Abstract
A comprehensive assessment of groundwater is carried out in parts of the Southern Gangetic Plain to identify the hydrogeochemical processes influencing groundwater geochemistry and to ascertain the suitability of groundwater for drinking and irrigational uses. Results of detailed chemical analysis reveal that groundwater of the study area is slightly acidic (pH values from 6.2 to 7.37) during post-monsoon, with dominant concentrations of Na+ and Ca2+ cations and HCO3 − and Cl− anions. Groundwater chemistry is largely controlled by rock-weathering and ion-exchange processes with secondary contribution from anthropogenic sources. In particular, values of EC, total hardness and concentrations of Na+ and HCO3 − exceed the prescribed limits of Indian and WHO water quality standards. Suitability of groundwater for irrigation purposes are evaluated on the basis of sodium adsorption ratio, sodium percent (%Na+), magnesium hazard, residual sodium carbonate and Kelley’s index. In this regard, nearly 40–50 % of the groundwater in the study area is found to be of excellent to good quality. In addition, principal component analysis, performed to identify the dominant geochemical processes, shows that the first three components are together account for 80.84 and 78.85 % of the total variance in the dataset for post-monsoon and pre-monsoon periods, respectively. This analysis also reveals key contributions from hydrogeochemical processes like mineral dissolution, silicate and carbonate weathering alongside anthropogenic activities like leaching of fertilizers from agricultural lands.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Groundwater is a vital natural resource and a primary source of freshwater for domestic, agricultural and industrial requirements in India. Roughly, 80 % of rural water use and ~50 % of urban water supply are met by extraction of groundwater only (Singh et al. 2013). Owing to rapid growth in industrialization, urbanization, population, and associated agricultural activities, groundwater resources in various parts of India are under severe stress almost permanently (Satapathy and Syed 2015). Over the past 50 years, expansion of groundwater-based irrigation has played a lead role in the country’s food security (CGWB 2010). With an increasing demand for groundwater, the quality of water has conjunctively deteriorated along with its quantity (Nandimandalam 2012). As a result, India is faced with serious issues related to the deteriorating quality of this vital natural resource (Sharma and Patel 2010).
Chemical characteristics of groundwater play an important role in assessing and classifying the quality of water. Groundwater quality depends on a number of factors such as geology, degree of chemical weathering of the various rock types, quality of recharge water, and water–rock interaction (Domenico 1972). Quality of groundwater is also influenced by it’s over exploitation, particularly in the coastal regions, where heavy withdrawal of freshwater leads to sea water intrusion and consequent increase in salinity of groundwater (CGWB 2010; Sophiya and Syed 2013). Temporal changes in the origin and contribution of recharged water alongside other hydrologic factors also cause periodic changes in groundwater quality (Laluraj and Gopinath 2006; Raju 2006; Rouxel et al. 2011). Water pollution not only affects the water quality but also threatens human health, economic development and social prosperity (Singh et al. 2013).
Geochemical studies of groundwater are essential to characterize variations in water quality as well as to assess its suitability for various purposes. During the past decade, various geochemical methods have been successfully used to assess groundwater quality (e.g., Park et al. 2005; Naik et al. 2009; Xiao et al. 2012). Consequently, numerous recent studies have focussed on analysing the natural concentrations of several ions and metals in groundwater, to isolate anthropogenic and natural sources that affect groundwater quality, and establish interactions that take place within the aquifer (e.g., Raju 2007; Singh et al. 2008; Ramesh and Elango 2011). In particular, a large number of groundwater studies have focused specifically on the assessments of its suitability for drinking and irrigation purposes (Subramani et al. 2005; Saha et al. 2008; Aghazadeh and Moghaddam 2010; Bozdag and Gocmez 2013). The majority of the previous work carried out in the Southern Gangetic Plain (SGP) has primarily focussed on the geochemical evolution of groundwater (Saha et al. 2008; Sahu and Saha 2015). These studies revealed localized occurrence of high salinity and nitrate and fluoride concentrations. Also of great concern is the issue of groundwater contamination due to the presence of arsenic in certain parts of Eastern India (e.g., Acharya et al. 1999; Sikdar and Chakraborty 2008; Chakraborty and Sikdar 2009; Saha et al. 2010). Overall, hydrogeochemical processes responsible for altering the chemical composition of groundwater vary rapidly with respect to space and time. Hence, comprehensive hydrogeochemical investigation is crucial to asses, identify and evaluate the chemical processes that affect the groundwater quality (Selvam et al. 2013).
Additionally, the composition of groundwater in the shallow aquifers of the SGP is potentially threatened by anthropogenic activities such as uncontrolled disposal of industrial effluents, urban wastes and enhanced utilization of fertilizers and pesticides (Saha and Alam 2014). Groundwater contamination by pesticides is highly unpredictable and influenced by various mechanisms (Shukla et al. 2006). Mostly carried by surface water, these contaminants further modify the composition and concentration of various constituents in groundwater (Prasanna et al. 2011). As a result, several studies have focused on hydrogeochemical characterization and contamination of groundwater due to anthropogenic activities (Raju 2007; Singh et al. 2008). Hence, monitoring and assessment of groundwater quality is one of the important facets for sustainable development and provides important information for water management.
