Abstract
The aim of this study was to determine the concentration of Pb, Zn, Cu, Cd, Cr, and Fe in sediments of the Ichkeul Lake and rivers ecosystem in northern Tunisia; their comparison with international sediment quality criteria was also proposed to assess the extent of metal pollution and their origin using various pollution indicators. For this purpose, sediment samples were collected in thirteen locations and characterized for metal content (Pb, Cr, Zn, Cu, Cd, and Fe) using the total digestion technique. Pollution level was assessed via the geoaccumulation index (Igeo), enrichment factor (EF), contamination factor (C ), potential ecological risk (PER), and pollution load index (PLI). Our results showed that all metals were lower than the threshold effect levels (TEL), the effect range low (ERL), the probable effect level (PEL), the effect range median (ERM), and the probable effect concentration (PEC) value, except Pb and Cr (higher than TEL), the threshold effect concentration (TEC), and the toxicity reference value (TRV). The geoaccumulation index (Igeo) indicated no pollution for Cr, Cu, Zn, and Fe, but moderate pollution for Pb. The enrichment factor showed no enrichment for most of the studied metals. This was further confirmed by the contamination factor that indicated low contamination levels, with the exception of Pb. The pollution load index (PLI) showed moderate pollution status in all the studied stations in the Ichkeul Lake and rivers ecosystem. The statistical results presented similar trends of Zn and Pb probably due to their similar pollution sources. It was found that the Ichkeul Lake and river ecosystems are characterized by moderate pollution status in all the studied sites except for that of a feeder river drained from an old lead mine which had relatively high metallic concentrations of Pb and Cd. It can be argued that effective remediation strategies and environmental management plans are required to control and reduce the input of environmentally hazardous toxic pollutants (Cd and Pb). This study may serve as a useful reference tool pertinent to approaches to the remediation of the old lead mine area surrounding the Ichkeul Lake as well as other areas under similar ecological conditions..
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Metal contamination in aquatic environments remains an issue of great concern due to its toxicity, wide source, abundance, persistence in the environment, and subsequent accumulation for a long period of time in aquatic habitats (Dong et al. 2011). Over the past century, heavy metals have been discharged into the world rivers and estuaries as a result of the rapid industrialization and urbanization (Salati and Moore 2010; Sdiri et al. 2012). River sediments therefore are important sources for the assessment of man-made contamination in rivers where heavy metals flow into lakes.
Sediments usually provide useful information for environmental and geochemical pollution status (Uluturhan et al. 2011). Heavy metals may settle down in the sediments and gradually release in water generating one of the most critical problems in aquatic environments (Zhang et al. 2014). Total heavy metal concentration in sediments can be therefore used for the identification of pollution source, pollution level, and ecological risks (Praveena et al. 2015). Protecting the quality of the sediment is an important part of restoring and maintaining biological integrity; it should always include the determination of the extent or degree of pollution by a given heavy metal (USEPA 2001). Consequently, the analysis of river sediments is a useful tool to study the metal pollution in a given area (Varol 2011).
The geoaccumulation index (I geo), enrichment factor (EF), contamination factor (C if ), potential ecological risk (PER), and pollution load index (PLI) are among different statistical indexes that can be used to determine the source and magnitude of metal pollution. Numerous researchers considered those parameters for assessing metal pollution (e.g., Amin et al. 2009; Wei and Yang 2010; Ghrefat et al. 2011; Varol 2011; Hasan et al. 2013; Zahra et al. 2014; Ghannem et al. 2014; Zarei et al. 2014; Zhang et al. 2014; Praveena et al. 2015; Aydi 2015). All these studies were of practical significance in terms of environmental protection.
The Ichkeul Lake (northern Tunisia) represents an original aquatic ecosystem whose biological balance has been altered due to the rapid anthropogenic development, anarchic urbanization, industrialization, and municipal waste water dumped directly into the lake, which can seriously affect water and sediments.
Furthermore, various unknown sources of pollutants can be emitted by industries that can have major negative effects on the ecosystem. Indeed, in the existing literature on ecosystems in Bizerte, Tunisia, some previous studies have carried out assessment of the water quality of Bizerte lagoon. However, to our knowledge, assessment of heavy metal pollution in surface sediments in Ichkeul Lake and its feeder rivers, along with the environmental risks they pose, has not received attention so far.
The main objectives of the present study are (1) to determine the concentration of Pb, Zn, Cu, Cd, Cr, and Fe in sediments of the Ichkeul Lake and its feeder rivers; (2) to assess heavy metal contamination levels and their origin; and (3) to discuss the pollution status. Hence, this study significantly contributes to evaluating the current status of sediment pollution in the Ichkeul ecosystem and providing updated information in support of effective environmental management in the area. This may help to develop beneficial water protection measures and better management of human activities in the Ichkeul Lake and its feeder rivers.
