scintillation principle is shown in Figure S1 in the supporting information. The detector has three photomultipliers that enable counting of the triple-to-double coincidence ratio, a method to obtain the counting efficiency of an unknown sample without any standard source. TDCR is a method that can be applied for both chemical and color quenching, aqua- and organic samples, cocktails, and isotopes. First, an appropriate scintillation cocktail (Aqualight Plus, Hidex, UK) was added to the scintillation vial. Then, the water sample was inserted below the scintillation cocktail by ensuring no aeration of the water and secured with a tight cap. The vial was kept for an hour so that radon’s short-term decay product entered equilibrium. Then, the scintillation counter technique was used to measure the activity of radon from the photon emission rate from the fluids. Before counting with the scintillation counter, the procedure below was followed.
The samples were always kept in cool and dark places since luminescence can have a significant effect. In addition, Hidex 300SL discharges static electricity from the sample to avoid the static electricity effect. Samples were prepared by adding 12 ml of a water-soluble cocktail to 8 ml of an aqueous sample. The scintillation device then measured the radon activity by emitting photons. It should be mentioned that special attention should be taken during the sample preparation; otherwise, the estimated result will be influenced by escaping the radon gas from the water sample.
Results and discussion
Before analysis of an unknown sample using an LSC, the device should be monitored by testing background radiation, calibration, and efficiency checked biweekly or monthly using a standard source. Such a controlled chart for calibration using a standard source of certified activity-free solution, carbon 14 (14C) source, and tritium source (3H) is shown in Fig. 3. The activity-free solution shows an almost constant reading with a minimal experimental error in the last eight years (Fig. 3a). This implies that radiation is always present in the activity-free solution and environment, even without a radioactive source. For the standard source of carbon 14, no significant changes in count per minute (CPM) were detected due to its longer half-life (approximately 5715±40 years) (Choppin et al. 2002).
On the other hand, for 3H, a significant decrease in CPM was found eight years after initializing the device (0.6162 times the initial count) (Fig. 3b). A rough estimation shows a half-life of approximately 11.75 years for 3H, which is close to the published data of 12.3 years reported in the literature (Lucas and Unterweger 2000). It should be mentioned that if the measured value is approximately 1000 times higher than the background count, the background effect can be ignored.
The measured concentrations of radon for 18 groundwater samples collected from the Hafr Al Batin, Thybiyah, and Qaisumah areas, Saudi Arabia, are tabulated in Table 1. It was found that the concentration varied in the range of 0.1 to 3.20 Bq/L, with an average of 0.96 Bq/L and a standard deviation of 0.82 Bq/L. Many national and international organizations have established their radon levels worldwide. For instance, the United States Environmental Protection Agency (USEPA) set a value of 11.1 Bq/L for the radon concentration in groundwater (USEPA 2000). The UNSCEAR set 40 Bq/L as a safe limit in their published report, (UNSCEAR 2000) while the European Commission (EC) and World Health Organization (WHO) have described a value of 100 Bq/L as an action limit (EC 2019; WHO 2011). As observed, all samples measured radon levels below those limits. Approximately 90% of the samples were far less than the radon levels reported earlier (Al-Jaseem et al. 2016; Aljaloud and ElBatouti 2021). Furthermore, the estimated radon concentrations in the present study are comparable to those found in the rest of Saudi Arabia (Abuelhia 2017; Abuelhia 2019; Aleissa et al. 2012; Aleissa et al. 2013). For example, a range of 1.45–9.15 Bq/L was found in the Al-Jawa area, as reported by Althoyaib et al. (2015); 0.76–4.69 Bq/L was found for groundwater from the Al-Qassim area, reported by El-Taher et al. (2016); (0.92–2.12) Bq/L was found in the Jeddah area reported by Tayyeb et al. (1998); 0.11–9.20 Bq/L was found in the Dammam area, reported by Abuelhia (2017); and 1.74–4.32 Bq/L was found in the Jazan area, as reported by El-Araby et al. (2019).
A comprehensive analysis of the data presented in Table 1 unveils a discernible trend: when examining radon concentrations, the average values in shallow wells consistently demonstrate a lower tendency compared to their counterparts in deep wells. This general pattern holds true with remarkable consistency, and it serves as valuable insight into the distribution of radon within the groundwater sources. However, it is important to note the presence of certain exceptions that defy this overarching trend. These exceptions are notable, as they deviate from the established pattern, adding complexity to the assessment. Notably, some shallow wells situated within the lower valley of the arid Al Batin River exhibit elevated radon concentrations that are atypical for their category. This localized variation suggests that geological and hydrogeological factors unique to this particular region may contribute to the observed discrepancies. Additionally, exceptions are not confined solely to shallow wells; they extend to certain deep wells as well. Furthermore, deep wells positioned atop the hillside display deviations from the anticipated pattern. These exceptions are indicative of the complex interplay of geological, hydrological, and environmental variables that influence radon concentration in groundwater (Massoud et al. 2020).
