Abstract
A hydrogeochemical approach has been carried out in the Mio-Plio-Quaternary aquifer system of northern Sfax to investigate the geochemical evolution, the origin of groundwaters and their circulation patterns. The groundwater samples collected from different wells seem to be dominated by sodium chloride type to sulphate chloride type. Detail analysis of chemical data including the thermodynamic calculations was used to assess that the chemical evolution of groundwater is primarily controlled by water–rock interactions. The values of sodium absorption ratio and electrical conductivity of the groundwater were plotted in the US Salinity Laboratory diagram for irrigation water. Most of the water samples in northern Sfax fall in the fields of C4S1, C4S2 and C4S3 indicating very high salinity and medium to high sodium alkalinity hazard. Thus, groundwater quality is ranging between doubtful to unsuitable for irrigation uses under normal condition, and further action for salinity control is required in remediating such problem. Principal component analysis of geochemical data used in conjunction with bivariate diagrams of major elements indicates that groundwater mineralization is mainly controlled by (1) water–rock interaction processes, (2) anthropogenic process in relation with return flow of NO3-rich irrigation waters and (3) domestic discharges.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coastal aquifers are considered as major sources for freshwater supply worldwide, especially in arid zones where the demands of the growing populations feed a continual need to develop new methods and plans to preserve groundwater resources. The weak rainfall and the intensive extraction of groundwater from unconfined aquifers reduce freshwater budget and create local water aquifer depression. The quality of groundwater is critical in such regions which are normally characterised by a variety of anthropogenic activities.
Groundwater quality concept in the northern of Sfax region has been evaluated in the last years by the natural processes (lithology, groundwater, quality of recharge waters and interactions with other types of water (Bouri et al. 2008)), the anthropogenic activities (agriculture, industry, urban development) and the increasing exploitation of water resources (Trabelsi 2003; Trabelsi et al. 2005; Ben Brahim 2006). In recent years, the deterioration of groundwater quality became an increasing serious problem in many parts in northern Sfax as a result of the expansion of agricultural irrigation return, the domestic effluents and the intensive pumping.
The original study objective is to evaluate the hydrochemical characteristics of the groundwater in the northern Sfax over a period of 1 year to include both the wet and the dry seasons. The data obtained over this period will be used to characterise the groundwater. This paper aims to identify the main processes controlling the hydrochemistry of the groundwater and to evaluate the suitability of the groundwater in the area for irrigation purposes.
This study will help current water resources planning and will provide a baseline for future monitoring programmes and studies of water quality.
Study area
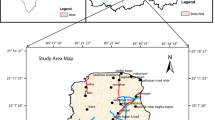
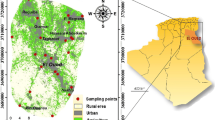
This study concerns three regions (El Hancha, Djebeniana and El Amra) of the northern Sfax area, located at the central east of Tunisia (Fig. 1). These regions have an arid Mediterranean climate, with extreme temperatures and rainfall variations with an average annual temperature and rainfall of 19°C and 205 mm, respectively.
The geology of the studied area is investigated by several authors (Hajjem 1979; Zbidi 1989; Beni Akhy 1994; Maliki 1994, 2000; Trabelsi 2003; Trabelsi et al. 2005). Most of the studied sites are located in the Mio-Plio-Quaternary layer system mainly formed by sand and sandy-clayey which considered by recent alluvial deposits conglomerates, gravels, silts calcareous etc.
The aquifer is recharged by direct infiltration (the permeability values are between 4.10−6 and 68.10−4 m/s), and its higher limit is at Medasse Sidi salah; the discharge limits coincide with the Mediterranean shore line and the sebkha of el Jem (Fig. 2). The groundwater flow is generally toward the southeast and may be locally disturbed by piezometric depressions due to the intensive exploitation (Ben Brahim 2006).
The pumping well density is about 100 to 110 wells/km2, whereas it was of 30 to 45 wells/km2 in the 1970s. The variations of annual rainfall, annual well withdrawal and groundwater chloride concentration in the study area indicate a parallel increase of the last two parameters versus time, while the first parameter decreases. This fact may be explained by seawater intrusion related to intensive exploitation (Trabelsi et al. 2005).