The study area, a part of the SGP, is a relatively flat terrain with intensive irrigation based agriculture. There are mainly two cropping seasons, monsoon (July–October) and winter (October–March), with cropping intensity of 69 and 74 %, respectively. Major part of irrigation in these seasons goes to wheat and paddy cultivation. Due to large-scale utilization of groundwater for cultivation, contamination and declination of groundwater resources is rampant in the study area. Since the groundwater of the study area is being used perilously to meet the requirements of rural and urban use, any degradation in the quality of groundwater would have critical baneful repercussions. Therefore, it is extremely important to characterize the geochemical constituents and understand the hydrogeochemical processes persistent in the area. In the present study, a detailed geochemical assessment of groundwater of the study area is carried out to define the quality of groundwater for drinking and irrigation purposes, based on major ion chemistry, elemental ratios, principal component analysis (PCA) and statistical correlations.
Study area and hydrogeological framework
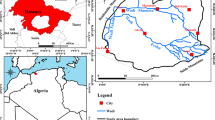
The study area covers approximately 600 km2 of the Southern Gangetic Plain (Rajgir-Nalanda area of Nalanda District) in the state of Bihar, India and covers a part of the Survey of India toposheet no. 72 G/8. The extent of the study area is from 85°15′E to 85°35′E longitudes and 25°15′N to 25°00′N latitudes (Fig. 1). The study area is flanked by the Paimar River in the west, Panchana River in the east and Rajgir Hills in the south, forming the Southern-most boundary. Physiographically the area varies from being hilly and undulating, in the south, to a flat terrain, in the north. The drainage of the area is primarily controlled by the Ganga river, flowing towards the east, and a number of ephemeral streams originating from the southern hilly regions, flowing towards the NNE direction. The study area experiences tropical to sub-tropical climate with an average annual rainfall of 1205 mm. The rainfall, primarily from the southwest monsoons, is also the major source of recharge into the aquifers of the study area (Saha et al. 2008). The Panchana and Paimar Rivers give rise to primarily sub-dendritic and sub-parallel drainage patterns. The study area consists of Pleistocene alluvial deposits of the Nawada Formation (Chakroborty and Chattopadhyay 2001) (Fig. 1). Stratigraphically, the alluvial deposits of Nalanda district are classified as (a) Nawada Formation (upper Holocene to Lower Pleistocene) and (b) Fatwa Formation (of middle to upper Holocene age) (Chakroborty and Chattopadhyay 2001). Lithologically, the Pleistocene deposits of Nawada Formation are made up of medium to coarse grained sands alternating with clay, sandy clay and silty clay. The top 30 m of the succession are pre-dominantly fine grained, essentially made up of clay, sandy-clay and silty-clay with thin lenses of intercalated sand, and constitutes the shallow aquifer of the study area. At a deeper level, layers of sand, of various size grades, forms the potential water-bearing zone and are referred to as the deep aquifer. Groundwater movement is sluggish within the shallow aquifer with hydraulic conductivity ranging from 3 × 10−2 to 9 × 10−2 m/day (Saha et al. 2008). The Precambrian basement rocks are exposed as Rajgir hills near the southern-most boundary, trending ENE-WSW. The maximum elevation attained by the Rajgir hills is 371 m above MSL (De 1986). The groundwater level in the study area varies between 0.7–5 and 1.70–8.20 mbgl during POM and PRM periods, respectively. The overall groundwater flow in the study area is towards north with some localized variation in the central part (see Fig. S1 for details).
Methodology
Sampling and geochemical analysis
A systematic sampling was carried out in the Post- and Pre-Monsoon periods, i.e., during the months of October 2012 and April 2013, respectively. Each time, thirty three (33) groundwater samples were collected in pre-washed 1 l polythene; narrow mouth, bottles from dug wells and the bottles were rinsed twice before sampling. All the samples were collected from wells tapping the shallow aquifer at depths of about 20–25 mbgl. 100 ml filtered groundwater samples were acidified with nitric acid for cation analysis where as non-acidified samples are used for anion analysis. The filtered samples were preserved at 4 °C and all the geochemical analysis were completed within a week. Samples are analyzed for physico-chemical attributes like pH, electrical conductivity (EC), total hardness (TH), total dissolved solids (TDS) and major cations and anions (e.g., calcium (Ca2+), magnesium (Mg2+), sodium (Na+), potassium (K+), bicarbonate (HCO3 −), chloride (Cl−), nitrate (NO3 −) and sulfate (SO4 2−) in the laboratory using the standard methods formalized by the American Public Health Association (2005). EC and pH are measured by an EC meter and pH meter, respectively. Major cations (Ca2+, K+, Na+) are measured using a flame photometer after calibrating the instrument with known standards while major anions (SO4 2−, NO3 −) are analyzed using UV spectro-photometer. Acid titration method is used to determine the concentration of bicarbonate (HCO3 −), chloride (Cl−) and total hardness of the samples. Chloride analysis is carried out by volumetric titration by using AgNO3 and K2CR2O7 solution, while bicarbonate is determined by HCl and methyl orange indicator solution by titration method. The results are evaluated in accordance with the drinking water quality standards given by the World Health Organization (2011) and Bureau of Indian Standards (2003).