The standard deviation (σ) was calculated mathematically for each groundwater sample. Statistically, the standard deviation of a series of samples indicates the deviation from the mean value and is a measure of the precision of the measurements. Radioactive decay follows the Poisson distribution law, from which it is possible to calculate the standard deviation as the square root of recorded counts by the RAD7 detector. When no decay is recorded, instead of reporting zero uncertainty, RAD7 reports an uncertainty value based on 2σ. In this study, the cycle time was increased to 30 min for maximum certainty. The radon concentration in the water sample obtained in Bq/m3 was converted to Bq/L.
If the sample was collected earlier but analyzed later, there must be a decay due to the delay in measuring radon concentration. Therefore, a correction should be made for the sample decay depending on the sampling time. The decay correction factor (DCF) is simply an exponential function with a time constant of 132.4 h and is calculated based on the following formula:
where T is the duration (in hours) between sampling and counting by the LSC instrument, and 132.4 h is the mean lifetime of the 222Rn atom (radon has a half-life of 3.825 days, multiplied by 24 h per day and divided by the natural logarithm of 2) (Salvato 2003). Such a decay correction factor with time is plotted in Fig. 4 (solid line). From the DCF plot, it can be seen that samples were appropriately sealed, stored, and counted by LSC within 24 h, and no significant correction was needed. However, a correction is needed if the sample is not counted immediately, and the DCF increases exponentially with time. In this study, in most cases, the sample was counted immediately within 4 h of sample collection except for six (open circle in Fig. 4) collected from Thybiyah and Qaisumah cities (those cities are far from the LSC measurement lab). A proper correction using the DCF equation was made and is tabulated in Table 1.
The estimated results using the LSC method are comparable to other countries surrounding the Kingdom. Table 2 compares the estimated radon concentrations of water in several countries. The radon concentration obtained in the current study follows the calculated values obtained from surrounding countries, such as Yemen, UAE, Kuwait, Iraq, and Jordan, as tabulated in Table 2. On the other hand, the results in the present study are far below those in Turkey, Algeria, India, Romania, Sudan, Iran, and China. The geological nature of the sampling area, soil nature, and environment might be the reason for the difference in radon concentrations (Asuni et al. 2021). Moreover, the irradiative emissions from radon, such as neutrons, neutron flux, water samples, contamination of other radioactive sources, and pH, can also influence the experimental results.(Aljaloud and ElBatouti 2021; Martin 2006) We are now estimating the radon concentration using other standard methods to better compare the advantages and disadvantages of the techniques, which will be reported elsewhere.
Conclusion
An advanced application using a liquid scintillation counter was used to ascertain the radon concentration in groundwater samples collected from diverse locations in the northeastern region of Saudi Arabia. The results of this meticulous analysis revealed that the estimated radon concentrations of these groundwater samples ranged from as low as 0.1 Bq/L to a maximum of 3.20 Bq/L, with a calculated average concentration of approximately 0.96 Bq/L and a standard deviation of 0.82 Bq/L. In the most northern part of the area, particularly within the lower valley, shallow wells were identified as having significantly higher radon concentrations. In contrast, wells situated in the hillside areas showed notably lower levels of radon. This variation in radon concentration is likely attributable to the geological composition of the rocks and sediments that the groundwater passes through. Remarkably, these findings indicate that the radon concentrations detected in the groundwater of this region consistently fall well below the safety thresholds established by both the United States Environmental Protection Agency (USPEA) and the European Atomic Energy Community (EAEC). Furthermore, they notably and comfortably remain significantly below the safe level recommendations established by two globally recognized authoritative bodies: the United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) and the World Health Organization (WHO). The results concluded that the potential radon exposure to humans residing within the tested areas of the Eastern Province of Saudi Arabia is unequivocally and substantially lower than the stringent safety limits stipulated by international standards. As a result, this comprehensive assessment offers reassuring evidence that there exists no discernible risk of radon exposure stemming from the groundwater within the sampled cities of this region. Such meticulous scientific investigations are paramount in ensuring the continued safety and well-being of the local population and serve as a testament to the rigorous scientific methods employed in environmental monitoring and risk assessment.
Data availability
The dataset used in this study is available from the corresponding author upon reasonable request except for data that are subject to third-party restrictions.