Materials and methods
Study area
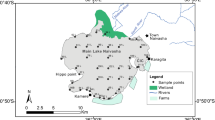
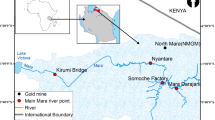
Ichkeul ecosystem is one of the four most important wetlands of the Western Mediterranean basins (i.e., Doñana in Spain, the Camargue in France, and Elklaa in Algeria) according to Tamasier (1987, 1992); (UNESCO 2009c) and Bird life International (2009). The studied area lies to the northwest of Tunisia (coordinates: 37° 02′ N 09° 33′ E) in the Mediterranean coast (Fig. 1). This region is internationally recognized as a protected area by Ramsar Convention 1980 (Ramsar Convention and Wetlands International 2009) and also designated as a biosphere reserve in 1977, and a natural world heritage site (UNESCO 2009a, b). Ichkeul Lake is an important stopover point for hundreds of thousands of migrating birds each year (Carp 1980). It comprises two physiographic units: an isolated wooded mountain and a permanent brackish water lake surrounded by marshland. It is connected to the sea via the river Tinja canal, and it is fed by six main rivers from the west and south, i.e., Douimis, Sejenane, Malah, Ghezala, Joumine (canalized across the marshes) and Tine, a tributary of the Joumine. The lake catchment and marshland soils are alluvial. The Ichkeul Mountain is composed of Triassic and Jurasssic formations, largely as metamorphosed limestones with pseudo-dolomitic marbles. Triassic limestones and other sedimentary deposits are exposed in the quarries on the south-western slopes of the mountain. The endorheic basin of the lake as well as the marshes is composed of Quaternary alluvia (Hollis 1977; Bousquet 1988).
The climate is typically Mediterranean; winter rainfalls fill up the rivers and the lake with freshwater from autumn and winter that overflow into the river Tinja which presents high contents of dissolved salts during the summer season. In summer, high evaporation lowers the water level and allows seawater intrusion. Salinity displays considerable seasonal changes from 3 ‰ in the innermost parts in spring to 38 ‰ at the mouth of the Tinja channel in autumn (Casagranda and Boudouresque 2007). The Ichkeul Lake is bordered by the old (Pb-Zn) Jalta mine.
The study area is surrounded by accumulation of solid mining wastes of the old Jalta mine after the processing of the Pb-Zn. Exposed to different meteoric factors, sulphides from the tailings dams and wastes are oxidized, thus generating acid mining drainage (AMD) able to mobilize and release heavy metals associated with sulphides. Sulfides are remobilized by run-off from the dump up to the surroundings of Ichkeul Lake (Gannouni et al. 2010).
The mining wastes correspond to large barren accumulations containing heavy metal contents that are responsible for the accumulation of the surface sediments of Ichkeul Lake. This area is also exposed to domestic sewages along with industrial, municipal, and recreational discharges. Also, agricultural activities in the surrounding Mateur Plain involve the use of pesticides and fertilizers to be transferred by the drainage system in the lake (Hollis 1986). These factors contribute to the environmentally unfriendly degradation of the lake water quality.
Sampling and analysis
Surface sediment samples (0–5 cm) were collected from 13 sites in three replicates from the Ichkeul Lake and rivers using a grab sampler in January 2014 (Fig. 1).
Geographical coordinates of sampling locations were recorded at each sampling point with a GPS (Table 1). The sampling sites were selected at the mouths of the main rivers which flow into the lake: Sejnane (S1), Douimis (S2), Melah (S3), Mallah (S4), Joumine (S5), Tinja (S10), Morra (S11), close to agricultural area to determine the direct runoff impact in the lake and at the thermal springs (S6, S7, S8, S9, S12). Finally, at site S13 from river directly draining the Jalta mine tailings, this site was selected as an example of a stream which can receive the soluble and particulate metals through river systems.
Sediment samples were taken from each site using stainless steel Van Veen grab. Immediately after collection, the samples were placed in polyethylene bags, refrigerated, and transported to the laboratory. Sediment samples were dried to a constant dry weight at 60 °C, and sieved with a 63-μm stainless steel sieve (Mudroch and Bourbonniere 1994). Finer sediments contain more heavy metals than the coarser ones. This enrichment is mainly due to surface adsorption and ionic attraction (Szefer et al. 1996).
The sediment samples were air dried and sieved to <2 mm for general analytical determinations. The pH was determined in a 1:5 suspension of sediment in Milli-Q quality water using a HANNA HI 2210 pH meter. Electrical conductivity (EC) (ms/cm at 25 °C) was measured using an Orion model 150 conductivity meter in the extracts obtained by filtering the 1:5 suspensions through a 0.45-μm cellulose acetate disk filter. Textural analysis was performed after dispersion of the fine sediment and by combining extraction by Robinson pipette and sieving. Analysis of TOC was carried out with 100 mg of powdered sample using a “Turbo” Rock-Eval 6 pyrolysis manufactured by Vinci Technologies. The full description of the method and of the parameters obtained is given by Espitalie et al. (1985).
The mineralogical composition of the samples was determined by X-ray diffraction (XRD) patterns and were acquired using a Bruker AXS D8 advance diffractometer equipped with a Cu tube, a Ge (1 1 1) incident beam monochromator (λ = 0.1541 nm), and a Vantec-1 PSD. Data were recorded in the 2θ range of 5–55° with an angular step size of 0.016° and a counting time of 6 s per step.
For the analyses of Pb, Cr, Cd, Cu, Zn, and Fe concentrations in sediment samples, about 0.5–1.0 g of the dried sediment were digested in a mixture of concentrated acids (nitric acid, fluorhydric acid, and perchloric acid) according to the EPA 3052 guideline (EPA 1996).