The quality of water used for irrigation is essential for the yield and health of crops, maintenance of soil productivity and protection of the environment (Singh et al. 2013; Vasanthavigar et al. 2012). Hence, chemical properties of groundwater play a significant role in assessing the quality of water suitable for irrigational purposes. Some of the most commonly used indices to assess the suitability of groundwater for irrigational purposes include sodium adsorption ratio (SAR), sodium percentage (%Na), residual sodium carbonate (RSC), magnesium hazard (MH), and Kelley’s index (KI) (Ramesh and Elango 2011; Singh et al. 2012). These indices are formalized as follows:
Statistical analysis
To characterize the interaction between groundwater quality parameters and to identify the processes controlling the variability of groundwater quality in Rajgir-Nalanda area of the SGP, principal component analysis (PCA) is performed on the physio-chemical attributes of the sampled data. The primary objective of PCA is to reduce the dimensionality of multivariate datasets. PCA is widely used for transforming a given set of interrelated variable into a new set of variables called principal components. The set of principal components generated presents uncorrelated linear combinations of the original variable and accounts for the total variance of the original data (Mahapatra et al. 2012). All the principal components are generated in such a way that they are orthogonal to each other. Principal components are generated in a sequentially ordered manner with decreasing contributions to the variance, i.e., the first principal component explains most of the variations present in original data and successive principal components account for decreasing proportions of variance. Previously, PCA has been used to understand the relationship between different water quality parameters and the interpretation of geochemical processes (e.g., Sikdar et al. 2001; Sikdar and Chakraborty 2008; Saha et al. 2008; Dudeja et al. 2013). The principal components are primarily eigen vectors of the variance–covariance matrix (Davis 1986; Syed et al. 2004). The rotation of principal components is widely used to transform the factors to simpler and more interpretable constructs (Saha et al. 2008). Varimax rotation procedure has been used here to compute the rotated factor matrix (Davis 1986). Even though, the maximum number of principal components is equal to the number of variables, only a few numbers of components are retained in the analysis for the purpose of interpretation (Sikdar and Chakraborty 2008). Factors with value close to ±1 indicate strong correlation between a variable and the factor, while those with values close to zero indicate weak correlation. The variables showing a rotated factor of value greater than 0.5 are considered as significant (Saha et al. 2008) in this study.
Results and discussion
Groundwater quality determines its suitability for different purposes depending upon specific standards. In this study, the drinking water standards of WHO (2011) and BIS (2003) forms the basis for the evaluation of groundwater quality for drinking purpose. Statistical measures of the analyzed groundwater quality parameters along with the limits prescribed by WHO (2011) and BIS (2003), for drinking water, are listed in Table 1.
Hydrogeochemical characteristics of groundwater
The pH of the collected groundwater samples varied slightly between the pre-monsoon (PRM) and post-monsoon (POM) periods. The value of pH ranges between 6.2–7.37 and 6.6–7.9 during POM and PRM, respectively, thereby indicating slightly acidic conditions during the POM period. The slightly acidic nature of groundwater is probably due to the combination of free CO2 and rainwater that form carbonic acid, which affects the pH of water. Majority of the groundwater samples are within the permissible limit of 6.5–8.5 prescribed for drinking water by BIS (2003) and WHO (2011). Electrical conductivity (EC) is an important parameter to isolate areas prone to salinity hazards and those with suitable groundwater quality for irrigational purposes. Values of EC vary from 10–2292 to 2.14–1982 µs/cm during POM and PRM, respectively. A slightly higher value is noted during POM, in comparison to PRM. Higher value of EC during POM can be due to the dissolution of minerals and the influence of anthropogenic contamination, causing increases in ionic concentration. Large variations in EC values are primarily attributed to geochemical process like ion-exchange, evaporation, sediment dissolution, and rainwater infiltration (Saha et al. 2008).
Total dissolved solid (TDS) in the groundwater samples of the study area vary from 6.7–1535 mg/l (during POM) to 1.43–1327 mg/l (during PRM). High concentration of TDS in the groundwater samples is probably due to the leaching of salts from the soil above and certain anthropogenic activities. As a primary assessment of its suitability for different purposes, groundwater is essentially classified on their TDS values (Freeze and Cherry 1979). The values of TDS obtained for almost all the groundwater samples collected in this study are within the range of fresh water type.
The hardness of water primarily depends upon the amount of divalent metallic cations, of which Ca2+ and Mg2+ are the most abundant in groundwater. Total hardness (TH) in the study area ranges from 4 to 820 mg/l, with an average value of 416.8 mg/l, in PRM and 10–726 mg/l, with an average of 303.2 mg/l, in POM. A generalized classification of groundwater based on the value of TH (Sawyer and McCarty 1967) shows that the majority of groundwater samples fall in the category of hard to very hard water.
Major cation concentration in the groundwater of the study area are in the order of Na+ > Ca2+ > Mg2+ > K+. The concentration of Na+ in the study area ranges from 15.6 to 184.6 mg/l, with an average of 90.75 mg/l, in POM and 7.7–275.5 mg/l, with an average 124.02 mg/l, in PRM period. According to WHO (2011) guidelines, the maximum admissible limit is 200 mg/l. Thus, ~20 % of the groundwater samples, of the PRM period, in the study area are above the permissible limit. Alongside negative impacts on human health, sodium concentration plays an important role in evaluating the quality of groundwater for irrigation. Increases in the concentration of sodium causes an increase in the hardness of soil and thus reduces its permeability. Significantly higher concentration of Na+ in groundwater of the study area is likely associated with weathering of silicate rocks and ion-exchange with clay. The concentration of Ca2+ and Mg2+ are within the permissible limit and are mostly related to the weathering of rocks. Concentration of K+ varies with a mean value of 33.41 and 20.71 mg/l in pre- and post-monsoon periods, respectively. As per WHO (2011) standards, the maximum allowable limit for K+ is 200 mg/l. The concentration of K+ in groundwater is possibly due to the occurrence of geogenically produced salt patches and/or fertilizer leaching through the subsurface.