References
Abojassim A (2014) Radon concentrations measurement for drinking water in Kufa City /Iraq using active detecting method. Advances in Physics, Theories and Applications 26:6
Abuelhia E (2017) Evaluation of annual effective dose from indoor radon concentration in Eastern Province, Dammam, Saudi Arabia.Radiat Phys Chem 140:137-140
Abuelhia E (2019) Assessment of radiation dose from radon ingestion and inhalation in commercially bottled drinking water and its annual effective dose in Eastern Province, Saudi Arabia. Int J Environ Health Res 29:164–172
Al-Bataina BA, Ismail AM, Kullab MK, Abumurad KM, Mustafa H (1997) Radon measurements in different types of natural waters in Jordan. Radiat Meas 28(1):591–594. https://doi.org/10.1016/S1350-4487(97)00146-7
Aleissa KA, Alghamdi AS, Almasoud FI, Islam MS (2012) Measurement of radon levels in groundwater supplies of Riyadh with liquid scintillation counter and the associated radiation dose. Radiat Prot Dosimetry 154:95–103
Aleissa KA, Alghamdi AS, Almasoud FI, Islam MS (2013) Measurement of radon levels in groundwater supplies of Riyadh with liquid scintillation counter and the associated radiation dose. Radiat Prot Dosimetry 154:95–103
Aljaloud KB, ElBatouti M (2021) Statistical analysis of 222Rn concentration in Zamzam and other water sources in the Kingdom of Saudi Arabia. Heliyon 7:e06057
Al-Jaseem QK, Almasoud FI, Ababneh AM, Al-Hobaib AS (2016) Radiological assessment of water treatment processes in a water treatment plant in Saudi Arabia: water and sludge radium content, radon air concentrations and dose rates. Sci Total Environ 563–564:1030–1036
Alsharhan AS, Rizk ZA, Nairn AEM, Bakhit DW, Alhajari SA (2001) ‘Preface’, hydrogeology of an arid region: the Arabian Gulf and adjoining areas. Elsevier Science B.V, Amsterdam, pp 287–309
Althoyaib S, El-Taher A (2015) Natural radioactivity measurements in groundwater from Al-Jawa, Saudi Arabia. J Radioanal Nucl Chem 304:547–552
Aly AA, Al-Omran AM, Alharby MM (2015) The water quality index and hydrochemical characterization of groundwater resources in Hafar Albatin, Saudi Arabia. Arab J Geosci 8:4177–4190
Amrani D (2002) Natural radioactivity in Algerian bottled mineral waters. J Radioanal Nucl Chem 252(3):597–600
Arun B, Viswanathan S, Venkatesan S, Jose MT, Balasubramaniam V (2021) Study of triple to double coincidence method for tritium measurements. Radiochemistry 63:221–226
Asuni G, Al Qahtani S, Khasawinah S (2021) Occupational radon assessment in underground storage facilities in Saudi Arabia. Health Physics Publish
Broda R (2003) A review of the triple-to-double coincidence ratio (TDCR) method for standardizing radionuclides. Appl Radiat Isot 58:585–594
Choppin GR, Liljenzin J-O, Rydberg JAN (2002) CHAPTER 5 - Radionuclides in Nature. In: Choppin GR, Liljenzin J-O, Rydberg JAN (eds) Radiochemistry and Nuclear Chemistry, 3rd edn. Butterworth-Heinemann, Woburn, pp 94–122
De Simone G, Galli G, Lucchetti C, Tuccimei P (2015) Calibration of Big Bottle RAD H2O set-up for radon in water using HDPE bottles. Radiat Meas 76:1–7
Durrani SA (1999) Radon concentration values in the field: correlation with underlying geology. Radiat Meas 31:271–276
EC (2019) Radon in workplaces: implementing the requirements in Council Directive. Publications Office
El-Araby E (2018) Direct measurement of the radioactive radon gas activity in water in Saudi Arabia, 020019 pp
El-Araby EH, Soliman HA, Abo-Elmagd M (2019) Measurement of radon levels in water and the associated health hazards in Jazan, Saudi Arabia. Journal of Radiation Research and Applied Sciences 12:31–36
El-Taher A (2012) Annual effective dose exposure in ground water from Qassim area, Saudi. J Environmen Sci Techn 5(6):475–481
El-Taher A, Al-Turki A (2016) Radon activity measurements in irrigation water from Qassim Province by RAD7. J Environ Biol 37:1299–1302
El-Taher AM, Abojassim AA, Najam LA, Mraity HAAB (2020) Assessment of annual effective dose for different age groups based on radon concentrations in the groundwater of Qassim, Saudi Arabia. Iranian Journal of Medical Physics 17:15–20
Gebresilasie KG, Berhe GG, Tesfay AH, Gebre SE (2021) Assessment of some physicochemical parameters and heavy metals in hand-dug well water samples of Kafta Humera Woreda, Tigray, Ethiopia. International Journal of Analytical Chemistry 2021:8867507