The digested samples were diluted to 25 ml with double distilled water and filtered through Whatman filter paper into acid-washed polyethylene sample bottles. After filtration, the samples were determined for Pb, Zn, Cu, Cd, Cr, and Fe using atomic emission spectrometer (Jobin Yvon Ultima C).
The analytical data quality was guaranteed through the implementation of laboratory quality assurance and quality control methods, including the use of standard operating procedures, calibration with standards, analysis of reagent blanks, recovery of known additions, and analysis of replicates. All analyses were run in triplicate, and the results were expressed as the mean.
Sediment contamination assessment methods
Geoaccumulation index
The geoaccumulation index (I geo) introduced by Müller (1969) was used as a reference to identify the extent of metal contamination in the studied sediments by comparing current concentrations with background levels. This index can be determined from the following equation:
where C n is the measured concentration of element n, B n is the background concentration value of the element n (Cd, Cr, Pb, Zn, Fe, and Cu in sediments were 0.2, 71, 16, 127, 35.900, and 32 mg/kg); (Martin and Whitfield 1983), and 1.5 is the background matrix correction factor that is included to correct possible background value variations due to lithogenic effects. The pollution levels derived through identifying I geo are listed in Table 2.
Enrichment factor
For the estimation of anthropogenic inputs, enrichment factor and geoaccumulation index are among the most widely used indicators (Covelli and Fontolan 1997; Ghrefat and Yusuf 2006; Gonzáles-Macías et al. 2006; Chen et al. 2007; Aprile and Bouvy 2008; Khuzestani and Souri 2013).
According to the enrichment factor technique, metal concentrations were normalized to metal concentrations of average shale (Ghrefat and Yusuf 2006; Maanan et al. 2015) or average crust (Karbassi et al. 2005; Gonzáles-Macías et al. 2006). The most commonly used elements for normalization are Al (Chen et al. 2007) and Fe (Ghrefat and Yusuf 2006; Ali 2015). In this study, the geochemical normalization was obtained using Fe (35.900 mg/kg) (Martin and Whitfield 1983). The enrichment factor (EF) was computed using the equation of Ergin et al. (1991):
where (C x/C Fe) sample is the ratio of concentration of the element being tested (C x) to that of Fe (C Fe) in the sediment sample, and (C x/C Fe)crust or background is the same ratio in unpolluted baseline samples. EF values were interpreted as suggested by Sakan et al. (2009), where: EF < 1 indicates no enrichment; <3 is minor enrichment; 3–5 is moderate enrichment; 5–10 is moderately severe enrichment; 10–25 is severe enrichment; 25–50 is very severe enrichment; and >50 is extremely severe enrichment.
Contamination factor
The contamination factor (C if ) is used to describe the contamination by a given toxic substance in the environment. In the version originally suggested by Hakanson (1980), the assessment of contamination was conducted through the reference of the elemental concentrations to pre-industrial levels (Hakanson 1980). This parameter is expressed as:
where Ci is the mean content of a substance i in sediments, and C in is the reference level for the substances (Cd, Cr, Pb, Zn, Fe, and Cu in sediments were 0.2, 71, 16, 127, 35.900, and 32 mg/kg; Martin and Whitfield 1983). The following criterion was used to describe the values of the contamination factor:
- C if <1:
-
low contamination factor
- 1≤ C if <3:
-
moderate contamination factors
- 3≤C if <6:
-
considerable contamination factors; and
- C if ≥6:
-
, very high contamination factor (Hakanson 1980).
Pollution load index
The PLI was originally proposed by Tomlinson et al. (1980) to determine the pollution load of sediments. It can also give a simple and relative means for the evaluation of the degree of metal pollution (Tomlinson et al. 1980). This parameter is expressed as:
where n is the number of metals and C if is the contamination factor. The pollution load index can be classified as no pollution (PLI < 1), moderate pollution (1 < PLI < 2), heavy pollution (2 < PLI < 3), and extremely heavy pollution (3 < PLI) (Tomlinson et al. 1980).
Potential ecological risk index (PER)
The potential ecological risk index (PER) was introduced to assess the ecological risk degree of heavy metals in the sediments studied. It was originally proposed by Hakanson (1980) and widely used as a diagnostic tool for water pollution control purposes (Guo et al. 2012; Saeedi et al. 2012; Suresh et al. 2012). The value of requested potential ecological risk index (PER) for the basin/lake can be calculated by the following formulas:
where C ifi is the single element pollution factor; Ci is the content of the element in samples and Ci n is the reference value of the element. The reference values of Cd, Cr, Pb, Zn, Cu, and Fe in sediments were 0.2, 71, 16, 127, 32, and 35.900 mg/kg (Martin and Whitfield 1983). The sum of C ifi for all the studied metals represents the integrated pollution degree (Cd) of the environment, E ir is the potential ecological risk index of an individual element, T ir is the biological toxic factor of an individual element, which are defined for Cu = Pb = 5, Cr = 2, Zn = 1, Cd = 30, and Fe = 6 (Guo et al. 2012; Fu et al. 2009; Zhu et al. 2013). PER is the comprehensive potential ecological index, which is the sum of Ei r (Table 3). It represents the sensitivity of the biological community to the toxic substance and illustrates the potential ecological risk caused by the overall contamination.