The dominance of anions in the sampled groundwater of the study area is in the order of HCO3 − > Cl− > SO4 2− > NO3 −. While the concentration of bicarbonate (HCO3 −) varies between 55.13 and 798 mg/l for samples collected during POM period and 25.13–784.14 mg/l for the samples collected during PRM period. The average concentration of HCO3 − for POM and PRM periods are estimated to be 445.23 and 484.14 mg/l, respectively. Higher concentration of HCO3 − in the groundwater samples can be attributed to agricultural return flows where dissolved carbonate minerals are being precipitated in the soil due to evaporation, which is a common process in such arid agricultural areas. Chloride (Cl−) is the second most dominant anion and its concentration range with an average of 55.16 and 58.23 mg/l during PRM and POM, respectively. Higher values of Cl− are indicative of secondary sources such as sewage effluent, breakdown of organic material and agricultural runoff. The sulfate (SO4 2−) ion concentration in the study area varies with an average value of 64.5 mg/l during PRM and 29.39 mg/l during POM period. Sulfate ions present in the groundwater of the study area are likely to have been generated from multiple sources, such as dissolution of sulfate minerals, oxidation of sulfide minerals and anthropogenic sources. Similarly, NO3 − concentration in the study area ranges between 0.29 and 14.55 mg/l during PRM, with an average of 5.89 mg/l, and between 0.36 and 14.17 mg/l during POM, with an average of 6.74 mg/l. The higher nitrate concentration during the post-monsoon period can also be attributed to surface water contamination of certain wells due to improper sealing of the well walls.
Hence, most of these values are well within the permissible limits prescribed by WHO (2011) for drinking water purposes. Approximately 97 % of the analyzed samples are having charge balance error within 5 % (ranges from 0.28–4.74 %), while the rest are having errors in the range of 5–10 % that are mostly due to some erroneous laboratory analysis or instrument error.
Hydrogeochemical facies
Evolution of the hydrogeochemical parameters of groundwater is examined by plotting the concentrations of major cations and anions in a Piper Diagram (Piper 1944). The analytical data obtained from the groundwater samples, plotted on a Piper trilinear diagram, also enables us to understand the hydrogeological regime of the study area during PRM and POM periods (Fig. 2).
Figure 2 illustrates a Piper Diagram with data points representing sampled groundwater for both PRM and POM periods in the study area. The distribution of cations in the Piper diagram shows that nearly 30 and 69 % of groundwater samples collected during POM and PRM period fall within the sodium and potassium field. In the anionic triangle, HCO3 − ions shows clear dominance. The diamond shaped field reveals that alkali (Na+ + K+) concentration surpasses the concentration of alkaline earth metals (Ca2+ + Mg2+) and weak acids exceeds the strong acids. Piper diagram reveals that nearly 65 and 30 % of the analyzed groundwater samples fall in the field of Ca2+–Mg2+–HCO3 − type for both POM and PRM period. The rest of the samples fall in the field of mixed Ca2+–Na+–HCO3 − type.
Hydrogeochemical processes
Weathering, ion-exchange processes, and inputs from atmospheric and anthropogenic sources are the major solute acquisition mechanisms controlling the concentration of chemical constituents in the groundwater (Xiao et al. 2012). Gibbs diagram (Gibbs 1970) represents the ratio of Na+/(Na+ + Ca2+) and Cl−/(Cl− + HCO3 −) as a function of total dissolved solids (TDS), which is widely used to assess functional sources of dissolved chemical constituents such as precipitation, rock-weathering and evaporation. The geochemical data of groundwater samples collected from Rajgir-Nalanda area are plotted in Gibb’s diagram shown in Fig. 3. The Gibb’s diagram portrays that most of the samples fall in the category of rock-weathering, with minor influence from evaporation. Hence, chemical weathering of minerals present in the aquifer rocks is the primary process involved in regulating the ionic composition of groundwater in the study area.
The source of dissolved constituents in groundwater can also be evaluated by looking at the relative abundance of individual ions, ionic ratios and their correlations. Ions of calcium, magnesium, sulfate, and bicarbonate are the primary constituents that are added to groundwater during rock-weathering, depending on the mineralogy of the aquifer rock. Cation exchange reactions are important geochemical reactions which control the distribution and occurrence of ions in groundwater. Cation exchange process can be identified using a relationship between the Na and Cl− ions. High concentration of Na+ with respect to Cl− or depletion of Na+ with respect to Cl− is the evident of cation exchange reactions (Senthilkumar and Elango 2013). The plot of Cl− versus Na+ (Fig. 4a, b) indicates that most of the sample points are near or above the 1:1 equiline, indicating that the area is characterized by the depletion of Na+ with respect to Cl−, which is conclusive of the dominance of ion–exchange reactions (Senthilkumar and Elango 2013).
The scatter plot of (Ca2+ + Mg2+) concentration against that of (HCO3 − + SO4 2−) is a quantitative indicator of the genesis of ionic concentration in groundwater. Groundwater samples lying close to the 1:1 line signifies the prevalence of mineral dissolution, such as that of calcite, dolomite and gypsum. Samples plotted towards the right of the 1:1 line, due to a large excess of (HCO3 − + SO4 2−), indicates the dominance of ion-exchange as the primary process (Singh et al. 2012). Most of the samples in this study fall near or below 1:1 line in Fig. 4c, d, thereby reconfirming the dominance of weathering and ion-exchange processes.