Harrison JD (2021) Lung cancer risk and effective dose coefficients for radon: UNSCEAR review and ICRP conclusions. J Radiol Prot 41:433–441
Hopke PK, Borak TB, Doull J, Cleaver JE, Eckerman KF, Gundersen LCS, Harley NH, Hess CT, Kinner NE, Kopecky KJ, McKone TE, Sextro RG, Simon SL (2000) Health risks due to radon in drinking water. Environ Sci Tech 34:921–926
Idriss H, Salih I, Sam A (2011) Study of radon in ground water and physicochemical parameters in Khartoum state. J Radioanal Nucl Chem 290(2):333–338
Kendall GM, Smith TJ (2002) Doses to organs and tissues from radon and its decay products. J Radiol Prot 22:389–406
Küçükönder E, Gümbür S (2022) Radon gas measurement in water samples in Kahramanmaras Province of Turkey. Water, Air, Soil Pollut 233:175
Kuo M-C (2023) ‘Methods of monitoring groundwater radon, pp. 5-20
Lucas LL, Unterweger MP (2000) Comprehensive review and critical evaluation of the half-life of tritium. J Res Nat Inst Stand Technol 105:541–549
Malakootian M, Nejhad YS (2017) Determination of radon concentration in drinking water of Bam villages and evaluation of the annual effective dose. Int J Radiat Res 15(1):81
Malik MFI, Rabaiee NA, Jaafar MS (2015) ‘Determination of radon concentration in water using RAD7 with RAD H2accessories’, National Physics Conference 2014 (PERFIK 2014), p. 120005
Maringer FJ, Baumgartner A, Rechberger F, Seidel C, Stietka M (2013) Activity measurement and effective dose modelling of natural radionuclides in building material. Appl Radiat Isot 81:279–283
Martin JE (2006) Physics for radiation protection: a handbook. John Wiley & Sons
Massoud E, El-Taher A, Elmoniem A, Elzain AEA, El-Taher A, Elzain A (2020) Estimation of environmental radioactivity and radiation dose from exposure to radon in groundwater for inhabitants in Qassim Area, Saudi Arabia. Desalin Water Treat 205:308–315
Mehra R, Bala P (2014) Estimation of annual effective dose due to Radon level in indoor air and soil gas in Hamirpur district of Himachal Pradesh. J Geochem Explor 142:16–20
Moldovan M, Nita DC, Cucos-Dinu A, Dicu T, Bican-Brişan N, Cosma C (2014) Radon concentration in drinking water and supplementary exposure in Băiţa-Ştei mining area, Bihor county (Romania). Radiat Prot Dosimetry 158(4):447–452
Salvato C (2003) The role of micro-strategies in the engineering of firm evolution. J Manag Stud 40:83–108
Seoud M (2018) Measurement of radon-222 concentration in bottled natural mineral drinking water in Kuwait using the nuclear track detector (CR-39)
Tayyeb Z, Kinsara A, Farid S (1998) A study on the radon concentrations in water in Jeddah (Saudi Arabia) and the associated health effects. J Environ Radioact 38:97–104
UNSCEAR (1988) ‘Sources’, Effects and Risks of ionizing Radiation 199
UNSCEAR (2000) The general assembly with scientific annex. United Nations
USEPA (1991) National primary drinking water regulations; Radionuclides, proposed rule. Fed. Reg. 56:33050–33127
USEPA (2000) National primary drinking water regulations; radionuclides; final rule. Fed. Reg. 65:76708
Vincent P (2008) Saudi Arabia: an environmental overview. CRC Press, London
WHO (2007) Desalination for safe water supply: Guidance for the Health and Environmental Aspects Applicable to Desalination. World Health Organization
WHO (2011) Guidelines for drinking-water quality. World Health Organization, pp 303–304
Xinwei L (2006) Analysis of radon concentration in drinking water in Baoji (China) and the associated health effects. Radiat Prot Dosimetry 121(4):452–455
Yalım HA, Sandıkcıoğlu A, Ünal R, Orhun Ö (2007) Measurements of radon concentrations in well waters near the Akşehir fault zone in Afyonkarahisar, Turkey. Radiat Meas (3):505–508
Acknowledgements
The authors thank the Deanship of Scientific Research, University of Hafr Al Batin, for the experimental facilities.
Funding
This work is financially supported by the Institutional Funding Project (No: IFP-A-202-1-3) from the Ministry of Education, Saudi Arabia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Broder J. Merkel
Supplementary information
ESM 1
(DOCX 107 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mamun, A., Alazmi, A.S. Advance application of liquid scintillation counter to estimate radon concentration in groundwater. Arab J Geosci 17, 34 (2024). https://doi.org/10.1007/s12517-023-11846-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-023-11846-8