Sediment quality guideline
Sediment quality assessment guidelines (SQGs) are very useful to screen sediment contamination by comparing sediment contaminant concentration with the corresponding quality guideline (Macdonald et al. 2000). In different studies, the numerical sediment quality guidelines were applied as predictors of contaminants in aquatic sediments (Sekabira et al. 2010). Sediment quality guidelines (SQGs) were used to evaluate the toxicological potential of metals in sediments and to classify areas that may represent a risk for their biota (García et al. 2008). These synthesized guidelines consist of the effect range low (ERL)/effect range median (ERM) and the threshold effect level (TEL)/probable effect level (PEL) values/threshold effect concentration (TEC)/probable effect concentration (PEC)/toxicity reference value (TRV). Low range values (i.e., ERLs or TELs) are concentrations below which adverse effects on sediment-dwelling fauna would be infrequently expected. However, the ERMs and PELs represent chemical concentrations above which adverse effects are likely to occur (MacDonald et al. 2000; Smith et al. 1996).
Statistical analysis
Data were statistically analyzed using the software Statistica 7. A Pearson’s bivariate correlation was used to evaluate the inter-element relationship in sediments. The means and standard deviations of the metal concentrations in sediments were calculated. Additionally, cluster analysis was performed in an attempt to understand sources of pollution.
Results and discussion
Physico-chemical properties and heavy metals in sediment samples
The data in Table 1 showed physico-chemical properties of the studied sediment samples. The pH values ranged between 6.5 and 8.76 with an average value of 8.01, suggesting alkaline conditions for most of the sediment samples. The electrical conductivity (EC) values ranged between 1.4 and 62.49 (ms/cm) with an average value of 15.86(ms/cm). Salinity values ranged from 0.01 to 0.63 % with an average of 0.18 %. The content of total organic carbon (TOC) varied from 0.07 to 5.51 % with an average of 1.46 %. The texture of the studied sediment samples was mainly dominated by sand fraction, ranging from 1 to 96 %.
Characterization of minerals in the sediments
Figure 2a depicts the major minerals found in the river sediments drained from the old mine of Pb-Zn. Based on the XRD analysis, the sediment sample of this river (S13) was mainly composed of calcite (CaCO3) with minor minerals such as quartz (SiO2), kaolinite [Al2Si2O5(OH)4], pyrite (FeS2), and Illite [(K,H3 O) (Al,Mg,Fe)2 (Si, Al)4 O10 ((OH)2 H2 O)], reflecting the geological characteristics of the mining area. Clay minerals found in the river often appeared as kaolinite, vermiculite, and illite.
Figure 2b shows that site S2, which is considered unpolluted, had almost the same mineral characterization. It was mainly composed of calcite and pyrite.
Concentration of heavy metals (Pb, Cr, Zn, Cu, Cd, and Fe) in sediments
Heavy metals concentrations (Pb, Cr, Zn, Cu, Cd, and Fe) in Ichkeul Lake and rivers ecosystem are shown in Table 4. The ranges of metals in sediments were: 1236.1–8086.21 mg/kg for Fe, 13.14–918.05 mg/kg for Zn, 6.7–894.62 for Pb, 1.8–894.04 mg/kg for Cr, 10.06–71.26 mg/kg for Cu and 0.25–1.37 mg/kg for Cd. In this study, the total metal concentrations followed the order of site S1 > site S5 > site S7 > site S6 > site S13 > site S9 > site S4 > site S8 > site S3 > site S2 > site S11 > site S10.
Table 4 was generated by comparing metal content measured in Ichkeul Lake and rivers ecosystem with similar studies of sediments in other regions of the world, global baseline values and sediment quality guidelines which are commonly applied as a management tool and very useful to track sediment contamination by comparing the concentration of heavy metals with the corresponding quality guideline (Caeiro et al. 2005) due to the lack of national guideline for Tunisia. The use of these guidelines enables evaluation of the degree to which the sediment-associated chemical status might adversely affect aquatic organisms and are designed to assist the interpretation of sediment quality (Wenning 2002). The SQGs reflect the importance of polluted sediments as a possible cause of adverse environmental effects; we compared the obtained metal contents in the surface sediments of Ichkeul Lake and rivers ecosystem with the criteria of the effect range low (ERL)/effect range median (ERM) and the threshold effect level (TEL)/probable effect level (PEL). The results showed that the mean contents of Pb and Cr were higher than their corresponding TEL, TEC, TRV; Cu was higher than TRV value; and Pb was higher than ERL and TRV values. The mean contents of other metals are below their corresponding TEL, PEL, ERL, TEC, TRV, PEC, TRV, and ERM values. Thus, there is rare or no biological effect that may occur due to metals contents in the Ichkeul lake sediments.
To measure possible anthropogenic effects, calculated enrichment factors for each element and station in the Ichkeul lake and rivers ecosystem were used. According to Zhang and Liu (2002), EF values between 0.05 and 1.5 indicate that the metal is entirely from crustal materials or natural processes, whereas EF values higher than 1.5 suggest that the sources are more likely to be anthropogenic. In this study, the mean EF values for all the metals studied were >1.5 in the sediments suggesting anthropogenic impact on the metal levels in these sites. The highest EF values were found at site S13 (Ghezala Mine) due to the drainage river by an old lead mine. The EF value for Pb in the sediments of site-S13 was 162.55, showing “extremely very severe enrichment”. Cd at all sites except site-S1 and site-S2 had the highest EF values among the six metals studied, while the EF mean values for Zn, Cr, and Cu were between 5 and 10, indicating “moderately severe enrichment”. Mean EF values presented in Table 5 show the following order: site S13 > site S10 > site S1 > site S12 > site S11 > site S2 > site S9 > site S4 > site S8 > site S4 > site S3 > site S5 > site S6 > site S7.