Correlation analysis
Here correlation coefficients are computed to demonstrate the degree of correspondence among the different hydrogeochemical parameters of groundwater in the study area. The computed correlation matrix of the 11 measured parameters is given in Table 2a, b for POM and PRM periods, respectively. During the POM period, strong correlation between Ca2+–Mg2+ (0.863), HCO3 −–Ca2+ (0.925), HCO3 −–Mg2+ (0.876), Na+ and HCO3 − (0.698), Na+–Cl− (0.692), NO3 2−–Mg2+ (0.688) and Ca2+–Na+ (0.616) indicates that Na+, Ca2+, Mg2+, HCO3 − are primarily derived from silicate and carbonate weathering. Moderate correlation observed between NO3 2−–SO4 2− (0.572) and Cl−–HCO3 −(0.561) suggests that anthropogenic activities are responsible for these relationships (Vasanthavigar et al. 2012). The pair-wise relationship between physico-chemical parameters show that carbonate and silicate weathering along with anthropogenic sources, probably leachates from fertilizer use, control the geochemistry of POM groundwater. It is also important to note that alongside leaching, groundwater concentrations can be impacted by surface water contamination, particularly during the post-monsoon period, due to the loss of well integrity. During the PRM period, good correlation is observed between Na+–Cl− (0.667), Mg2+–SO4 2− (0.642), HCO3 −–SO4 2− (0.821), Cl−–SO4 2− (0.775), suggesting dissolution of sulfate minerals and weathering of magnesium and sodium sulfate minerals as the primary source (Esmaeili and Moore 2011).
Evaluation of water quality for irrigation
The suitability of groundwater for irrigational purposes depends upon the effect of mineral constituents on both plants and soils. Excess concentration of dissolved ions in water can affect plants as well as the physico-chemical properties of soils, which would lead to lower productivity and destruction of soil structure (Bozdag and Gocmez 2013; Ravikumar et al. 2010). The general criteria for assessing irrigation water quality are total salt concentration as measured by EC and relative proportions of Na+ as expressed by sodium adsorption ratio (SAR), sodium percentage (%Na+) and residual sodium carbonate (RSC). Sodium percent and electrical conductivity are important parameters that can control the use and classification of groundwater for irrigation. The presence of excess Na+ negatively affects plant growth, soil structure, permeability and aeration (Singh et al. 2008).
There is a significant relationship between SAR values and the extent to which sodium is absorbed by soils. The SAR values computed for this area ranges from 0.9 to 4.9 during POM and 0.6 to 15.4 during PRM periods (Table 3). Groundwater with SAR values greater than 9, when used for irrigational purposes, significantly affects the permeability and the shrinking and swelling properties of clayey soils (Saleh et al. 1999). Plotting of sampled groundwater data in the US salinity diagram shows that approximately 65 % of the groundwater belongs to the C3S1 category, indicating that the groundwater of the study area is of medium to high salinity and with low sodium content (Fig. 5). Samples belonging to C2S1 and C1S1 categories are low in salinity and sodium hazard and are therefore best suited for irrigation purposes.
Shown in Fig. 6 is the disposition of the sampled groundwater in a Wilcox diagram (Wilcox 1955). Figure 6 clearly illustrates that about 36 % of the samples fall in excellent to good category and 12 % of the samples fall in the category of good to permissible for irrigational purposes, during the PRM period. During the POM period, about 36 % of the samples are of the category of excellent to good, while 54 % of the samples are of good to permissible quality. The remaining 52 % (of PRM) and 6 % (of POM) fall in the permissible to doubtful and doubtful to unsuitable category.
The quantity of bicarbonate and carbonate in excess of alkaline earths (Ca2+ + Mg2+) is expressed as RSC (Eq. 3), which also influences the suitability of water for irrigation. The presence of anions such as HCO3 − and CO3 2− in irrigation water tend to precipitate calcium and magnesium ions in the soil, resulting in an increased proportion of sodium ions (Karanth 1989). According to the US Salinity Laboratory (USSL), RSC values less than 1.25 meq/l (Milliequivalent per liter; Milliequivalent weight is the atomic or molecular weight divided by the valence or charge of the ion) is safe for irrigation, a value between 1.25 and 2.5 meq/l is of marginal quality and a value higher than 2.5 meq/l is unsuitable for irrigation (USSL 1954). In this study, ~58 % of the samples have RSC values less than 1.25 meq/l, which indicates that the samples are of safe quality for irrigation, 21 % of the samples are of marginal quality for irrigational purposes and rest of the samples are unsuitable for irrigational purposes.
Further, groundwater of the study area is classified, for irrigational purpose, using Kelley’s index (Eq. 5). This index is computed on the basis of Na+ concentration measured against those of Ca2+ and Mg2+ (Kelley 1957). Groundwater with Kelley’s index less than 1 is considered suitable for irrigation and those with Kelley’s index greater than 1 indicates dominance of Na+ in groundwater and therefore unsuitable for irrigation. While 57 % of the groundwater samples are observed to be suitable for irrigation during POM, only 24 % of them are found to be suitable during the PRM season. Overall interpretation of all these classifications reveal that nearly 40–50 % of the sampled groundwater in the area are suitable for irrigation, while the remaining are marginal/doubtful or unsuitable for irrigation.