The synthetic geological cross section along the three regions presented in Fig. 1 shows the presence of a horizontal structure composed of a slightly clayey sand level of Mio-Plio-Quaternary age (Fig. 3). This latter is limited of the bottom by a clay layer, representing the aquifer substratum. On the top, heterogeneous clastic materials presented essentially by sand and gravel are encountered, implying a generally permeable aquifer.
From the new piezometric maps developed in pre- and post-rainfall status (Figs. 4 and 5), it can be inferred that the groundwater flow direction is generally towards the southeast, implying discharge to the Mediterranean Sea, the natural outlet. Nevertheless, the coastal zone of Djebeniana and El Amra regions appears to be highly influenced by overexploitation because the isopiestic lines show values lower than 0 m. This can be attributed to the increase of the exploitation rate.
Materials and methods
Forty monitoring wells ranging in depth from 15 to 45 m were sampled in 2007; the geographical location of the sampling site is shown (Fig. 1). The physicochemical parameters (pH, electrical conductivity (EC) and the total dissolved solids (TDS)) were measured in situ using a WTW multi-parameter device (multi-P3PH/LF-SET). Alkalinity was determined using colorimetric titration with sulphuric acid. The major elements have been analysed in the LARSEN Laboratory at the National School of Engineers in Sfax. The analytical methods which were used are reported in Table 1. Only analyses of water whose charge balance errors are less than 5% are used in this study (Table 2).
Principal component analysis (PCA) method used to extract related variables and infer the processes that control water chemistry (Helena et al. 2000; Hidalgo and Cruz-Sanjulian 2001; Muller et al. 2008) was performed using SPSS 10.0 statistical programme.
Saturation indexes of some common minerals were calculated using the programme PHREEQC (Parkhurst and Apello 1999) interfaced with Aquachem Version 5.
The rest of analytical data were processed, using the computer programmes: HydroGEN 32 and ARC GIS 9.2.
The groundwater salinity mapping was obtained by Surfer programme interpolation using the database of monitoring wells provided by the district water of resources of Sfax (2007).
The methods are successfully used to group the data to evaluate the influence of various environs in the study area. Various classification methods such as piper, Schoeller, expanded Durov, correlation method and salinity hazard measurements are also employed to the critical study of geochemical characteristics of groundwater to identify the vulnerable parts of the aquifer.
Results and discussion
Principal component analysis
PCA, as a multivariate data analytic technique, reduces a large number of variables (measured physical parameters, major anions and cations in water samples) to a small number of variables which are the principal components (PCs). More concisely, PCA combines two or more correlated variables into one variable. This approach has been used to extract related variables and infer the processes that control water chemistry. Varimax rotation is applied to the PCs in order to determine the factors that can be more easily explained in terms of hydrochemical or anthropogenic processes (Helena et al. 2000). This rotation is called Varimax because the goal is to maximize the variance (variability) of the new variable, while minimizing the variance around the new variable XLSTAT (2008). The number of PCs extracted (to explain the underlying data structure) is defined by using the “Kaiser criterion” Kaiser (1958) where only the PCs with eigenvalues greater than unity are retained. In other words, unless a PC extracts at least as much information as the equivalent of one original variable, it is dropped XLSTAT (2008). The reader is referred to the work of Davis and Dewiest (1966) for an in-depth account of the theory.
In this study, PCA was applied to nine normalized variables datasets separately, for the period 2007 (EC, Ca2+, Mg2+, Na+, K+, Cl−, SO42−, HCO −3 and NO −3 ). According to the eigenvalue, one-criterion three principal components were selected, which explain 58.022% of the total variance in the water quality datasets (Table 3). The analysis indicates that the most relevant variables defining water quality are related to water dissolved salts (Cl−, Na+, Ca2+, K+, NO −3 and EC) and the less relevant ones are Mg2+, SO 2−4 and HCO −3 .
From Table 4, PC1 which explains 26.977% of the total variance is mainly driven by Cl− Na+, Ca2+, K+, NO −3 and EC− which are chemical variables; PC2 is strongly and positively related to Mg2+ and SO 2−4 . PC3 which explains 15.383% of the total variance is positively related to HCO −3 and negatively to Ca2+.
There are evidences that PC1 and PC2 are related to dissolution of the evaporate minerals and deterioration of water quality by the transfer of pollution by domestic septic tanks and probably by the seawater intrusion caused by the overexploitation.