The I geo values for heavy metals in the Ichkeul Lake and rivers ecosystem are shown in Table 6. We have chosen the widely used average earth crust’s metals concentration reported by Martin and Whitfield (1983). Based on geoaccumulation index, it was found that major parts of the studied samples could be considered unpolluted for Cr, Cu, Zn, and Fe. The pollution levels of these metals based on I geo values were assessed as unpolluted (I geo < 0) (The variations of the I geo were −1.25 to −0.55 for Cr, −2.22 to −1.60 for Zn and −2.52 to −1.85 for Cu). While the values of Pb were demonstrated to have moderate contamination based on I geo values (I geo > 0) (The variation of the I geo for Pb was 0.07 to 0.69). The I geo values of Cd at sites S2, S3 and S8, Cr at all sites except site-S1, Pb, and Zn at all sites except site S13, Cu at all sites except site S5, and Fe at all sites were less than zero, suggesting that these sites were not polluted by these metals. The I geo values for Zn at site S13 and Cu in the sediments of site S5 were below 1, which can be classified as “unpolluted to moderately polluted”. Among the six metals studied Pb, Cr and Cd had the highest I geo values. The highest I geo values of the metals studied were found in the sediments of site S13. The I geo class of Pb was “heavily to extremely polluted” for sediments of site S13 (I geo = 5.22). These differences might be caused by waste water drained from old mining industries located in the upper reach. The I geo class of Cr was “moderately to heavily polluted” for sediments of site S1 (I geo = 3.06), while the I geo class of Cd were “moderately polluted” for sediments of site S13 (Igeo = 2.1) (Table 5).
Table 6 presents Er and PER values of the metals studied. The potential Er indices of Fe, Zn, Cr, and Cu were lower than 40, which indicate a slight potential ecological risk of these elements in the Ichkeul Lake. The average potential ecological risk Er indicates that metal pollution in the sediments followed the sequence of Cd > Pb > Cu > Cr > Zn > Fe. The Er values of Cd and Pb at site S13 showed high levels. In the case of these metals, most of the Er values were at a very high level, while the Er value for the rest of the examined metals was at a relatively low level (Er < 40). However, in the present study, Cd showed higher ecological risk. Generally, applications of phosphate fertilizers to the agricultural field beside the river and waste disposal from the Mateur town are the main sources of cadmium in sediment, thus the proper drainage discharge treatment systems prior to the release of polluted waste into Ichkeul Lake should be upgraded to preserve the lake water quality. Potential ecological risk index (PER) in the sampling sites varied from 42.94 to 494.77, indicating low to considerable risk (Table 6).
The CF and PLI are widely used to evaluate the degree of heavy metal pollution in the sediments (Bhuiyan et al. 2010). The calculated CF and PLI for the studied metals in sediments of Ichkeul Lake and rivers ecosystem are listed in Table 6. The CF values for Pb (55.91) and Cd (6.84) at site S12 are relatively high, indicating that site S12 is highly polluted by both metals originating from deep geological formations. The mean CF values for the metals in the study area followed the sequence Pb (5.04) > Cd (2.13) > Cr (1.03) > Zn (0.78) > Cu (0.54) > Fe (0.09) and demonstrated low pollution levels. The PLI values calculated for each site are also shown in Table 7.
The values of PLI were low in all the studied samples and varied between 1.15 and 2.03, indicating moderate pollution, considering the total of the studied metals (Fig. 3). Sekabira et al. (2010) reported that a PLI > 1 indicated anthropogenic inputs.
Pearson correlation analysis
Table 8 shows the results of Pearson’s correlation coefficient of the calculated Er of individual metals and PER. The correlation suggested that Cd, Pb and Cd, Zn may originate from the same pollution source, with a correlation coefficient of 0.97 (p > 0.01) and 0.98 (p > 0.01), respectively, which leads us to hypothesize that metals with a high positive correlation are possibly from the same pollutant source (Khuzestani and Souri 2013; Nowrouzi et al. 2012). Similarly, Pb and PER had a high correlation coefficient of 0.99 (p > 0.01). Significant correlation also occurred between Zn and PER (0.99 (p > 0.01)). However, Cu shows very low correlations with other variables, indicating the possibility of different source. The absence of correlation between these heavy metals illustrates that the metals are not controlled by a single factor (Table 8).
Cluster analysis was conducted to evaluate the correlation between the calculated Er and PER. As shown in Fig. 4, variables are grouped into four statistically significant clusters: cluster 1 consists of Pb, Cu, Zn, and PER, which means that Pb, Cu and Zn are closely related to each other and mainly determine the PER value; cluster 2 consists of Cr, while cluster 3 contains only Fe, implying that it is independent from the other metals and did not closely relate to the PER; and cluster 4 contains Cd.