Principal component analysis (PCA)
For a better understanding of the groundwater system and its chemistry, multivariate statistical analysis is performed using hydrogeochemical data. The main objective of principal component analysis (PCA) is to identify patterns by compressing the data without much loss of information (Vasanthavigar et al. 2012). It is designed to convert the original variables into new uncorrelated variables called the principal components, which are the linear combination of original variables. In this analysis, correlations among variables are used to estimate principal components (PCs) with common associations and associated constituent loading on the principal component axis (Lucas and Jauzein 2008). The aim of PCA in hydrocgeohemistry is to explain the observed relations amongst the variables, also called factors. The factor loadings, also called component loadings, in PCA are the correlation coefficients between the variables (rows) and factors (columns) in the data matrix. The Kaiser criterion (Kaiser 1960) is applied here to determine the number of significant components in the data set. The data matrix consisting of 10 variables and 33 observations have been used for the PCA. Three components are identified to statistically represent the contributions influencing the chemical composition of groundwater during POM and PRM periods. Results indicate that the first three components together explain about 80.84 and 78.85 % of the variances in the dataset (Table 4) for POM and PRM periods, respectively. The eigen values of the respective principal components are represented in a scree plot for POM (Fig. 7a) and PRM (Fig. 7b) periods. A scree plot is used to identify the number of useful principal components. This identification is made by noting the component number at which there is a sharp break in the slope of the scree plot. The change in the slope of a scree plot is resultant of episodic change in the magnitude of eigen values. In Fig. 7, slope of the plots change from steep to shallow after the first three factors. Hence, in this study we have considered only the first three components. Factor loadings, eigen values, percentage of variance and cumulative percent for first three components considered in this study are given in Table 5.
The first principal component accounts for 57.80 and 48.06 % of the total variance observed in the groundwater quality of POM and PRM periods. While the highest loading values of the first principal component are observed for TDS, Ca2+, Mg2+, HCO3 −, K+, NO3 2−, and SO4 2− for the POM period; the highest value for the PRM period observed for the variables like K+, Ca2+, Mg2+, SO4 2−, HCO3 −, and NO3 2−. High loadings of calcium, magnesium and bicarbonate indicate the dominance of silicate and carbonate weathering in the study area. Sources of sulfate include rainfall, fertilizers and dissolution of sulfide minerals present in the study area. High loadings of K+ and NO3 − indicate the infiltration of fertilizer-rich irrigation water to the groundwater regime (Saha et al. 2008). The second component is observed to account for 13.79 and 18.54 % of total variance during POM and PRM periods, respectively. This component is essentially composed of Na+ and Cl− during POM and PRM period, respectively. A high loading of sodium indicates natural weathering of minerals and ion-exchange processes in the groundwater (Drever 1997). Loading of chloride indicates the anthropogenic input in the groundwater system from leaching of industrial effluents, domestic and agricultural and from natural sources such as rainfall and Cl− bearing minerals. The third component accounts for 9.24 and 12.23 % of the total variance, with high loading on pH for both post- and pre-monsoon periods, respectively. The pH depends on the CO2–CO3–HCO3 equilibrium and hence the pH of groundwater refers to the form in which CO2 is present. While the presence of carbonic acid is indicated by pH values less than 4.5, presence of bicarbonate and carbonate is signified by pH values in the range of 4.5–8.2 and greater than 8.2, respectively (Singh et al. 2012). Since the pH range of the analyzed groundwater samples vary from 6.2 to 7.97, it indicates heavy influence of bicarbonate ions.
Summary and conclusion
In recent decades, demand for freshwater, particularly for agricultural and domestic purposes, has led to drastic depletion and deterioration of groundwater in several parts of eastern India. In the present study a detailed investigation is carried out with the objectives of identifying hydrochemical processes and their relation with groundwater quality using various statistical and analytical metrics. The results presented here highlight the quality aspect of groundwater for human consumption as well as for agricultural purposes.
Groundwater in the Rajgir-Nalanda area of the Southern Gangetic Plain is generally slightly acidic, hard to very hard and fresh to brackish in nature. The concentration of cations and anions are in the order of, Na+ > Ca2+ > Mg2+ > K+ and HCO3 − > Cl− > SO4 2− > NO3 −, respectively. Concentrations of pH, TDS, TH, Na, and HCO3 − exceed the desirable limit at some places and require treatment before utilization. In a majority of the groundwater samples the concentration of alkalis exceeds that of alkaline earth metals. Piper diagram reveals that nearly 65 and 30 % of the analyzed groundwater samples fall in the field of Ca2+–Mg2+–HCO3 − type for POM and PRM periods, respectively. The rest of the samples are falls in the field of mixed Ca2+–Na+–HCO3 − type. Water chemistry of the study area strongly reflects the dominance of rock-weathering with minor effects of evaporation.
SAR, %Na and RSC values along with USSL salinity diagram and Wilcox diagram reflect on the suitability of groundwater for irrigation purposes. The calculated SAR value in this area ranges between 0.9–4.9 in post-monsoon (POM) and 0.6–15.4 in pre-monsoon (PRM) periods. Plotting of hydrogeochemical data in USSL diagram shows that approximately 65 % of the groundwater samples fall in the field of C3S1 category, indicative of groundwater with medium to high salinity. Based on %Na in Wilcox diagram, nearly 36 % of groundwater is found to be of excellent to good category for use in irrigation. Overall, results exhibit that nearly 40–50 % of the sampled groundwater in the study area is suitable for irrigation, while the remaining are marginal/doubtful or unsuitable for irrigation. Principal component analysis (PCA) of geochemical parameters reveals the dominance of the first three principal components (PCs), representing 80.84 and 78.85 % of cumulative variance, during the POM and PRM periods, respectively. These three components identify hydrogeochemical processes like mineral dissolution, silicate and carbonate weathering with anthropogenic activities like leaching of fertilizers from agricultural lands as primary drivers controlling the overall variance in the geochemical properties of groundwater in the study area.