PC2 is related to common sources of natural processes of dissolution of geological rocks components. The PC1 is related to the transfer of pollution by organic fertilizers and manner.
Water type
The physicochemical data of the investigated groundwater showed large spatial variations. The pH is close to neutrality (between 6.8 and 7.95), electrical conductivity ranging from 1.024 to 10.000 μS/cm and salinities (TDS) between 3 and 12.6 g l−1 (Table 2).
The analyses of cations and anions by Piper trilinear diagram (Piper 1944) are shown in Fig. 6. Dominant cations are mainly calcium and sodium, while dominant anions are sulphate and chloride. The sum of cations and anions shows two main groundwater types: Na–Cl and Ca–SO4–Cl.
The Schoeller plots of Fig. 7 indicate that all of the waters sampled from the El Hancha, Djebeniana and El Amra regions are generally high concentrations of Na+, K+, Ca2+, Cl− and SO 2−4 . Here, the second is highly concentrated in Cl− and Na+ (in Djebeniana region). Therefore, the increase of chlorinated and sodium concentrations in Djebeniana region might be explained by the possible mixing with seawater (Ben Brahim 2006).
Bivariate diagrams of major elements versus chloride are used in order to separate the different mechanisms that contribute to groundwater salinization. In particular, sodium (Fig. 8a), sulphate (Fig. 8b) and calcium (Fig. 8c) are positively correlated with chloride. This indicates the possible dissolution of halite, gypsum and/or anhydrite-bearing rocks relatively abundant especially in the coastal part of aquifer. The dissolution of these evaporitic rocks is confirmed by mineral saturation indexes, which show a saturation of water samples with respect to the mentioned minerals. The leaching of salts during subsequent rainfall events in combination with extensive evaporation losses increases the concentrations of Na and Cl in the soil and in groundwaters, especially those of the Mio-Plio-Quaternary aquifer bordering the Mediterranean Sea.
Normally, gypsum or anhydrite dissolution produces Ca and SO4 in 1:1 equivalent ratio. However, for all the groundwater samples, the plot of SO4 versus Ca (Fig. 9) shows a relative SO4 enrichment, which is probably derived from typical water–soil–rock interaction.
On the other hand, cation exchange probably generates a Ca deficiency with respect to SO4 concentration. The phenomenological evidence for this exchange is given by the bivariate plot of (Ca2+ + Mg2+) − (HCO −3 + SO 2−4 ) in function of (Na+ + K+ − Cl−) (Garcia et al. 2001) as shown in Fig. 10. In the absence of these reactions, all data should plot close to the origin (Mc Lean et al. 2000). However, Fig. 10 indicates an increase of Na+ + K+ related to the decrease in Ca2+ + Mg2+.
In this case, the expanded Durov diagram was used to identify the hydrochemical processes and reaction paths such as mixing, ion exchange and dissolution affecting groundwater composition. Indeed, Durov (1948) and Lloyd and Heathcotte (1985) demonstrated that the position of the samples in the sub-square of expanded Durov diagram was used to identify processes and chemical reactions of the corresponding aquifer. The significance of each of the nine fields on the expanded Durov diagram is as follows: field 1 indicates recharging waters, fields 2 and 3 indicate ion exchange, fields 4 and 5 indicate waters exhibiting simple dissolution or mixing, field 6 indicates probable mixing influences, fields 7 and 8 indicate that groundwaters may be related to reverse ion exchange and field 9 indicates the mixing by seawater.
Plotted in the expanded Durov diagram (Fig. 11), the groundwater types of the Mio-Plio-Quaternary aquifer belong to two hydrochemical characteristics, according to their order of importance. Most of the samples are concentrated in sub-square 5 which indicate that groundwater chemistry is related to the mixing of different origins. Water type plotted in sub-square 5 is mainly mixed-SO4 type, and this composition probably evolved from the interaction of mixing waters with direct cation exchange and/or conservative reactions.
The water type is the mixed water. In this water type, neither a particular cation nor anion is dominant. This water type plots in the middle of the diagram.
However, four samples are in field 8 because mixing reverse ion exchange affects their composition. Indeed, samples taken from Djebeniana and El Amra which are localised in field 9 proving the water of high salinity have the typical Na–Cl–SO4 composition, commonly characterising the dissolution of evaporate sediments.