Conclusion
The geochemical analysis of selected heavy metals (Pb, Cr, Cd, Cu, Zn and Fe) in the sediments of the Ichkeul Lake and rivers ecosystem (northern Tunisia) ranged from 1236.1-8086.21 mg/kg for Fe, 13.14-918.05 mg/kg for Zn, 6.7-894.62 for Pb, 1.8-894.04 mg/kg for Cr, 10.06-71.26 mg/kg for Cu and 0.25-1.37 mg/kg for Cd. The sediment quality guidelines (SQGs) showed that all the studied metals were lower than TEL, PEL, ERL, PEC, and ERM values, except Pb and Cr. Pollution levels of Cr, Cd, Cu, Zn and Fe were assessed as unpolluted based on I geo values (I geo < 0), but not for Pb (I geo > 0). The potential ecological risk index (PER) values of each single metal had low potential ecological risk with the exception of Cd and Pb at site S13 which showed high levels of Cd. Higher potential ecological risk was observed at site S13; the old lead mine indicated moderate to considerable risk level. The contamination factor (C if ) also demonstrated low contamination levels for Fe, Cr, Zn, and Cu, while most of the values of Pb were found to have moderate contamination level based on (C if ) analysis. The values of PLI, determining the overall metal pollution in sediments, showed moderate pollution status in all the studied stations in the Ichkeul Lake border. The statistical results showed that Zn and Pb present a clear trend and have the same potential pollution source. It is recommended that effective remediation strategy and environmental management plan are required to control and reduce the input of toxic metals (Cd and Pb), which would significantly minimize the potential of further pollution of the environment. This study may serve as a useful reference tool for decision-makers in agronomic activities planning, future reclamation, and remediation efforts for the old lead mine area surrounding the Ichkeul Lake.
References
Ali BNM, Lin CY, Cleophas F, Abdullah MH, Musta B (2015) Assessment of heavy metals contamination in Mamut river sediments using sediment quality guidelines and geochemical indices. Environ Monit Assess 187(1):1–11
Amin B, Ismail A, Arshad A, Kong YC, Kamarudin MS (2009) Anthropogenic impacts on heavy metal concentrations in the coastal sediments of Dumai, Indonesia. Environ Monit Assess 148:291–305
Aprile FM, Bouvy M (2008) Distribution and enrichment of heavy metals in sediments at the Tapacurá river basin, Northeastern Brazil. Braz J Aquat Sci Technol 12(1):1–8
Aydi A (2015) Assessment of heavy metal contamination risk in soils of landfill of Bizerte (Tunisia) with a focus on application of pollution indicators. Environ Earth Sci 74(4):3019–3027
Bhuiyan MA, Parvez L, Islam MA, Dampare SB, Suzuki S (2010) Heavy metal pollution of coal mine-affected agricultural soils in the northern part of Bangladesh. J Hazard Mater 173(1):384–392
Bird Life International (2009) Important bird area factsheet: Ichkeul, Tunisia. Available at: http://www.birdlife.org/datazone/sites/index.html?action=SitHTMDetails.asp&sid=6919&m=0
Bousquet B (1988) Rapport de mission du Parc National de l’Ichkeul et autres aires protégées de Tunisie septentrionale et centrale. MAB-UNESCO et Fonds du Patrimoine Mondial
Caeiro S, Costa MH, Ramos TB, Fernandes F, Silveira N, Coimbra A, Medeiros G, Painho M (2005) Assessing heavy metal contamination in Sado Estuary sediment: an index analysis approach. Ecol Indic 5:151–169
Carp E (1980) Directory of wetlands of international importance in the western Palearctic. IUCN
Casagranda C, Boudouresque CF (2007) Biomass of Ruppia cirrhosa and Potamogeton pectinatus in a Mediterranean brackish lagoon, Lake Ichkeul, Tunisia. Fundam Appl Limnol/Arch Hydrobiol 168(3):243–255
Chen CW, Kao CM, Chen CF, Dong CD (2007) Distribution and accumulation of heavy metals in the sediments of Kaohsiung Harbor, Taiwan. Chemosphere 66(8):1431–1440
Covelli S, Fontolan G (1997) Application of a normalization procedure in determining regional geochemical baselines. Environ Geol 30(1/2):34–45
Dong JH, Yu M, Bian ZF, Wang Y, Di CL (2011) Geostatistical analyses of heavy metal distribution in reclaimed mine land in Xuzhou, China. Environ Earth Sci 62(1):127–137
EPA, Environmental Protection Agency (1996) Method 3052. microwave assisted digestion of siliceous and organically based matrices. In: test methods for evaluating solid wastes: physical/chemical methods, EPA SW-846, 3rd edn. Chapt. 3, Section A, vol. I (Inorganic Analytes), Office of Solid Waste and Emergency Response, Washington, DC 1–20
Ergin M, Saydam C, Basturk O, Erdem E, Yoruk R (1991) Heavy metal concentrations in surface sediments from the two coastal inlets (Golden Horn Estuary and Izmit Bay) of the northeastern sea of Marmara. Chem Geol 91:269–285
Espitalie J, Deroo G, Marquis F (1985) La pyrolyse Rock-Eval et ses applications. Deuxième partie. Oil Gas Sci Technol 40(6):755–784