References
Acharya SK, Chakraborty P, Lahiri S, Ray MBC, Guha S, Bhowmik A (1999) Arsenic poisoning in Ganges Delta. Nature 401:545
Aghazadeh N, Moghaddam AA (2010) Investigation of hydrochemical characteristics of groundwater in the Harzandat aquifer, Northwest of Iran. Environ Monit Assess 176:183–195
APHA (2005) Standard methods for Examination of Water and Waste water 21st (ed) Washington, DC
BIS (2003) Indian Standard drinking water specifications IS 10500:1991, 2.2 (edn) (2003–2009). Bureau of Indian standards, New Delhi
Bozdag A, Gocmez G (2013) Evaluation of groundwater quality in the Cihanbeyli basin, Konya, Central Anatolia, Turkey. Environ Earth Sci 69:921–937
CGWB (2010) Groundwater quality in shallow aquifers of India. Ministry of Water Resources GOI, Faridabad
Chakraborty S, Sikdar PK (2009) Geological framework and isotope tracing of the arsenious quarternary aquifer of the southwestern north bengal plain, West Bengal, India. Environ Earth Sci 59(4):723–736
Chakroborty C, Chattopadhyay GS (2001) Quarternary geology of South Ganga Plain in Bihar. Ind Min 55:133–142
Davis JC (1986) Statistics and data analysis in geology. John Wiley & Sons, New York
De P (1986) Quarternary geology and geomorphology of Son-Ganga Basin, Nalanda district, Bihar. Geological Survey of India unpublished report no. UE9971, Geological Survey of India, Calcutta, p 7
Domenico PA (1972) Concepts and models in groundwater hydrology. McGraw-Hill, New York
Drever JI (1997) The geochemistry of natural waters, 3rd edn. Prentice Hall, Englewood Cliffs, p 436
Dudeja D, Bartarya SK, Khanna PP (2013) Ionic sources and water quality assessment around a reservoir in Tehri, Uttarakhand, Garhwal Himalaya. Environ Earth Sci 69:2513–2527. doi:10.1007/s12665-012-2076-2
Esmaeili A, Moore F (2011) Hydrogeochemical assessment of groundwater in Isfahan province, Iran. Environ Earth Sci. doi:10.1007/s12665-011-1484-z
Freeze RA, Cherry JA (1979) Groundwater. Prentice Hall, Englewood Cliffs
Gibbs RJ (1970) Mechanisms controlling world’s water chemistry. Science 170:1088–1090
Kaiser HF (1960) The application of electronic computers to factor analysis. Educ Psychol Measur 20:141–151
Karanth KR (1989) Groundwater assessment development and management. Tata McGraw-Hill Publ. Comp. Ltd, New Delhi
Kelley WP (1957) Adsorbed sodium cation exchange capacity and percentage sodium sorption in alkali soils. Science 84:473–477
Laluraj CM, Gopinath G (2006) Assessment on seasonal variation of groundwater quality of phreatic aquifers—a river basin system. Environ Monit Assess 117:45–57
Lucas L, Jauzein M (2008) Use of principal component analysis to profile temporal and spatial variations of chlorinated solvent concentration in groundwater. Environ Pollut 151:205–212. doi:10.1016/j.envpol.2007.01.054
Mahapatra SS, Sahu M, Patel RK, Panda BN (2012) Prediction of water quality using principal component analysis. Water Qual Expo Health 4:93–104. doi:10.1007/s12403-012-0068-9
Naik P, Awashti AK, Anand AVSS, Behera PN (2009) Hydrogeochemistry of the Koyna River basin, India. Environ Earth Sci 59:613–629
Nandimandalam JR (2012) Evaluation of hydrogeochemical processes in the Pleistocene aquifers of Middle Ganga Plain, Uttar Pradesh, India. Environ Earth Sci 65:1291–1308
Park SC, Yun S, Chae GT, Yoo IS, Shin KS, Heo CH (2005) Regional hydrochemical study on salinization of coastal aquifers, western coastal area of South Korea. J Hydrol 313:182–194
Piper AM (1944) A graphical procedure in the geochemical interpretation of water analysis. Trans Am Geophys Union 25:914–928
Prasanna MV, Chidambaram S, Senthil KG, Ramanathan AL, Nainwal HC (2011) Hydrogeochemical assessment of groundwater in Neyveli Basin, Cuddalore District, South India. Arab J Geosci 4:319–330
Raju NR (2006) Seasonal evaluation of hydro-geochemical parameters using correlation and regression analysis. Curr Sci 91(6):820–826
Raju NJ (2007) Hydrogeochemical parameters for assessment of groundwater quality in the upper Gunjanaeru River basin, Cuddapah district, Andhra Pradesh, South India. Environ Geol 52:1067–1074
Ramesh K, Elango L (2011) Groundwater quality and its suitability for domestic and agricultural use in Tondiar river basin, Tamil Nadu, India. Environ Monit Assess. doi:10.1007/s10661-011-2231-3
Ravikumar P, Venkatesharaju K, Prakash KL, Somashekar RK (2010) Geochemistry of groundwater and groundwater prospects evaluation, Anekal Taluk, Banglore Urban District, Karnataka, India. Environ Monit Assess 179:93–112
Rouxel M, Molenat J, Ruiz L, Legout C, Faucheux M, Gascuel-Odoux C (2011) Seasonal and spatial variation in groundwater quality along the hillslope of an agricultural research catchment (Western France). Hydrol Process 25(6):831–841
Saha D, Alam F (2014) Groundwater vulnerability assessment using DRASTIC and Pesticide DRASTIC models in intense agriculture area of the Gangetic plains. Environ Monit Assess, India. doi:10.1007/s10661-014-4041-x