Figure 12 shows that the salinity tends to increase downstream. The TDS values range from 3 to 12 g l−1, with an average value of 4.66 g l−1. Salinity is relatively high along the coastal zone, where TDS generally exceeds 12 g l−1. This might be explained by the overexploitation process, which tends to be quite important zones of low groundwater depths. A salinity rate of less than 3 g l−1 is observed in the upstream of Djebeniana and El Amra zones. This is probably the result of infiltration of rainwater. The composition of the aquifer material and the intensive pumping led to various processes of salinization by geochemical interaction of the sediment with water and by seawater intrusion observed near the coastal zone.
Water–rock interaction processes
Most transformations on the earth’s surface are controlled by interactions between water and rocks. The minerals that make up the rocks react with aqueous solutions; primary minerals are dissolved, thus freeing ionic species in solution and secondary minerals precipitate at thermodynamic equilibrium or in oversaturated domain. The alteration processes are usually very complex which is the reason why geochemical models have been developed, to understand and simulate the reaction occurring between a set of minerals, constituting a rock and an aqueous solution at thermodynamic disequilibrium (Clement et al. 1994).
In the present study, saturation indexes (SI) with respect to carbonate (dolomite, calcite and aragonite) and evaporate (gypsum and anhydrite) minerals, as well as activities of soluble species, were calculated by using the chemical programme PHREEQC (Parkhurst and Apello 1999); the expression of Debye and Huckel is used for computation of coefficient activities because all investigated groundwaters have generally higher total dissolved solids.
Figures 13 and 14 show the plots of SI against total dissolved solids, for all the investigated groundwater in the following discussion. We may assume that SI values falling with range ±0.5 U from 0 indicate the equilibrium state (Plummer et al. 1976). Most of the considered groundwaters are undersaturated with respect to dolomite, aragonite and calcite indicating that these carbonate mineral phases could not have influenced the composition of groundwater of this study area (Fig. 13). On the other hand, groundwater samples are found to be saturated with respect to evaporate minerals (Fig. 14) indicating phases going dissolution for anhydrite and gypsum, which explains the high concentration of evaporate elements in groundwater investigated.
Groundwater assessment for irrigation use
The water quality evaluation in the area of study is carried out to determine their suitability for agricultural purposes. Nitrate is a major contaminant of water; the agricultural irrigation effluent, industrial wastewater discharge and domestic effluents have largely contributed to contamination of groundwater. In addition, the high nitrate in humans or animal drinking water is associated with a number of health problems like methemoglobinemia. High nitrate and phosphorus levels present in groundwater that discharges into surface waters (McCobb et al. 2003) can contribute to long-term eutrophication of water bodies.
Nitrate concentrations of waters (Table 2) were found above the World Health Organization (WHO 1998) recommended limit (45 mg l−1), especially for those samples accruing in rural areas. Almost 40% of samples for the period 2007 showed concentrations greater than 45 mg l−1. The massive usage of artificial fertilizers is not only the direct consequences of high nitrate concentration in groundwater of study area but also domestic septic tanks are a source of contamination. The absence of sanitation network in the whole region contributes to the nitrification of ground waters according to the process. Nitrate concentrations vary greatly from 1.3 mg l−1 (P30) to 378 mg l−1 (P16) for the period 2007 (Fig. 15).
Levels of nitrates in groundwater in some wells are higher than the safe levels proposed by the WHO and thus pose a threat to human health. Due to the detrimental biological effects, treatment and prevention methods must be considered to protect groundwater aquifers from nitrate leaching and high concentrations. While prevention, such as reduced dependence on nitrogen-rich fertilizers, imposed a protection, perimeter particularly around rural private wells can lower the risk of nitrates in the study area.
The suitability of groundwater for irrigation is dependent on the mineral constituents of water on both the plant and the soil. The sodium adsorption ratio (SAR) of the water is defined by the following equation (Richards 1954):
Ions in the equation are expressed in milliequivalents per litre.