Fu C, Guo J , Pan J, Qi J, Zhou W (2009) Potential Ecological Risk Assessment of Heavy Metal Pollution in Sediments of the Yangtze River Within the Wanzhou Section, China. Biol Trace Elem Res 129:270–277
Gannouni S, Mammou AB, Belghith A, Eljaoued SA (2010) La Spectrométrie, outil d’étude des impacts des rejets miniers sur l’environnement. Teledetection 8(4):257–269
García E, Cruz-Motta J, Farina O, Bastidas C (2008) Anthropogenic influences on heavy metals across marine habitats in the western coast of Venezuela. Cont Shelf Res 28(20):2757–2766
Ghannem N, Gargouri D, Sarbeji MM, Yaich C, Azri C (2014) Metal contamination of surface sediments of the Sfax–Chebba coastal line, Tunisia. Environ Earth Sci 72(9):3419–3427
Ghrefat HA, Yusuf N (2006) Assessing Mn, Fe, Cu, Zn, and Cd pollution in bottom sediments of Wadi Al-Arab Dam, Jordan. Chemosphere 65(11):2114–2121
Ghrefat HA, Abu-Rukah Y, Rosen MA (2011) Application of geoaccumulation index and enrichment factor for assessing metal contamination in the sediments of Kafrain Dam, Jordan. Environ Monit Assess 178(1–4):95–109
Gonzáles-Macías C, Schifter I, Lluch-Cota DB, Méndez-Rodríguez L, Hernández-Vázquez S (2006) Distribution, enrichment and accumulation of heavy metals in coastal sediments of Salina Cruz Bay, Mexico. Environ Monit Assess 118:211–230
Guo G, Wu F, Xie F, Zhang R (2012) Spatial distribution and pollution assessment of heavy metals in urban soils from southwest China. J Environ Sci 24(3):410–418
Hakanson L (1980) Ecological risk index for aquatic pollution control, a sedimentological approach. Water Res 14:975–1001
Hasan AB, Kabir S, Reza AS, Zaman MN, Ahsan A, Rashid M (2013) Enrichment factor and geo-accumulation index of trace metals in sediments of the ship breaking area of Sitakund Upazilla (Bhatiary–Kumira), Chittagong, Bangladesh. J Geochem Explor 125:130–137
Hollis GE (1977) A management plan for the proposed Park National de l’Ichkeul, Tunisia. Conservation report:11
Hollis GE, Agnew CT, Battarbee RW, Chisnal N, Fisher RC, Flower R, Smart M (1986) Modelling and management of the internationally important wetlands at Garaet el Ichkeul. Tunisia IWRB, Slimbrige, UK
Karbassi AR, Nabi-Bidhendi GR, Bayati I (2005) Environmental geochemistry of heavy metals in a sediment core off Bushehr, Persian Gulf. Iran J Environ Health Sci Eng 2(4):255–260
Khuzestani RB, Souri B (2013) Evaluation of heavy metal contamination hazards in nuisance dust particles, in Kurdistan Province, western Iran. J Environ Sci 25(7):1346–1354
Liu J, Li Y, Zhang B, Cao J, Cao Z, Domagalski J (2009) Ecological risk of heavy metals in sediments of the Luan River source water. Ecotoxicology 18:748–758
Maanan M, Saddik M, Maanan M, Chaibi M, Assobhei O, Zourarah B (2015) Environmental and ecological risk assessment of heavy metals in sediments of Nador lagoon, Morocco. Ecol Indic 48:616–626
Mac Donald DD, Ingersoll CG, Berger TA (2000) Development and evaluation of consensus-based sediment quality guidelines for freshwater ecosystems. Arch Environ Contam Toxicol 39:20–31
Martin JM, Whitfield M (1983) The significance of the river input of chemical elements to the ocean. In: Wong CS, Boyle E, Brul KW, Burton JD, Goldberg ED (eds) Trace metals in sea water. Plenum Press, New York, pp 265–296
Mudroch A, Bourbonniere RA (1994) Sediment preservation processing and storage, In: Mudroch A, Mackinght SD (eds) Hand book of techniques for aquatic sampling, 2nd edn. Boca Raton
Müller G (1969) Index of geoaccumulation in sediments of the Rhine River. Geol J 2(3):109–118
Müller G (1981) The heavy metal pollution of the sediments of Neckars and its tributary: a stocktaking. Chemical Zeitung 105:157–164
Nowrouzi M, Pourkhabbaz A, Rezaei M (2012) Bioaccumulation and distribution of metals in sediments and Avicenna marina tissues in the Hara Biosphere Reserve, Iran. Bull Environ Contam Toxicol 89:799–804
Praveena SM, Pradhan B, Ismail SNS (2015) Spatial assessment of heavy metals in surface soil from Klang District (Malaysia): an example from a tropical environment. Hum Ecol Risk Assess: An Inter J 21(7):1980–2003
Saeedi M, Li LY, Salmanzadeh M (2012) Heavy metals and polycyclic aromatic hydrocarbons: pollution and ecological risk assessment in street dust of Tehran. J Hazard Mater 227–228:9–17
Sakan SM, Dordević DS, Manojlović DD, Predrag PS (2009) Assessment of heavymetal pollutants accumulation in the Tisza river sediments. J Environ Manag 90:3382–3390
Salati S, Moore F (2010) Assessment of heavy metal concentration in the Khoshk River water and sediment, Shiraz, Southwest Iran. Environ Monit Assess 164(1–4):677–689
Sdiri A, Higashi T, Jamoussi F, Bouaziz S (2012) Effects of impurities on the removal of heavy metals by natural limestones in aqueous systems. J Environ Manag 93:171–179