Saha D, Dhar YR, Sikdar PK (2008) Geochemical evolution of groundwater in the Pleistocene aquifers of South Ganga Plain Bihar. J Geo Soc Ind 71:473–482
Saha D, Sarangam SS, Dwivedi SN, Bhartariya KG (2010) Evaluation of hydrogeochemical processes in arsenic-contaminated alluvial aquifers in parts of Mid-Ganga Basin, Bihar, Eastern India. Environ Earth Sci 61:799–811
Sahu S, Saha D (2015) Role of shallow alluvial stratigraphy and Holocene geomorphology on groundwater arsenic contamination in the Middle Ganga Plain. Environ Earth Sci, India. doi:10.1007/s12665-014-3637-3
Saleh A, Al-Ruwaih F, Shehata M (1999) Hydrogeochemical processes operating within the main aquifers of Kuwait. J Arid Environ 42:195–209
Satapathy I, Syed TH (2015) Characterization of groundwater potential and artificial recharge sites in Bokaro District, Jharkhand (India), using remote sensing and GIS-based techniques. Environ Earth Sci. doi:10.1007/s12665-015-4474-8
Sawyer CN, McCarty DL (1967) Chemistry of sanitary engineers, 2nd edn. McGraw-Hill, New York, p 518
Selvam S, Manimaran G, Sivasubramanian P, Balasubramanian N, Seshunarayana T (2013) GIS-based evaluation of water quality index of groundwater resources around Tuticorin coastal city. Environ Earth Sci, South India. doi:10.1007/s12665-013-2662-y
Senthilkumar M, Elango L (2013) Geochemical processes controlling the groundwater quality in lower Palar river basin, southern India. J Earth Syst Sci 122:419–432
Sharma ND, Patel JN (2010) Evaluation of groundwater quality index of the urban segments of Surat city India. Int J Geol 4:1–4
Shukla G, Kumar A, Bhanti M, Joseph PE, Taneja A (2006) Organochlorine pesticide contamination of groundwater in the city of Hyderabad. Environ Int 32:244–247
Sikdar PK, Chakraborty S (2008) Genesis of arsenic in groundwater of North Bengal Plain using PCA: a case study of English Bazar block, Malda District, West Bengal, India. Hydrol Process 22(13):1796–1809
Sikdar PK, Sarkar SS, Palchoudhury S (2001) Geochemical evolution of groundwater in the quarternary aquifer of Calcutta and Howrah, India. J Asian Earth Sci 19:579–594
Singh UK, Kumar M, Chauhan R, Jha PK, Ramanathan AL, Subramanian V (2008) Assessment of the impact of landfill on groundwater quality: a case study of the Pirana site in western India. Environ Monit Assess 141:309–321
Singh AK, Mondal GC, Singh TB, Singh S, Tewary BK, Sinha A (2012) Hydrogeochemical processes and quality assessment of groundwater in Dumka and Jamtara districts, Jharkhand, India. Environ Earth Sci 67:2175–2191. doi:10.1007/s12665-012-1658-3
Singh K, Singh D, Hundal HS, Khurana MPS (2013) An appraisal of groundwater quality for drinking and irrigation purposes in southern part of Bathinda district of Punjab, northwest India. Environ Earth Sci. doi:10.1007/s12665-013-2272-8
Sophiya MS, Syed TH (2013) Assessment of vulnerability to sweater intrusion and potential remediation measures for coastal aquifers: a case study from eastern India. Environ Earth Sci 70(3):1197–1209. doi:10.1007/s12665-012-2206-x
Subramani T, Elango L, Damodarasamy SR (2005) Groundwater quality and its suitability for drinking and agricultural use in Chitar River Basin, Tamilnadu, India. Environ Geol 47:1099–1110
Syed TH, Lakshmi V, Paleologos E, Lohmann D, Mitchell K, Famiglietti JS (2004) Analysis of process controls in land surface hydrological cycle over the continental United States. J Geophys Res 109:D22105. doi:10.1029/2004JD004640
USSL (1954) Diagnosis and improvement of saline and alkali soils. United states Development Agency handbook 60. Government Printing Office, Washington, DC, p 147
Vasanthavigar M, Srinivasamoorthy K, Prasanna MV (2012) Identification of groundwater contamination zones and its sources by using multivariate statistical approach in Thirumanimuthar sub-basin, Tamil Nadu, India. Environ Earth Sci. doi:10.1007/s12665-012-1868-8
WHO (2011) Guidelines for drinking water quality, vol. 4, Recommendations, 3rd edn, Geneva
Wilcox LV (1955) Classification and use of irrigation waters. USDA, Circular 969, Washington, DC, USA
Xiao J, Jin ZD, Zhang F, Wang J (2012) Solute geochemistry and its sources of the groundwaters in the Qinghai Lake Catchment, NW China. J Asian Earth Sci 52:21–30
Acknowledgments
The Junior Research Fellow grant of the Indian School of Mines, Dhanbad has sponsored a part of this research. We are grateful to the four anonymous reviewers for their valuable comments which helped us in improving the quality of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sethy, S.N., Syed, T.H., Kumar, A. et al. Hydrogeochemical characterization and quality assessment of groundwater in parts of Southern Gangetic Plain. Environ Earth Sci 75, 232 (2016). https://doi.org/10.1007/s12665-015-5049-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-015-5049-4