The SAR is used to classify the risks associated with sodium in irrigation water sources. Water with a SAR over 10 will accumulate sodium in the soil. High SAR can act to disperse soil aggregates, which in turn reduces the number of large pores in soil (Grattan 2002). Sodium affects the deflocculation of a clay soil, thus decreasing its capillary porosity (air porosity) and its water infiltration rate. Such effects on the soil structure are particularly harmful on plants.
Using the SAR, irrigation waters can be divided into four classes. Such classification is primarily based upon the sodium effects on the soil physical conditions (Ayers and Westcot 1988). The sodium adsorption ratio was plotted on the Wilcox diagram (USA Salinity Laboratory) (Fig. 16). The waters were found mostly confined in five classes of water type: C4S1 which has low sodium alkalinity hazards and C3S2, C4S2 and C4S3 which have medium to high sodium alkalinity hazards and very high salinity. Therefore, the water quality in the study area is ranging between doubtful to unsuitable for irrigation uses Table 5.
Conclusions
The hydrochemical features of the Mio-Plio-Quaternary aquifer system of the northern Sfax have been investigated based on multivariate statistical analysis and bivariate conventional diagrams to determine the main hydrochemical processes of the salinization and to identify the origin of groundwaters. It was possible to demonstrate that groundwater mineralization is mainly controlled by water–rock interaction and anthropogenic process. Indeed, waters are generally of calcium sulphate and sodium-chlorinated types. The quality of groundwater, mainly saturated with gypsum and anhydrite minerals, is controlled by the dissolution of evaporates minerals. The study revealed that many private wells suffer from nitrate contamination. The anthropogenic sources are really the ones that most often cause the amount of nitrate to rise to a dangerous level. Many local sources of potential nitrate contamination of groundwater exist, such as sites used for disposal of human and animal sewage and septic tanks.
PCA were used to differentiate the water samples on the basis of their chemical compositions. It rendered considerable variable reduction and clearly distinguish between variables group.
These components revealed that the hydrochemical process was the major factor responsible for shallow groundwater quality; the second factor identified is attributed to salinization, due to the overexploitation process which induced the deterioration of groundwater quality and nitrate pollution, due to fertilization and absence of sanitation network.
US Salinity Laboratory Staff diagram reveals that most of the groundwater samples are not suitable for irrigation purposes under normal condition. Also, the salinity hazard for the most water wells is classified as medium and high salinity.
Therefore, salinity is the principal concern in irrigated agriculture in northern Sfax area. High salinity may be related to precipitation and dissolution processes within Mio-Plio-Quaternary formations, evaporate deposits and overexploitation in the study area.
Since the water is used in variable human activities in the northern Sfax, the groundwater quality is thus threatened; the cartography of water characteristics should help to control the quality and guide modes of water utilization.
References
Ayers RS, Westcot DW (1988) Water quality for agriculture. FAO Irrigation and Drainage Paper No, 29 (Rev 1), Food and Agriculture Organization of the United Nations
Ben Brahim F (2006) Actualisation de l’étude hydrogéologique des nappes phréatiques de Sfax-Nord. Mémoire de Mastère, faculté des Sciences de Sfax
Beni Akhy R (1994) Etude de l'évolution et modélisation mathématique de la nappe phréatique urbaine de Sfax. Mémoire DEA, faculté des Sciences de Tunis, Université de Tunis II, Tunisie, pp 117
Bouri S, Abida H, Khanfir H (2008) Impacts of wastewater irrigation in arid and semi arid regions, Tunisia. J Environ Geol 53:1421–1432
Clement A, Fritz B, Made B (1994) Thermodynamic and kinetic modelling of digenetic reactions in sedimentary basins. Description of the geochemical Code KINDISP. French Institute of Petroleum 49:569–602
Davis SN, Dewiest RJM (1966) Hydrogeology. Wiley, New York, p 463