Sekabira K, Oryem Origa H, Basamba T, Mutumba G, Kakudidi E (2010) Assessment of heavy metal pollution in the urban stream sediments and its tributaries. Int J Environ Sci Technol 7(3):435–446
Smith SL, MacDonald DD, Keenleyside KA, Ingersoll CG, Field LJ (1996) A preliminary evaluation of sediment quality assessment values for freshwater ecosystems. J Great Lakes Res 22:624–638
Suresh G, Sutharsan P, Ramasamy V, Venkatachalapathy R (2012) Assessment of spatial distribution and potential ecological risk of the heavy metals in relation to granulometric contents of Veeranam lake sediments, India. Ecotoxicol Environ Saf 84:117–124
Szefer P, Szefer K, Glasby GP, Pempkowiak J, Kaliszan R (1996) Heavy-metal pollution in surficial sediments from the southern Baltic Sea off Poland. J Environ Sci Health A31:2723–2754
Tamisier A, Pradel R (1992) Analyse statistique de l’habitat hivernal diurne du canard siffleur Anas penelope L. en Camargue. Perspectives de gestion. Revue d’écologie 47:135–150
Tamisier A, Bonnet P, Bredin D, Dervieux A, Rehfish M, Rocamora G, Skinner J (1987) L’Ichkeul (Tunisie), quartier d’hiver exceptionnel d’Anatidés et de foulques. Importance, fonctionnement et originalité. Oiseau et la Revue Francaise d’Ornithologie 57(4):296–306
Tomlinson D, Wilson J, Harris C, Jeffrey D (1980) Problems in the assessment of heavy-metal levels in estuaries and the formation of a pollution index. Helgoländer Meeresun 33(1–4):566–575
Uluturhan E, Kontas A, Can E (2011) Sediment concentrations of heavy metals in the Homa Lagoon (Eastern Aegean Sea): assessment of contamination and ecological risks. Mar Pollut Bull 62(9):1989–1997
UNESCO (2009a) Biosphere reserve information Tunisia, Ichkeul. UNESCO-MAB Biosphere Reserves Directory. Available at: http://www.unesco.org/mabdb/br/brdir/directory/biores.asp?code=TUN+03&mode=all
UNESCO (2009b) List of biosphere reserves which are wholly or partially world heritage sites and Ramsar wetlands. UNESCO-MAB Biosphere Reserves Directory. Available at: http://www.unesco.org/mab/doc/brs/brs_ramsar_whc.pdf
UNESCO (2009c) World Heritage Centre Ichkeul National Park. Available at: http://whc.unesco.org/en/list/8/
USEPA, United States Environmental Protection Agency (1999) Screening level ecological risks assessment protocol for hazardous waste combustion facilities vol. 3. Appendix E: toxicity reference values, EPA
USEPA, United States Environmental Protection Agency (2001) Methods for collection, storage and manipulation of sediments for chemical and toxicological analyses: technical manual. USEPA, Washington
Varol M (2011) Assessment of heavy metal contamination in sediments of the Tigris River (Turkey) using pollution indices and multivariate statistical techniques. J Hazard Mater 195:355–364
Wei B, Yang L (2010) A review of heavy metal contaminations in urban soils, urban road dusts and agricultural soils from China. Microchem J 94(2):99–107
Wenning RJ (2002) In: Ingersoll CG (ed) Executive summary of the SETAC Pellston Workshop on use of sediment quality guidelines and related tools for the assessment of contaminated sediments. Pensacola: Society of Environ Toxico and Chem (SETAC)
Zahra A, Hashmi MZ, Malik RN, Ahmed Z (2014) Enrichment and geoaccumulation of heavy metals and risk assessment of sediments of the Kurang Nallah—feeding tributary of the Rawal Lake Reservoir, Pakistan. Sci Total Environ 470–471:925–933
Zarei I, Pourkhabbaz A, Khuzestani RB (2014) An assessment of metal contamination risk in sediments of Hara Biosphere Reserve, southern Iran with a focus on application of pollution indicators. Environ Monit Assess 186(10):6047–6060
Zhang J, Liu CL (2002) Riverine composition and estuarine geochemistry of particulate metals in China weathering features, anthropogenic impact and chemical fluxes. Estuar Coast Shelf Sci 54:1051–1070
Zhang J, Hua Li Z, Che J, Wang M, Tao R, Liu D (2014) Assessment of heavy metal contamination status in sediments and identification of pollution source in Daye Lake, Central China. Environ Earth Sci 72(4):1279–1288
Zhu X, Ji H, Chen Y, Qiao M, Tang L (2013) Assessment and sources of heavy metals in surface sediments of Miyun Reservoir, Beijing. Environ Monit Assess 185(7):6049–6062
Acknowledgments
The authors would like to thank the editor and anonymous reviewers for their useful constructive feedback. Also, special thanks go to Mr Zied Tlili, a teacher of English for Specific Purposes at the Higher Institute of Business Administration of Gafsa, Tunisia, for his diligent professional English language editing.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ouchir, N., Aissa, L.B., Boughdiri, M. et al. Assessment of heavy metal contamination status in sediments and identification of pollution source in Ichkeul Lake and rivers ecosystem, northern Tunisia. Arab J Geosci 9, 539 (2016). https://doi.org/10.1007/s12517-016-2560-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-016-2560-1