Durov SA (1948) Natural waters and graphic representation of their compositions. Dokl Akad Nauk SSSR 59:87–90
Garcia MG, Del Hidalgo M, Blesa MA (2001) Geochemistry of groundwater in the alluvial plain of Tucuman province, Argentina. J Hydrol 9:597–610
Grattan SR (2002) Irrigation water salinity and crop production. Publication 8066, FWQP references sheet 9.10. Division of Agriculture and Natural Resources, University of California (USA)
Hajjem A (1979) Etude hydrogéologique de la plaine de Djebeniana. Rapport interne du Commissariat régional de développement agricole, Sfax, Tunisie
Helena B, Pardo R, Vega M, Barrado E, Fernandez JM, Fernandez L (2000) Temporal evolution of groundwater composition in an alluvial aquifer (Pisuerga river, Spain) by principal component analysis. Water Res 34:807–816
Hidalgo MC, Cruz-Sanjulian J (2001) Ground water composition, hydrochemical evolution and mass transfer in a regional detrital aquifer (Baza Basin, southern Spain). Appl Geochem 16(7–8):745–758
Kaiser HF (1958) The Varimax criteria for analytical rotation in factor analysis. Psychometrika 23:187–200
Lloyd WJ, Heathcotte JA (1985) Natural inorganic hydrochemistry in relation to groundwater. Clarendon, Oxford
Maliki A (1994) Etude hydrochimique et isotopique des nappes phréatiques de Skhira et de Djebeniana et de la nappe profonde de Sfax. Mémoire de DEA, Faculté des Sciences de Tunis, Université de Tunis II, Tunisie, pp 127
Maliki A (2000) Etude hydrogéologique, hydrochimique et isotopique de la nappe profonde de Sfax (Tunisie). Thèse de Doctorat, Faculté des Sciences de Tunis, Université de Tunis II, Tunisie, pp 300
Mc Lean W, Jankowski J, Lavitt N (2000) Groundwater quality and sustainability in an alluvial aquifer, Australia. In: Sielilo et al (eds) Groundwater, past achievement and future challenges. Balkema, Rotterdam, pp 567–573
McCobb TD, LeBlanc DR, Walter DA, Hess KM, Kent DB, Smith R (2003) Phosphorus in a groundwater contaminant plume discharging to Ashumet Pond, Cape Cod, Massachusetts, 1999. Water Resources Investigations Report 02-4306, US Geological Survey
Muller J, Kylandern M, Martinez-Cortizas A, Wust RAJ, Weiss D, Blake K, Coles B, Garcia-Sanchez R (2008) The use of principle component analyses in characterising trace and major elemental distribution in a 55 kyr peat deposit in tropical Australia: implications to paleoclimate. Geochim Cosmochim Ac 72(2):449–463
Parkhurst DL, Apello CAJ (1999) User guide to PHREEQC (version2) a computer program for speciation, batch reaction, one-dimensional transport, and inverse geochemical calculations. U.S. Geological Survey, Water Resources Investigations Report 99-4259.
Piper AM (1944) A graphic procedure in the geochemical interpretation of water analyses. Trans Am Geophys Union 25:914–923
Plummer LN, Jones BF, Trusedall AH (1976) WATEQ-a Fortran IV version of WATEQ a computer program for calculating chemical equilibrium of natural waters. U.S. Geol-Surv. Water Res, Washington, DC, vol 76, pp 13–61 (Revised 1978, 1984).
Richards LA (1954) Diagnosis and improvement of saline and alkali soils. US Department of Agriculture handbook 60. US Department of Agriculture, Washington, DC, p 159
Trabelsi R (2003) Hydrogéologie de la nappe phréatique nord du sahel de Sfax : Evolution de la minéralisation et de l'intrusion marine. Mémoire de mastère, Faculté des Sciences de Tunis
Trabelsi R, Zaïri M, Smida H, Ben Dhia H (2005) Salinisation des nappes côtières: cas de la nappe nord du Sahel de Sfax, Tunisie. Geoscience 337:515–524
WHO (1998) Guidelines for drinking water quality. Addendum to vol 2, 2nd edn. Health criteria and other supporting information (WHO/EOS/98.1). World Health Organization, Geneva
XLSTAT (2008) Data analysis and statistical solution for MS Excel, Web. http://www.xlstat.com. Accessed 4 Nov 2008.
Zbidi H (1989) Hydrogéologie de la nappe profonde de Sfax. Rapport DGRE, Tunis
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ben Brahim, F., Bouri, S. & Khanfir, H. Hydrochemical analysis and evaluation of groundwater quality of a Mio-Plio-Quaternary aquifer system in an arid regions: case of El Hancha, Djebeniana and El Amra regions, Tunisia. Arab J Geosci 6, 2089–2102 (2013). https://doi.org/10.1007/s12517-011-0481-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12517-011-0481-6